Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review
Abstract
:1. Introduction
2. PFAS Applications and Related Concerns
2.1. What Are PFASs Used for?
2.2. Reasons for Concern
2.3. Physical Separation vs. Advanced Oxidation Processes
2.4. Analytical Techniques for PFAS Detection in Water
2.4.1. Chromatographic Techniques
2.4.2. Sensing Systems for PFAS Detection
2.4.3. Total Fluorine Analysis (TF)
3. Advanced Oxidation Processes (AOPs) for PFAS
3.1. Heterogeneous Photocatalysis Materials
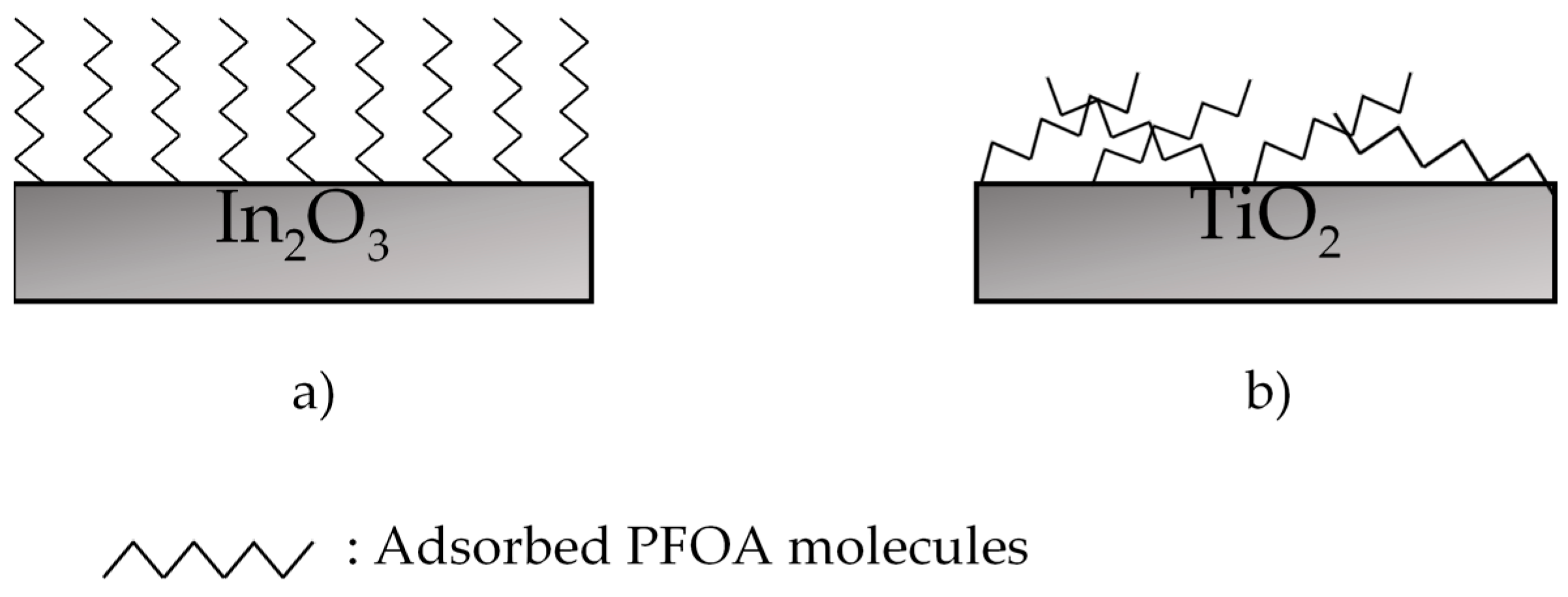
3.1.1. Metal Oxides
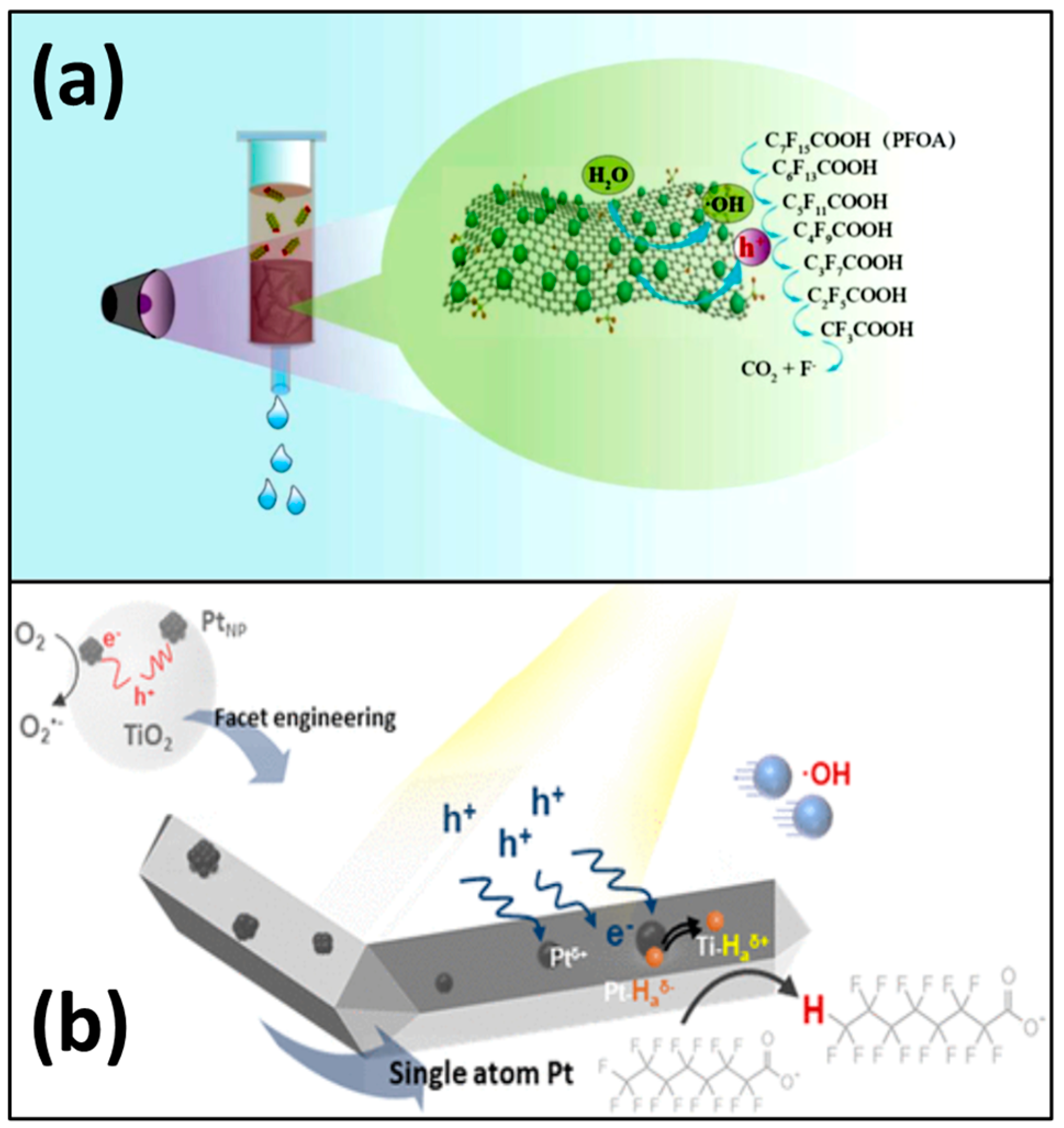

3.1.2. Other Photocatalysts
| Photocatalytic Performance | Photocatalytic Reaction Condition | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Photocatalyst | Reaction Time (h) | Rate Constant (min−1) | % Degradation | % Defluorination | Concentration of PFOA/PFOS | Catalyst Dosage (g/L) | pH of Solution | Light Source | |
| TiO2 | 12 | 0.0001 | 15% | --- | 50 mg/L | 0.5 | 5 | 254 nm and 400 W | [57] |
| Titanate nanotubes | 24 | --- | 59% | --- | 120.8 µM | 0.125 | 4 | 254 nm and 400 W | [58] |
| P25 TiO2 | 3 | 0.0005 | 30% | ---- | 12 mM | 0.66 | 3 | 310–400 nm and 75 W/m2 | [59] |
| TiO2-HClO4 | 7 | 0.0047 | 97% | 38% | 120 µM | 0.66 | 2.47 | 254 nm and 16 W | [56] |
| TiO2-Oxalic acid | 3 | --- | 86.7% | 16.5% | 24 µM | 0.5 | 2.47 | 254 nm and 23 W | [60] |
| TiO2-MWCNT | 8 | --- | 100% | ---- | 72.5 µM | 0.4 | 5 | 365 nm and 300 W | [61] |
| TiO2-rGO | 12 | 0.0027 | 93 ± 7% | 20% | 240 µM | 0.1 | 3.8 | 254 nm and 150 W | [63] |
| TiO2-MIP | 10 | 0.0044 | 81% | 30.2% | 72.5 µM | -- | 5 | 254 nm and 23 W | [65] |
| TiO2 QD/Graphene | 10 | 0.0098 | ~90% | ---- | 300 µM | 0.02 | 5 | UV light 150 W | [64] |
| TiO2-Pt | 7 | 0.0121 | 100 | 34.8 | 144.9 µM | 0.5 | 3 | 365 nm and 135 W | [66] |
| TiO2-Pd | 0.0073 | 94.2 | 25.9 | ||||||
| TiO2-Ag | 0.0021 | 57.7 | 8.1 | ||||||
| TiO2-Cu | 12 | 0.0031 | 91 | 19 | 121 µM | 0.5 | 5 | 254 nm and 400 W | [57] |
| TiO2-Fe | 4 | 0.0015 | 60 | -- | |||||
| TiO2-Pb | 4.5 | 0.0086 | 99 | 22.4 | 121 µM | 0.5 | 5 | 254 nm and 400 W | [68] |
| TiO2-Pt single atom | 2 | 40 | 35 | 100 µM | 0.25 | 7 | 254 nm and 7.87 mW/cm2 | [67] | |
| Fe/TNT@AC | 4 | 0.0153 | 90 | 62 | 100 µM | 1 | 7 | 254 nm and 21 mW/cm2 | [69] |
| Ga/TNT@AC | 4 | 75 | 66.2 | 0.1 mg/L | 3 | 7 | 254 nm and 210 W/m2 | [70] | |
| ZnO | 3 days | --- | 95 | --- | 25.5 µM | 1 | 6.5–7.0 | 365 nm and 13 W/m2 | [71] |
| Ga2O3 | 3 | 0.0020 | 36 | 15 | 0.5 mg/L | 0.5 | -- | 254 nm and 14 W | [73] |
| Ga2O3 needle-like | 40 min | 0.0380 | 100 | 58 | 0.5 mg/L | 0.5 | -- | 254 nm and 14 W | [74] |
| Ga2O3 sheaf-like | 45 min | 0.0240 | 100 | 60 | 0.5 mg/L | 0.5 | --- | 254 nm and 14 W | [75] |
| β-Ga2O3 nanorods | 1.5 | 0.0425 | 98.8 | 56.2 | 10 mg/L | 0.5 | 3 | 254 nm and 50 W | [76] |
| In-doped Ga2O3 nanosheets | 1 | 0.0416 | 100 | -- | 20 mg/L | 0.5 | -- | Hg lamp | [77] |
| In2O3 | 4 | 0.006 | 75 | 33 | 40 mg/L | 3.4 | 5 | 254 nm and 23 W | [10] |
| In2O3 microspheres | 17 min | 0.130 | 100 | -- | 30 mg/L | 0.5 | 3 | 254 nm and 15 W | [78] |
| In2O3 nanocubes | 2 | 0.030 | 100 | -- | |||||
| In2O3 nanoplates | 42 min | 0.073 | 100 | -- | |||||
| In2O3 nanoporous nanospheres | 30 min | 0.100 | 100 | 71 | 30 mg/L | 0.5 | 3.9 | 254 nm and 23 W | [9] |
| In2O3 porous nanoplates | 30 min | 0.158 | 100 | --- | 30 mg/L | 0.5 | --- | 254 nm and 15 W | [79] |
| MOF-derived In2O3 nanospheres | 3 | --- | 100 | 50 | 10 mg/L | 0.2 | --- | 254 nm and 32 W | [80] |
| In2O3/graphene | 3 | 0.008 | 100 | 60.9 | 30 mg/L | 0.5 | 3 | 254 nm and 15 W | [81] |
| In2O3/Ce2O3 | 1 | 0.063 | 100 | 53.3 | 100 mg/L | 0.4 | -- | 254 nm and 500 W | [82] |
| In2O3/MnOx | 3 | 0.301 | 100 | 17.4 | 50 mg/L | 0.5 | 3.8 | Xenon lamp and 500 W | [83] |
| InOOH | 3 | 0.008 | 83.4 | --- | 20 mg/L | 0.25 | 254 nm and 18 W | [84] | |
| BiOCl | 12 | --- | 100 | 59.3 | 20 µM | 0.5 | 4.8 | 254 nm and 10 W | [87] |
| BiOCl with OV | 8.5 | --- | 68.8 | 12.86 | 50 µM | 1 | 2 | Xenon lamp and 300 W | [88] |
| BiOF | 6 | 0.006 | 100 | 56.8 | 15 mg/L | 3–5 | UV light | [89] | |
| BiOI | 2 | 0.0048 | 66 | 37% in 3h | 20 mg/L | 0.4 | -- | Hg lamp and 300 W | [90] |
| BiOI0.95Br0.05 | 2 | 0.0205 | 100 | 65% in 3h | 20 mg/L | 0.4 | -- | Hg lamp and 300 W | [90] |
| Bi/BiOI1-xFx | 2 | 0.0375 | 100 | 10 | 40 mg/L | 0.4 | 3–5 | Xenon lamp and 800 W | [91] |
| BiOI@Bi5O7I | 6 | 0.0041 | 81 | 60 | 15 mg/L | 0.5 | 3 | Xenon lamp and 800 W | [92] |
| BiOI/ZnO | 6 | 0.0002 | 91 | 52.2 | 1 mg/L | 0.5 | 4 | Xenon lamp and 500 W | [93] |
| BOHP | 1 | 0.1000 | 100 | 60 | 0.13 mM | 1.8 | 4 | 254 nm and 18 W | [94] |
| BOHP/CS | 4 | --- | 100 | 32.5 | 0.2 mg/L | 1.0 | 7 | 254 nm and 18 W | [95] |
| FeO/CS | 1 | --- | 95.2 | 57.2 | 0.2 mg/L | 1.0 | 7 | Stimulated solar light | [96] |
| Boron nitride | 4 | 100 | 52 | 0.12 mM | 2.5 | 6.5 | 254 nm and 24 W | [97] | |
| H3PW12O40/bimodal mesoporous silica | 4 | 0.0018 | 50 | 10 mg/L | 0.2 | 4 | 254 nm and 8 W | [98] | |
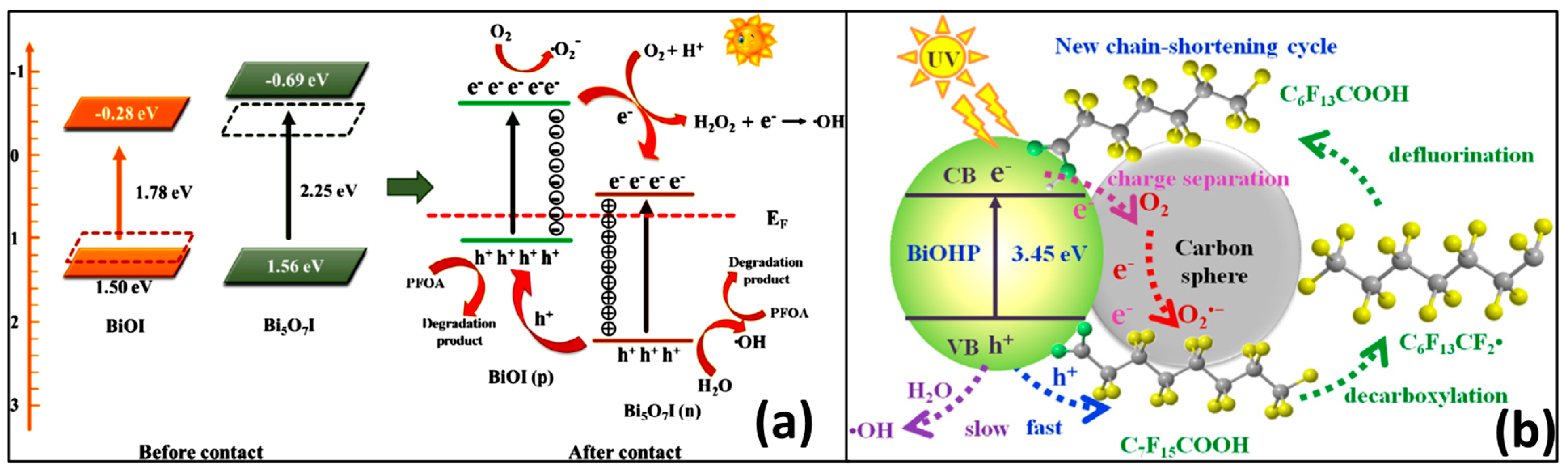
3.1.3. Reaction Mechanisms
4. Direct Photolysis
5. Methods Based on a Sacrificial Radical Source
6. Light Sources
7. Economic Aspects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Baran, J.R. Fluorinated Surfactants and Repellents, 2nd ed.Revised and Expanded Surfactant Science Series; American Chemical Society: Washington, DC, USA, 2001; Volume 123, p. 8882. [Google Scholar] [CrossRef]
- Interstate Technology and Regulatory Council (ITRC), PFAS Technical and Regulatory Guidance Document and Fact. 2020. Available online: https://pfas-1.itrcweb.org/ (accessed on 5 November 2020).
- Kirk, M.; Smurthwaite, K.; Braeunig, J.; Trevenar, S.; D’Este, C.; Lucas, R.; Lal, A.; Korda, R.; Clements, A.; Mueller, J. The PFAS Health Study: Systematic Literature Review. 2018. Available online: https://rsph.anu.edu.au/files/PFAS%20Health%20Study%20Systematic%20Review_1.pdf (accessed on 11 September 2021).
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chem. Eng. J. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Xu, Y.; Fletcher, T.; Pineda, D.; Lindh, C.H.; Nilsson, C.; Glynn, A.; Vogs, C.; Norström, K.; Lilja, K.; Jakobsson, K.; et al. Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ. Health Perspect. 2021, 128, 77004. [Google Scholar] [CrossRef]
- Kirsch, P. Front Matter. In Modern Fluoroorganic Chemistry; Wiley-VCH: Baden-Württemberg, Germany, 2004; pp. i–xii. [Google Scholar] [CrossRef]
- Guo, R.; Sim, W.-J.; Lee, E.-S.; Lee, J.-H.; Oh, J.-E. Evaluation of the fate of perfluoroalkyl compounds in wastewater treatment plants. Water Res. 2010, 44, 3476–3486. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, P.; Shao, T.; Li, X. In2O3 nanoporous nanosphere: A highly efficient photocatalyst for decomposition of perfluorooctanoic acid. Appl. Catal. B Environ. 2012, 125, 350–357. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Jin, L.; Shao, T.; Li, Z.; Cao, J. Efficient Photocatalytic Decomposition of Perfluorooctanoic Acid by Indium Oxide and Its Mechanism. Environ. Sci. Technol. 2012, 46, 5528–5534. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.L.; Altaee, A.; Ahmed, M.B.; Johir, M.A.H.; Ren, J.; Li, X. Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate. Chemosphere 2020, 239, 124722. [Google Scholar] [CrossRef]
- Henry, B.J.; Carlin, J.P.; Hammerschmidt, J.A.; Buck, R.C.; Buxton, L.W.; Fiedler, H.; Seed, J.; Hernandez, O. A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers. Integr. Environ. Assess. Manag. 2018, 14, 316–334. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Washington, J.W.; Jenkins, T.M.; Rankin, K.; Naile, J.E. Decades-Scale Degradation of Commercial, Side-Chain, Fluorotelomer-Based Polymers in Soils and Water. Environ. Sci. Technol. 2015, 49, 915–923. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- C8 Science Panel. 2019. Available online: www.c8sciencepanel.org (accessed on 22 January 2020).
- Emerging Chemical Risks in Europe—‘PFAS’. Europen Environment Agency, 12 December 2019. Last modified 9 March 2021. Available online: https://www.eea.europa.eu/publications/emerging-chemical-risks-in-europe (accessed on 31 March 2021). [CrossRef]
- ATSDR. Toxicological Profile for Perfluoroalkyls Draft for Public Comment; US Dept. of Health and Human Sciences: Atlanta, GA, USA, 2018. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 15 April 2021).
- WHO. Some Chemicals Used as Solvents and in Polymer Manufacture; WHO: Geneva, Switzerland, 2016; Volume 110, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; ISBN 978-92-832-0148-9. Available online: https://www.iarc.who.int/news-events/iarc-monographs-volume-110-some-chemicals-used-as-solvents-and-in-polymer-manufacture/ (accessed on 20 April 2021).
- Vaughn, B.; Andrea, W.; Kyle, S. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living Near a Chemical Plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Fenton, S.E.; Reiner, J.L.; Nakayama, S.F.; Delinsky, A.D.; Stanko, J.P.; Hines, E.P.; White, S.S.; Lindstrom, A.B.; Strynar, M.J.; Petropoulou, S.-S.E. Analysis of PFOA in dosed CD-1 mice. Part 2: Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009, 27, 365–372. [Google Scholar] [CrossRef] [Green Version]
- White, S.; Stanko, P.; Kayoko, K.; Calafat, A.M.; Hines, E.P.; Fenton, S. Gestational and Chronic Low-Dose PFOA Exposures and Mammary Gland Growth and Differentiation in Three Generations of CD-1 Mice. Environ. Health Perspect. 2011, 119, 1070–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, N.B.; Khalid, A.; Tian, Y.; Ayres, C.; Sabaraya, I.V.; Pietari, J.; Hanigan, D.; Chowdhury, I.; Apul, O.G. Removal of poly- and per-fluoroalkyl substances from aqueous systems by nano-enabled water treatment strategies. Environ. Sci. Water Res. Technol. 2019, 5, 198–208. [Google Scholar] [CrossRef]
- ECHA Guidance on Information Requirements and Chemical Safety Assessment. 2016. Available online: https://echa.europa.eu/guidance-documents/guidance-on-information-requirements-and-chemical-safety-assessment (accessed on 12 February 2020).
- Fourth National Report on Human Exposure to Environmental Chemicals; 2021. Available online: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2021-508.pdf (accessed on 30 April 2021).
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the Applications, Environmental Release, and Remediation Technologies of Per- and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public Heal. 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Mahinroosta, R.; Senevirathna, L. A review of the emerging treatment technologies for PFAS contaminated soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef] [PubMed]
- Appleman, T.D. The Removal of Poly- and Perfluoroalkyl Substances by North American Water Treatment Practices, Colorado School of Mines. 2012. Available online: http://hdl.handle.net/11124/78755 (accessed on 10 December 2020).
- Lu, D.; Sha, S.; Luo, J.; Huang, Z.; Jackie, X.Z. Treatment train approaches for the remediation of per- and polyfluoroalkyl substances (PFAS): A critical review. J. Hazard. Mater. 2020, 386, 121963. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Thelakkat Kochunarayanan, P.; Kalve, E.; Hurst, J.; Dasgupta, S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—A review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- Jahnke, A.; Berger, U. Trace analysis of per- and polyfluorinated alkyl substances in various matrices—How do current methods perform? J. Chromatogr. A 2009, 1216, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, H.A.; Vo Duy, S.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.; Sory, T.K.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616–617, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Barzen-Hanson, K.A.; Field, J.A. Discovery and Implications of C2 and C3 Perfluoroalkyl Sulfonates in Aqueous Film-Forming Foams and Groundwater. Environ. Sci. Technol. Lett. 2015, 2, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Yeung, L.W.Y.; Stadey, C.; Mabury, S.A. Simultaneous analysis of perfluoroalkyl and polyfluoroalkyl substances including ultrashort-chain C2 and C3 compounds in rain and river water samples by ultra performance convergence chromatography. J. Chromatogr. A 2017, 1522, 78–85. [Google Scholar] [CrossRef]
- Shao, M.; Ding, G.; Zhang, J.; Wei, L.; Xue, H.; Zhang, N.; Li, Y.; Chen, G.; Sun, Y. Occurrence and distribution of perfluoroalkyl substances (PFASs) in surface water and bottom water of the Shuangtaizi Estuary, China. Environ. Pollut. 2016, 216, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.; Nödler, K.; Brauch, H.-J.; Zwiener, C.; Lange, F.T. Robust trace analysis of polar (C2-C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: Method development and application to surface water, groundwater and drinking water. Environ. Sci. Pollut. Res. 2019, 26, 7326–7336. [Google Scholar] [CrossRef]
- Gellrich, V.; Brunn, H.; Stahl, T. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in mineral water and tap water. J. Environ. Sci. Health Part A 2013, 48, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Pereira, A.D.S.; Martin, J.W. Discovery of C5–C17 Poly- and Perfluoroalkyl Substances in Water by In-Line SPE-HPLC-Orbitrap with In-Source Fragmentation Flagging. Anal. Chem. 2015, 87, 4260–4268. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, H.; Pan, Y.; Shi, Y.; Cai, Y. Chitosan-Coated Octadecyl-Functionalized Magnetite Nanoparticles: Preparation and Application in Extraction of Trace Pollutants from Environmental Water Samples. Anal. Chem. 2010, 82, 2363–2371. [Google Scholar] [CrossRef]
- Xia, W.; Wan, Y.-J.; Wang, X.; Li, Y.; Yang, W.-J.; Wang, C.-X.; Xu, S. Sensitive bioassay for detection of PPARα potentially hazardous ligands with gold nanoparticle probe. J. Hazard. Mater. 2011, 192, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Takayose, M.; Akamatsu, K.; Nawafune, H.; Murashima, T.; Matsui, J. Colorimetric Detection of Perfluorooctanoic Acid (PFOA) Utilizing Polystyrene-Modified Gold Nanoparticles. Anal. Lett. 2012, 45, 2856–2864. [Google Scholar] [CrossRef]
- Niu, H.; Wang, S.; Zhou, Z.; Ma, Y.; Ma, X.; Cai, Y. Sensitive Colorimetric Visualization of Perfluorinated Compounds Using Poly(ethylene glycol) and Perfluorinated Thiols Modified Gold Nanoparticles. Anal. Chem. 2014, 86, 4170–4177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Buck, R.C.; Hungerbühler, K. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environ. Int. 2014, 70, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Megharaj, M.; Naidu, R. Surface-enhanced Raman scattering (SERS) detection of fluorosurfactants in firefighting foams. RSC Adv. 2016, 6, 11140–11145. [Google Scholar] [CrossRef]
- Okaru, A.O.; Brunner, T.S.; Ackermann, S.M.; Kuballa, T.; Walch, S.G.; Kohl-Himmelseher, M.; Lachenmeier, D.W. Application of 19F NMR Spectroscopy for Content Determination of Fluorinated Pharmaceuticals. J. Anal. Methods Chem. 2017, 2017, 9206297. [Google Scholar] [CrossRef]
- Guo, W.; Jin, L.; Hu, S.; Guo, Q. Method Development for the Determination of Total Fluorine in Foods by Tandem Inductively Coupled Plasma Mass Spectrometry with a Mass-Shift Strategy. J. Agric. Food Chem. 2017, 65, 3406–3412. [Google Scholar] [CrossRef]
- Boča, M.; Barborík, P.; Mičušík, M.; Omastová, M. X-ray photoelectron spectroscopy as detection tool for coordinated or uncoordinated fluorine atoms demonstrated on fluoride systems NaF, K2TaF7, K3TaF8, K2ZrF6, Na7Zr6F31 and K3ZrF7. Solid State Sci. 2012, 14, 828–832. [Google Scholar] [CrossRef]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Liang, Y. Nanotechnology in remediation of water contaminated by poly- and perfluoroalkyl substances: A review. Environ. Pollut. 2019, 247, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Wu, M.; Xu, G. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors. Chem. Eng. J. 2017, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, W.; Xu, J.; Wang, D.; Song, L.; Ni, B.-J. Photochemical decomposition of perfluorochemicals in contaminated water. Water Res. 2020, 186, 116311. [Google Scholar] [CrossRef]
- Panchangam, S.C.; Lin, A.Y.-C.; Shaik, K.L.; Lin, C.-F. Decomposition of perfluorocarboxylic acids (PFCAs) by heterogeneous photocatalysis in acidic aqueous medium. Chemosphere 2009, 77, 242–248. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Huang, C.-C. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide. J. Hazard. Mater. 2015, 288, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lo, S.-L.; Kuo, J. Effects of titanate nanotubes synthesized by a microwave hydrothermal method on photocatalytic decomposition of perfluorooctanoic acid. Water Res. 2011, 45, 4131–4140. [Google Scholar] [CrossRef]
- Sansotera, M.; Persico, F.; Pirola, C.; Navarrini, W.; Di Michele, A.; Bianchi, C.L. Decomposition of perfluorooctanoic acid photocatalyzed by titanium dioxide: Chemical modification of the catalyst surface induced by fluoride ions. Appl. Catal. B Environ. 2014, 148–149, 29–35. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P. Photocatalytic decomposition of perfluorooctanoic acid (PFOA) by TiO2 in the presence of oxalic acid. J. Hazard. Mater. 2011, 192, 1869–1875. [Google Scholar] [CrossRef]
- Song, C.; Chen, P.; Wang, C.; Zhu, L. Photodegradation of perfluorooctanoic acid by synthesized TiO2-MWCNT composites under 365nm UV irradiation. Chemosphere 2012, 86, 853–859. [Google Scholar] [CrossRef]
- Rivero, M.J.; Ribao, P.; Gomez-Ruiz, B.; Urtiaga, A.; Ortiz, I. Comparative performance of TiO2-rGO photocatalyst in the degradation of dichloroacetic and perfluorooctanoic acids. Sep. Purif. Technol. 2020, 240, 116637. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Xu, J.; Song, S.; Wang, J.; Li, Y.; Liu, R.; Shen, Y. TiO2 quantum dots loaded sulfonated graphene aerogel for effective adsorption-photocatalysis of PFOA. Sci. Total Environ. 2020, 698, 134275. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Tian, A.; Mao, K.; Liu, J. Selective Removal of Perfluorooctanoic Acid Using Molecularly Imprinted Polymer-Modified TiO2 Nanotube Arrays. Int. J. Photoenergy 2016, 2016, 7368795. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yu, Z.; Liu, Q.; Sun, L.; Huang, W. Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2. Chem. Eng. J. 2016, 286, 232–238. [Google Scholar] [CrossRef]
- Weon, S.; Suh, M.-J.; Chu, C.; Huang, D.; Stavitski, E.; Kim, J.-H. Site-Selective Loading of Single-Atom Pt on TiO2 for Photocatalytic Oxidation and Reductive Hydrodefluorination. ACS ES&T Eng. 2021, 1, 512–522. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Kuo, J.; Wu, C.-H. Decomposition of perfluorooctanoic acid by ultraviolet light irradiation with Pb-modified titanium dioxide. J. Hazard. Mater. 2016, 303, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Abada, B.; Alivio, T.E.G.; Shao, Y.; O’Loughlin, T.E.; Klemashevich, C.; Banerjee, S.; Jayaraman, A.; Chu, K.-H. Photodegradation of fluorotelomer carboxylic 5:3 acid and perfluorooctanoic acid using zinc oxide. Environ. Pollut. 2018, 243, 637–644. [Google Scholar] [CrossRef]
- Ong, C.B.; Mohammad, A.W.; Ng, L.Y.; Mahmoudi, E.; Azizkhani, S.; Hayati Hairom, N.H. Solar photocatalytic and surface enhancement of ZnO/rGO nanocomposite: Degradation of perfluorooctanoic acid and dye. Process Saf. Environ. Prot. 2017, 112, 298–307. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, P. Photocatalytic decomposition of perfluorooctanoic acid with β-Ga2O3 wide bandgap photocatalyst. Catal. Commun. 2009, 10, 1184–1187. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, P.; Li, Z.; Jin, L. Photocatalytic decomposition of perfluorooctanoic acid in pure water and wastewater by needle-like nanostructured gallium oxide. Chin. J. Catal. 2013, 34, 1551–1559. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, P.; Jin, L.; Li, Z. Photocatalytic decomposition of perfluorooctanoic acid in pure water and sewage water by nanostructured gallium oxide. Appl. Catal. B Environ. 2013, 142–143, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Li, X.; Yang, L.; Wang, F.; Li, J.; Xia, W.; Li, W.; Zhou, L.; Zhao, C. ß-Ga2O3 Nanorod Synthesis with a One-step Microwave Irradiation Hydrothermal Method and its Efficient Photocatalytic Degradation for Perfluorooctanoic Acid. Photochem. Photobiol. 2015, 91, 42–47. [Google Scholar] [CrossRef]
- Tan, X.; Chen, G.; Xing, D.; Ding, W.; Liu, H.; Li, T.; Huang, Y. Indium-modified Ga2O3 hierarchical nanosheets as efficient photocatalysts for the degradation of perfluorooctanoic acid. Environ. Sci. Nano 2020, 7, 2229–2239. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Shao, T.; Wang, J.; Jin, L.; Li, X. Different nanostructured In2O3 for photocatalytic decomposition of perfluorooctanoic acid (PFOA). J. Hazard. Mater. 2013, 260, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, P.; Li, J.; Shao, T.; Wang, J.; Jin, L. Synthesis of In2O3 porous nanoplates for photocatalytic decomposition of perfluorooctanoic acid (PFOA). Catal. Commun. 2014, 43, 42–46. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Duan, X.; Hao, Q.; Wei, W.; Wang, S.; Ni, B.-J. Facile preparation of hydrophilic In2O3 nanospheres and rods with improved performances for photocatalytic degradation of PFOA. Environ. Sci. Nano 2021, 8, 1010–1018. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Li, J.; Shao, T.; Jin, L. Synthesis of In2O3-graphene composites and their photocatalytic performance towards perfluorooctanoic acid decomposition. J. Photochem. Photobiol. A Chem. 2013, 271, 111–116. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, H.; Chen, H.; Xu, C.; Chen, J. Enhancement of photocatalytic decomposition of perfluorooctanoic acid on CeO2/In2O3. RSC Adv. 2016, 6, 72015–72021. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fang, C.; Li, C. Highly Efficient Degradation of Perfluorooctanoic Acid over a MnOx-Modified Oxygen-Vacancy-Rich In2O3 Photocatalyst. ChemCatChem 2019, 11, 2297–2303. [Google Scholar] [CrossRef]
- Xu, J.; Wu, M.; Yang, J.; Wang, Z.; Chen, M.; Teng, F. Efficient photocatalytic degradation of perfluorooctanoic acid by a wide band gap p-block metal oxyhydroxide InOOH. Appl. Surf. Sci. 2017, 416, 587–592. [Google Scholar] [CrossRef]
- Verma, S.; Mezgebe, B.; Sahle-Demessie, E.; Nadagouda, M.N. Photooxidative decomposition and defluorination of perfluorooctanoic acid (PFOA) using an innovative technology of UV–vis/ZnxCu1-xFe2O4/oxalic acid. Chemosphere 2021, 280, 130660. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef]
- Song, Z.; Dong, X.; Wang, N.; Zhu, L.; Luo, Z.; Fang, J.; Xiong, C. Efficient photocatalytic defluorination of perfluorooctanoic acid over BiOCl nanosheets via a hole direct oxidation mechanism. Chem. Eng. J. 2017, 317, 925–934. [Google Scholar] [CrossRef]
- Song, Z.; Dong, X.; Fang, J.; Xiong, C.; Wang, N.; Tang, X. Improved photocatalytic degradation of perfluorooctanoic acid on oxygen vacancies-tunable bismuth oxychloride nanosheets prepared by a facile hydrolysis. J. Hazard. Mater. 2019, 377, 371–380. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Underneath mechanisms into the super effective degradation of PFOA by BiOF nanosheets with tunable oxygen vacancies on exposed (101) facets. Appl. Catal. B Environ. 2021, 286, 3–5. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Wang, T.; Zhu, L. Highly efficient photocatalytic degradation toward perfluorooctanoic acid by bromine doped BiOI with high exposure of (001) facet. Appl. Catal. B Environ. 2020, 268, 118442. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Decomposition of highly persistent perfluorooctanoic acid by hollow Bi/BiOI1-xFx: Synergistic effects of surface plasmon resonance and modified band structures. J. Hazard. Mater. 2021, 402, 123459. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, C.; Wang, Y.; Wang, Y.; Sun, B.; Zhu, L. In situ preparation of p-n BiOI@Bi5O7I heterojunction for enhanced PFOA photocatalytic degradation under simulated solar light irradiation. Chem. Eng. J. 2020, 391, 123530. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, W.; Li, X.; Zheng, Z.; Bi, F.; Yang, M.; Xu, J.; Zhang, X. Insights into the degradation mechanism of perfluorooctanoic acid under visible-light irradiation through fabricating flower-shaped Bi5O7I/ZnO n-n heterojunction microspheres. Chem. Eng. J. 2021, 420, 129934. [Google Scholar] [CrossRef]
- Sahu, S.P.; Qanbarzadeh, M.; Ateia, M.; Torkzadeh, H.; Maroli, A.S.; Cates, E.L. Rapid Degradation and Mineralization of Perfluorooctanoic Acid by a New Petitjeanite Bi3O(OH)(PO4)2 Microparticle Ultraviolet Photocatalyst. Environ. Sci. Technol. Lett. 2018, 5, 533–538. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced photocatalytic degradation of perfluorooctanoic acid using carbon-modified bismuth phosphate composite: Effectiveness, material synergy and roles of carbon. Chem. Eng. J. 2020, 395, 124991. [Google Scholar] [CrossRef]
- Xu, T.; Ji, H.; Gu, Y.; Tong, T.; Xia, Y.; Zhang, L.; Zhao, D. Enhanced adsorption and photocatalytic degradation of perfluorooctanoic acid in water using iron (hydr)oxides/carbon sphere composite. Chem. Eng. J. 2020, 388, 124230. [Google Scholar] [CrossRef]
- Duan, L.; Wang, B.; Heck, K.; Guo, S.; Clark, C.A.; Arredondo, J.; Wang, M.; Senftle, T.P.; Westerhoff, P.; Wen, X.; et al. Efficient Photocatalytic PFOA Degradation over Boron Nitride. Environ. Sci. Technol. Lett. 2020, 7, 613–619. [Google Scholar] [CrossRef]
- You, X.; Yu, L.; Xiao, F.; Wu, S.; Yang, C.; Cheng, J. Synthesis of phosphotungstic acid-supported bimodal mesoporous silica-based catalyst for defluorination of aqueous perfluorooctanoic acid under vacuum UV irradiation. Chem. Eng. J. 2018, 335, 812–821. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, W.; Meng, Y.; Xia, S. A direct Z-scheme heterojunction with boosted transportation of photogenerated charge carriers for highly efficient photodegradation of PFOA: Reaction kinetics and mechanism. Appl. Catal. B Environ. 2021, 285, 119851. [Google Scholar] [CrossRef]
- Banks, D.; Jun, B.-M.; Heo, J.; Her, N.; Park, C.M.; Yoon, Y. Selected advanced water treatment technologies for perfluoroalkyl and polyfluoroalkyl substances: A review. Sep. Purif. Technol. 2020, 231, 115929. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.B.; Alam, M.M.; Zhou, J.L.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Rahman, M.S.; Hossen, J.; Hasan, A.T.M.K.; Moni, M.A. Advanced treatment technologies efficacies and mechanism of per- and poly-fluoroalkyl substances removal from water. Process Saf. Environ. Prot. 2020, 136, 1–14. [Google Scholar] [CrossRef]
- Gatto, S.; Sansotera, M.; Persico, F.; Gola, M.; Pirola, C.; Panzeri, W.; Navarrini, W.; Bianchi, C.L. Surface fluorination on TiO2 catalyst induced by photodegradation of perfluorooctanoic acid. Catal. Today 2015, 241, 8–14. [Google Scholar] [CrossRef]
- Parrino, F.; De Pasquale, C.; Palmisano, L. Influence of Surface-Related Phenomena on Mechanism, Selectivity, and Conversion of TiO2-Induced Photocatalytic Reactions. ChemSusChem 2019, 12, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaggia, A.; Ameduri, B. Recent advances on synthesis of potentially non-bioaccumulable fluorinated surfactants. Curr. Opin. Colloid Interface Sci. 2012, 17, 188–195. [Google Scholar] [CrossRef]
- Panchangam, S.C.; Lin, A.Y.-C.; Tsai, J.-H.; Lin, C.-F. Sonication-assisted photocatalytic decomposition of perfluorooctanoic acid. Chemosphere 2009, 75, 654–660. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Ding, S.; Zhang, L. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes. Water Res. 2012, 46, 2281–2289. [Google Scholar] [CrossRef]
- Kutsuna, S.; Hori, H. Rate constants for aqueous-phase reactions of SO4− with C2F5C(O)O− and C3F7C(O)O− at 298 K. Int. J. Chem. Kinet. 2007, 39, 276–288. [Google Scholar] [CrossRef]
- Talaeemashhadi, S.; Sansotera, M.; Gambarotti, C.; Famulari, A.; Bianchi, C.L.; Antonio Guarda, P.; Navarrini, W. Functionalization of multi-walled carbon nanotubes with perfluoropolyether peroxide to produce superhydrophobic properties. Carbon N. Y. 2013, 59, 150–159. [Google Scholar] [CrossRef]
- de Bruyn, W.J.; Shorter, J.A.; Davidovits, P.; Worsnop, D.R.; Zahniser, M.S.; Kolb, C.E. Uptake of Haloacetyl and Carbonyl Halides by Water Surfaces. Environ. Sci. Technol. 1995, 29, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Sansotera, M.; Navarrini, W.; Gola, M.; Bianchi, C.L.; Wormald, P.; Famulari, A.; Avataneo, M. Peroxidic perfluoropolyether for the covalent binding of perfluoropolyether chains on carbon black surface. J. Fluor. Chem. 2011, 132, 1254–1261. [Google Scholar] [CrossRef]
- Wallington, T.J.; Ellermann, T.; Nielsen, O.J.; Sehested, J. Atmospheric Chemistry of FCOx Radicals: UV Spectra and Self-Reaction Kinetics of FCO and FC(O)O2 and Kinetics of Some Reactions of FCOx with O2, O3, and NO at 296 K. J. Phys. Chem. 1994, 98, 2346–2356. [Google Scholar] [CrossRef]
- Sansotera, M.; Navarrini, W.; Magagnin, L.; Bianchi, C.L.; Sanguineti, A.; Metrangolo, P.; Resnati, G. Hydrophobic carbonaceous materials obtained by covalent bonding of perfluorocarbon and perfluoropolyether chains. J. Mater. Chem. 2010, 20, 8607–8616. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Morigaki, T.; Taniguchi, S.; Takanami, R. UV photolysis of perfluorooctanoic acid (PFOA) in dilute aqueous solution. Water Sci. Technol. 2011, 63, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Hori, H.; Hayakawa, E.; Einaga, H.; Kutsuna, S.; Koike, K.; Ibusuki, T.; Kiatagawa, H.; Arakawa, R. Decomposition of Environmentally Persistent Perfluorooctanoic Acid in Water by Photochemical Approaches. Environ. Sci. Technol. 2004, 38, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater. 2010, 179, 1143–1146. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Dillert, R.; Bahnemann, D.; Hidaka, H. Light-induced degradation of perfluorocarboxylic acids in the presence of titanium dioxide. Chemosphere 2007, 67, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Hoffmann, M.R. Novel Photocatalytic Mechanisms for CHCl3, CHBr3, and CCl3CO2− Degradation and the Fate of Photogenerated Trihalomethyl Radicals on TiO2. Environ. Sci. Technol. 1997, 31, 89–95. [Google Scholar] [CrossRef]
- Fessi, N.; Nsib, M.F.; Cardenas, L.; Guillard, C.; Dappozze, F.; Houas, A.; Parrino, F.; Palmisano, L.; Ledoux, G.; Amans, D.; et al. Surface and Electronic Features of Fluorinated TiO2 and Their Influence on the Photocatalytic Degradation of 1-Methylnaphthalene. J. Phys. Chem. C 2020, 124, 11456–11468. [Google Scholar] [CrossRef]
- Schröder, H.F.; Meesters, R.J.W. Stability of fluorinated surfactants in advanced oxidation processes—A follow up of degradation products using flow injection–mass spectrometry, liquid chromatography–mass spectrometry and liquid chromatography–multiple stage mass spectrometry. J. Chromatogr. A 2005, 1082, 110–119. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liao, X.; Yan, X.; Huling, S.G.; Chai, T.; Tao, H. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. J. Hazard. Mater. 2013, 254–255, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Rodríguez, S.; Pardo, F.; Romero, A. Use of Fenton reagent combined with humic acids for the removal of PFOA from contaminated water. Sci. Total Environ. 2016, 563–564, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, J.; Sun, J.; Hu, Y.; Liang, X. Defluorination of Aqueous Perfluorooctanesulfonate by Activated Persulfate Oxidation. PLoS ONE 2013, 8, e74877. [Google Scholar]
- Lee, Y.-C.; Lo, S.-L.; Chiueh, P.-T.; Liou, Y.-H.; Chen, M.-L. Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation. Water Res. 2010, 44, 886–892. [Google Scholar] [CrossRef]
- Lee, Y.; Lo, S.; Kuo, J.; Hsieh, C. Decomposition of perfluorooctanoic acid by microwaveactivated persulfate: Effects of temperature, pH, and chloride ions. Front. Environ. Sci. Eng. 2012, 6, 17–25. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, S.-H.; Kim, H.Y.; Doudrick, K.; Yu, S.; Kim, S.D. Decomposition of perfluorooctane sulfonate (PFOS) using a hybrid process with electron beam and chemical oxidants. Chem. Eng. J. 2019, 361, 1363–1370. [Google Scholar] [CrossRef]
- Taniyasu, S.; Yamashita, N.; Yamazaki, E.; Petrick, G.; Kannan, K. The environmental photolysis of perfluorooctanesulfonate, perfluorooctanoate, and related fluorochemicals. Chemosphere 2013, 90, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, C.; Li, F.; Chen, J.; Zhou, Q. Photo-reductive defluorination of perfluorooctanoic acid in water. Water Res. 2010, 44, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem. Eng. J. 2012, 180, 197–203. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Liu, J. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar] [CrossRef]
- Hori, H.; Yamamoto, A.; Koike, K.; Kutsuna, S.; Osaka, I.; Arakawa, R. Photochemical decomposition of environmentally persistent short-chain perfluorocarboxylic acids in water mediated by iron(II)/(III) redox reactions. Chemosphere 2007, 68, 572–578. [Google Scholar] [CrossRef]
- Chen, Z.; Teng, Y.; Mi, N.; Jin, X.; Yang, D.; Wang, C.; Wu, B.; Ren, H.; Zeng, G.; Gu, C. Highly Efficient Hydrated Electron Utilization and Reductive Destruction of Perfluoroalkyl Substances Induced by Intermolecular Interaction. Environ. Sci. Technol. 2021, 55, 3996–4006. [Google Scholar] [CrossRef]
- Javed, H.; Lyu, C.; Sun, R.; Zhang, D.; Alvarez, P.J.J. Discerning the inefficacy of hydroxyl radicals during perfluorooctanoic acid degradation. Chemosphere 2020, 247, 125883. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.-J.; Lyu, X.-J.; Yin, H.; Sheng, G.-P. Complete mineralization of perfluorooctanoic acid (PFOA) by γ-irradiation in aqueous solution. Sci. Rep. 2014, 4, 7418. [Google Scholar] [CrossRef] [Green Version]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S.; Kiatagawa, H.; Arakawa, R. Efficient Decomposition of Environmentally Persistent Perfluorocarboxylic Acids by Use of Persulfate as a Photochemical Oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P. Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate. Water Sci. Technol. 2006, 54, 317–325. [Google Scholar] [CrossRef]
- Liang, C.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate–thiosulfate redox couple. Chemosphere 2004, 55, 1213–1223. [Google Scholar] [CrossRef]
- Tran, T.; Abrell, L.; Brusseau, M.L.; Chorover, J. Iron-activated persulfate oxidation degrades aqueous Perfluorooctanoic acid (PFOA) at ambient temperature. Chemosphere 2021, 281, 130824. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Xie, Z.; Wu, L.; Huang, M.; Zhang, Z. Persulfate-based degradation of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in aqueous solution: Review on influences, mechanisms and prospective. J. Hazard. Mater. 2020, 393, 122405. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Tang, Y.; Lu, P.; Chen, W.; Xu, X.; Li, L. Mechanism insight of PFOA degradation by ZnO assisted-photocatalytic ozonation: Efficiency and intermediates. Chemosphere 2017, 180, 247–252. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- National Association of Clean Water Agencies (NACWA). Available online: https://www.nacwa.org/ (accessed on 22 January 2021).
- Whitby, P.; Yu, R.; Mackey, E. Consider the Hidden Costs of PFAS Treatment. Opflow 2021, 47, 10–15. [Google Scholar] [CrossRef]
- Olatunde, O.C.; Kuvarega, A.T.; Onwudiwe, D.C. Photo enhanced degradation of polyfluoroalkyl and perfluoroalkyl substances. Heliyon 2020, 6, e05614. [Google Scholar] [CrossRef] [PubMed]



| Irradiation Wavelength (nm) | Power (Electrical, W) | Substrates | Reaction Time | Degradation/Defluorination Yield (%) | Ref. |
|---|---|---|---|---|---|
| 254 | 200 | PFOA 1.35 mM | 72 h | 89/33 | [116] |
| 254 | 15 | PFOA 0.025 mM | 6 h | 13/2 | [131] |
| 185 (prevalent) | 20 | PFOA 0.12–2.42 μM | 3 h | 87/21 | [132] |
| 254 (prevalent) | 31/0.5 | ||||
| 185 | 15 | PFOA 60 μM | 2 h | 62/17 | [133] |
| 220–460 | 200 | perfluoropropionic acid, perfluorobutyric acid, perfluoropentanoic acid | 24 h | 16–24/9–12 | [134] |
| 254 | 36 | PFOA 0.024 mM | 8 h | 20/9 | [135] |
| 254 | 24 | PFOA 1 mg/L | 24 h | 21/9 | [136] |
| Technology | Stage of Development | Energy (KWh/m3) | Relative Cost (USD) | Removal Efficiency (%) | Time to 90% Degradation (min) |
|---|---|---|---|---|---|
| Photolysis (185 nm) | Emerging | 99 | 14 | 82 | 216 |
| Photochemicals (persulfates) | Research | 864 | 121 | 99 | 460 |
| Photocatalysis (indium oxides + 254 nm) | Emerging | 2106 | 295 | 89 | 705 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Appl. Sci. 2021, 11, 8458. https://doi.org/10.3390/app11188458
Leonello D, Fendrich MA, Parrino F, Patel N, Orlandi M, Miotello A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Applied Sciences. 2021; 11(18):8458. https://doi.org/10.3390/app11188458
Chicago/Turabian StyleLeonello, Domenico, Murilo Alexandre Fendrich, Francesco Parrino, Nainesh Patel, Michele Orlandi, and Antonio Miotello. 2021. "Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review" Applied Sciences 11, no. 18: 8458. https://doi.org/10.3390/app11188458
APA StyleLeonello, D., Fendrich, M. A., Parrino, F., Patel, N., Orlandi, M., & Miotello, A. (2021). Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Applied Sciences, 11(18), 8458. https://doi.org/10.3390/app11188458










