Abstract
Ineffective healing and treatment of foot ulcers can lead to an infection and gangrene of the wound area that ultimately results in the loss of the limb. The incidence of foot ulcers is higher in patients with diabetes, peripheral vascular disease and kidney disease. Accordingly, this study was undertaken to assess the ability of foot bathing in CO2-enriched water to heal foot ulcers. The design was a double-blinded, randomized, placebo-controlled study. Patients with at least one foot ulcer were randomized to receive either a treatment with bath therapy at 37 ± 0.5 °C containing either 1000–1200 ppm CO2-enriched tap water (the intervention) or non-carbonated tap water at 37 ± 0.5 °C (the control group). Treatment was conducted three times/week for 15 min per session for up to 16 weeks for a total of 48 treatment sessions. Before and at the end of every treatment month, wound size, wound area oxygenation and the ankle brachial index were measured. In addition, the McGill pain questionnaire was conducted. Blood was also collected at these time points (for a total of five collections) for the measurement of different biomarkers. While no significant differences (p < 0.05) in the group/time interaction effect were observed, a clear separation within the wound area reduction/wound area/oxygenated Hb outcomes was seen between placebo (control) and treatment (CO2) group. This pilot study is suggestive that bathing in CO2-enriched water may accelerate the healing of foot ulcers.
1. Introduction
It has been estimated that 4% annually, and up to 30% in the entire lifetime, of patients with diabetes may experience a foot ulcer [1,2]. The data suggest a surge in global incidence of diabetes, with which there is a concomitant increase in the global burden of diabetic foot ulcers [3]. A number of factors including bacterial infection, tissue ischemia, ongoing trauma and poor management are major aspects precluding the physiological ulcer healing, often resulting in a chronic, non-healing ulcer formation [4]. The chronic, non-healing foot ulcers are associated with several complication including infections and gangrenes. Furthermore, the diabetic patients with foot ulcers are at a higher risk of amputation [1,2], as well as a higher risk of requiring re-amputation [5]. Besides, diabetic patients developing foot ulcers are at a much higher risk of death, experiencing an increase in three-year mortality risk from 13% to 28% [6]. Furthermore, such higher morbidity and mortality burden is associated with a significant psychological and physical stress to the patient, resulting in a poor quality of life [7], as well as with a significant increase in healthcare utilization. The diabetic foot ulcer is thus a major health care concern, incurring significant health care costs [8,9,10].
The risk for foot ulceration is increased in patients with diabetes and chronic kidney disease (CKD) [11]. In this regard, it has been reported that foot ulceration, as well as amputation increased in diabetic patients undergoing dialysis [12]. In addition, the occurrence of foot ulcers in patients with diabetes and CKD receiving dialysis is five times higher compared to pre-dialysis diabetic patients [13]. Hemodialysis has also been linked to a higher risk for peripheral arterial disease (PAD); indeed, hemodialysis causes a reduction in skin microcirculation and tissue oxygenation, leading to ulceration [14]. Interestingly, an improvement in peripheral blood flow in PAD patients in response to CO2 treatment has been reported [15,16,17,18,19,20].
Impairment of microcirculation and the resulting ischemia is at the core of the occurrence of diabetic foot ulcers. Similarly, in patients with PAD, vasoconstriction leads to ischemia and is considered to play a key role in the pathogenesis of foot ulceration [21]. These foot ulcers, secondary to PAD affecting tibial and peroneal arteries, are found in about 50% of cases. The management approach for foot ulcers in patient with diabetes and PAD is to relieve ischemic pain, heal the ulcers and to prevent amputation [22,23]. CO2 is known to causes vasodilation and therefore bathing limbs in CO2-enriched water will increase blood flow in the skin, given that CO2 can permeate through the skin [20]. The efficacy of CO2-enriched water in healing of diabetic foot ulcers has also been evaluated [24,25]; however, there are limitations in case studies. Thus, there is a need for an appropriately designed clinical study to determine the efficacy of CO2-enriched water for the treatment of foot ulcers. Furthermore, while several different approaches have been undertaken to examine wound healing [26], only 24–50% of all ulcers are reported to heal after 12 weeks to 6 months of standard treatment [27,28]. Therefore, there is a need for evaluating novel approaches that may exhibit higher efficacy for the treatment of foot ulcers.
Accordingly, the present study was undertaken to assess the potential of CO2-enriched water in the treatment of foot ulcers. The study tested the hypothesis that bathing of foot ulcers in CO2-enriched water bath will reduce the size of the foot ulcer. The objectives of the study following treatment with CO2-enriched water were to (1) determine wound area reduction; (2) measure peripheral blood flow; (3) measure oxygen saturation level in the wound and (4) measure blood levels of vascular endothelial growth factor and tumor necrosis factor-α, as well as blood lipid profile, fasting blood glucose and HbA1c.
2. Materials and Methods
2.1. Study Design
This study was a randomized, double-blinded, placebo controlled clinical trial. Study participants were those with at least one foot ulcer. The inclusion and exclusion criteria for the study are shown in Table 1. Patients were randomly assigned to either receive foot bathing with CO2-enriched tap water (1000–1200 ppm CO2, the treatment group), or normal tap water (the control group). Treatment was carried out at 37 ± 0.5 °C, 3 times/week, for 15 min sessions for up to 16 weeks. Blood (approximately 20 mL) was also collected at the beginning of the treatment period and at the end of every month of the treatment period (a total of 5 collections) for measuring different biomarkers. It should be noted that the standard of care for wound healing (i.e., debridement, use of povidone iodine and new dressing) continued throughout the study period and was not interrupted by participation in the study.

Table 1.
Criteria used for inclusion and exclusion for the study.
2.2. Institutional Review Board Approvals
The study was conducted according to the guidelines of the Declaration of Helsinki, and written approvals from the Research Ethics Board at the University of Manitoba, St. Boniface Hospital Research Review Committee, Seven Oaks Hospital and Health Sciences Centre Research Impact Committee were obtained prior to start of the study. The tenets of Good Clinical Practice and Good Laboratory Practice were followed in the conduct of this study.
2.3. Participant Selection, Recruitment and Informed Consent
Potential study participants were screened for eligibility by review of their medical history and approached for written informed consent prior to study enrolment. Participants were in-patients or out-patients at St. Boniface Hospital, Seven Oaks Hospital and Health Sciences Centre and were given both written and verbal information describing the the study. Ample time was allowed for participants to provide their consent. Patient demographics (diabetes, PAD, hemodialysis) were also recorded at screening.
2.4. Foot Bathing
Foot bathing was performed with either CO2-enriched tap water (37 ± 0.5 °C; CO2 concentration of 1000–1200 ppm) or regular tap water (37 ± 0.5 °C) with an immersion time of 15 min for 3 times/week for up to 16 weeks (4 months) for a total of 48 treatment sessions. Study participants were rested on a chair for 15 min prior to the start of foot bathing treatment. Table 2 shows the primary and secondary outcome measures for the study. The following procedures were conducted at before and at end of 1, 2, 3 and 4 months of the treatment period. It should be noted that at the end of each foot bathing treatment session feet were meticulously towel dried and new dressing applied. Povidone iodine was applied if indicated.

Table 2.
Primary and secondary outcome measures.
2.5. Measurement of Ulcer Size
The area, volume and depth of the foot ulcer were measured by using the Silhouette Mobile camera (ARANZ Medical, Christchurch, New Zealand). The Silhouette Mobile camera is a portable handheld computer with an integrated high-resolution digital camera and embedded laser light. The accuracy of measurements obtained with this device have been reported to be within 2% of surface area on wound models with user variability of only 2–5% in clinical use [29].
2.6. Ankle/Brachial Index (ABI)/Blood Flow
The ankle brachial index (ABI) was measured to assess peripheral blood flow [30]. The study participants were requested to rest in supine position for 10 min before taking pressure measurements for calculating the ABI. A continuous wave, handheld Doppler machine along with a conventional sphygmomanometer were used to measure the systolic pressure in both the posterior tibial and dorsalis pedis arteries. Brachial systolic and diastolic pressures were also measured. The ABI was calculated as a ratio of the ankle systolic pressure and brachial systolic blood pressure [30].
2.7. Limb Oxygenation
Noninvasive near infra-red spectroscopy (NIRS) [31,32,33,34] was used to measure the extent of oxygen saturation in the microcirculation of the foot ulcer. This technology can measure the percentage of hemoglobin oxygen saturation up to a distance of 30 mm below the skin [32].
2.8. Biochemical Measurements
Blood samples collected from study participants at baseline and at the end of each month of the treatment period were analyzed for C-reactive protein, glucose, total cholesterol, triglycerides, high density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol levels by Laboratory Services at St. Boniface Hospital. TNF-α, and VEGF were measured by ELISA method in the Albrechtsen Research Centre at St. Boniface Hospital as described elsewhere [35].
2.9. Questionnaires
The degree of rest pain was determined by using the short form McGill Pain Questionnaire [36].
2.10. Premature Withdrawal/Discontinuation Criteria
Study participants were permitted to withdraw from the study at any time for any reason. The clinical investigators were also allowed to withdraw any participant from the study at any time for medical reasons, or if the participant was consistently not compliant with the study protocol.
2.11. Statistical Analysis
Baseline characteristics and outcome measures of the patient population were summarized using means and standard errors or medians and interquartile ranges for continuous variables where appropriate. Categorical variables were summarized using percentages. The primary and secondary outcomes were analyzed using generalized estimating equations (GEE) to account for dependency of observations. Utilizing this methodology, a group effect, time effect, and group/time effect was calculated for each of the study outcomes of interest. All statistical analyses were performed using SAS version 9.3. For all statistical analyses, the significance level was set at p < 0.05.
3. Results
The baseline characteristics of the 16 participants that were compliant with the baseline visit are presented in Table 3. The population has an average age of 59.9 ± 1.5 years and there are 11 males and 5 female participants. 94% (15/16) were diabetic, as evidenced by an approximate 2-fold elevation of the mean FBG and an approximate 1.5-fold increase in the mean values for HbA1c. 63% (10/16) of the participants were undergoing dialysis for ESRD. Baseline analysis revealed no significant differences in the mean values of FBG, HbA1c, total cholesterol, triglycerides, HDL-cholesterol, LDL-cholesterol, total cholesterol/HDL-cholesterol ratio and the LDL-cholesterol/HDL-cholesterol ratio were observed between the control and those randomized into the CO2-treated group. It should be noted that 80% (12/15) of the participants have elevated C-reactive protein levels (Table 3).

Table 3.
Baseline characteristics, fasting blood glucose, HbA1c, lipid profile and wound/limb characteristics of patients enrolled into the study.
Table 3 shows some of the baseline characteristics of the study participants. It was observed that 38% (6/16) patients are below knee amputees (BKA). All patients had at least one foot ulcer. The McGill Questionnaire revealed that the baseline pain intensity scores were significantly higher in the participants randomized into the CO2 group. It was also noted that the mean value for oxygen saturation at the site of the wound was approximately 42%. ABI was not measurable in the placebo (control) group and in some patients in the CO2 group, due to non-compressibility of arteries. However, based on the pre- and post- treatment, an increase in the ABI was observed in the CO2 group (mean values ± S.E.M: baseline = 0.84 ± 0.08 (n = 80; treatment = 1 month: 0.97 ± 0.12 (n = 4)); 2 months: 0.97 ± 0.28 (n = 2); 3 months: 0.97 ± 0.40 (n = 2) and 4 months: 1.02 ± 0.26 (n = 2)).
Figure 1a shows the % wound area reduction in control and CO2 treated groups. It can be seen that the median wound area reduction was greater in the CO2 treated group in a time-dependent manner as compared to the control group. Similarly, the median wound area was reduced with increasing extent over the time course of the treatment with CO2-enriched as compared to control (Figure 1b). These improvements in the CO2 treated group were associated with a time-dependent increase in the % median oxygenated Hb levels (Figure 2a). Interestingly, the % median O2 saturation in the wound area was higher in the CO2 treated group up to 2 months of the intervention but were similar to the control group thereafter (Figure 2b). It can be seen from Figure 3 that patients randomly assigned to the CO2 intervention group exhibited higher pain scores at baseline, which gradually decreased with the course of the treatment with CO2-enriched water. No differences in the serum levels of TNFα and VEGF were seen in control and CO2-treated groups (Figure 4).
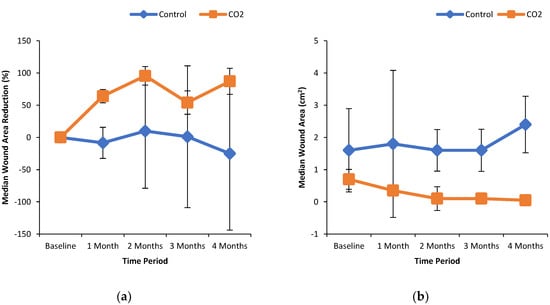
Figure 1.
Effect of CO2-water bathing on the dimensions of foot ulcers. Panel (a) percent wound area reduction of foot ulcers and panel (b) wound area in patients treated without and with CO2-enriched water. Study participants were randomized to undergo foot bathing either with CO2-enriched tap water (37 ± 0.5 °C; CO2 concentration of 1000–1200 ppm) or with regular tap water (37 ± 0.5 °C) with an immersion time of 15 min for 3 times/week for up to 16 weeks for a total of 48 treatments. The Silhouette Mobile camera (ARANZ Medical, Christchurch, New Zealand) was used to measure the dimensions of the ulcer.
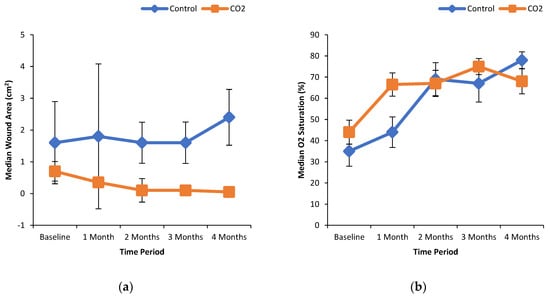
Figure 2.
Oxygenated hemoglobin and oxygen saturation levels in the wound area in patients treated without and with CO2-enriched water. Panel (a) oxygenated hemoglobin levels in the wound area and panel (b) oxygen saturation levels of the wound in patients treated without and with CO2-enriched water. Participants underwent foot bathing with CO2-enriched tap water (37 ± 0.5 °C; CO2 concentration of 1000–1200 ppm) or a regular tap water (37 ± 0.5 °C) with an immersion time of 15 min for 3 times/week for up to 16 weeks for a total of 48 treatment sessions. Non-invasive near infra-red spectroscopy was used to measure the percentage of oxygenated hemoglobin and oxygen saturation levels in the microcirculation of tissue up to 3 cm below the skin as described elsewhere [32].
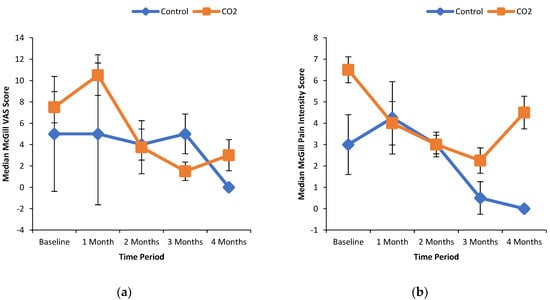
Figure 3.
McGill VAS and pain intensity scores in patients treated with and without CO2-enriched water. The short form McGill Pain Questionnaire was used to measure the degree of pain (a) and degree of pain intensity (b) experienced by the study participants.
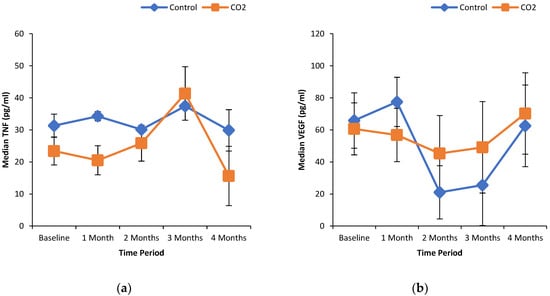
Figure 4.
Effect of CO2-water bathing on markers of inflammation and angiogenesis in the serum of patients with foot ulcers. TNF-α (a) and VEGF (b) were measured by ELISA method as per manufacturers (Invitrogen) instructions and as described elsewhere [35]. Blood samples were collected before and at the end of every month of the treatment period (for a total of 5 collections) for the measurement of VEGF and TNFα.
The most important p-value for the purposes of this study in likely the group/time interaction effect, as this represents the difference between the groups experienced over time. While no statistically significant differences (p < 0.05) in the group/time interaction effect were observed, from the data presented it can be seen that there is a clear separation within the wound area reduction/wound area/oxygenated Hb outcomes. The lack of statistical difference at the level of p < 0.05 could very well be due to the small sample size. Nonetheless, some inference can be made. A p-value essentially represents the probability that the difference observed is due to random chance. Thus, the p-value of 0.072 for the group/time effect for wound area reduction represents that there is a 7.2% chance that the differences observed is due to random chance. This essentially means that there is a 93% confidence that there is a difference being observed between both groups.
4. Discussion
We have earlier described the rationale and design of this study undertaken to evaluate the clinical utility, efficacy and safety of a novel approach involving bathing in CO2-enriched water for the treatment of foot ulcers [37]. Although several different approaches have been undertaken to facilitate healing of foot ulcers, it is clear that such approaches require multidisciplinary management if amputation is to be avoided [38]. Several therapies are available in clinical practice and include topical agents, dressings, engineered tissue, cell therapy, growth factors, devices and herbal/natural remedies [1]. Furthermore, novel therapies such as magnetic fields have been employed and show great potential in promoting wound healing and have already been utilized in the management of diabetic wounds [39]. The potential of polymeric wound dressing has also been evaluated in wound care management [40]. In addition, improvements in hydrogel dressings have enabled advancements for wound healing [41]. With the development of nanotechnology, specifically glass/hydrogel nanoparticles, incorporating bioactive agents or cells, has allowed a prolonged availability of target molecules at the wound site, and thereby facilitating wound healing [41,42]. Novel therapies including traction force-activated payloads local delivery of short-interfering RNA are also potentially of value in the healing of wounds [42].
The evidence for the beneficial actions of topical oxygen therapy in healing of diabetes-related foot ulcers and the likelihood of healing has recently been examined; however, the effect on amputation and cost-effectiveness of such therapy was unclear [43]. An improved lower limb ulcer healing in response to increase tissue oxygenation via hyperbaric oxygen has been reported [44]. Negative pressure wound therapy (NPWT) is one of the most effective techniques for the treatment of foot wounds. Although some advancements in this technique have been achieved, due to the complex pathogenesis and management of diabetic foot, irregular application of NPWT has been linked to complications, such as infections, bleeding and necrosis that seriously affect its treatment outcomes [45]. Based on the aforementioned information, it is apparent that several different approaches are available for the healing of foot ulcers, however their efficacy is dependent on an adequate arterial perfusion [46].
The present foot care study has generated data on the safety, tolerability, vascular efficacy and capacity to heal ulcerated foot lesions. While no statistically significant differences (p < 0.05) in the group/time interaction effect were observed, there is a clear separation within the wound area reduction/wound area/oxygenated Hb outcomes. The lack of statistical difference at the level of p < 0.05 may be attributed to the small sample size. There is likely an acceleration in the process of wound healing in response to foot bathing in carbonated (1000 ppm–1200 ppm) water at 37 ± 0.5 °C. The facilitated wound healing seen in the treatment group may be associated with an increase in O2 saturation and oxygenated hemoglobin levels at site of wound. Interestingly, in some cases, we also observed that foot bathing in carbonated water alleviated ischemic discoloration of skin (data not shown). Although ABI was largely not measurable in the placebo (control) group, as well as in some patients in the treatment arm, due to non-compressibility of arteries [47,48], based on the pre- and post- treatment values of ABI in the CO2 treated group, the accelerated healing of wounds in this group may be associated with an increase in peripheral circulation. However, it should be mentioned that while no significant differences in the serum levels of VEGF were observed between control and CO2 groups, the median VEGF serum concentration was higher in the CO2 treated group following longer duration (2–4 months) of treatment, suggesting the possibility of new collateral blood vessel formation [49].
In support of our notion that bathing of feet in CO2-enriched water, several clinical studies have demonstrated an increase in blood flow subsequent to treatment with CO2. In patients with mild, bilateral, peripheral occlusive arterial disease (intermittent claudication, femoral or iliac type) immersion of the lower leg and foot in fresh CO2-enriched water (1200 mg CO2) for 20 min has shown increases in microcirculation and oxygen tension in the skin of the foot [15]. Interestingly, topical applications of CO2 have been shown to increase skin blood flow in a concentration-dependent manner, providing additional support for the clinical use of CO2 bathing in the treatment of disturbances of skin circulation as well as skin ulcers and wounds [50]. It has been suggested that the effects of CO2-enriched water on the subcutaneous microcirculation may be due to peripheral vasodilation as evidenced by increased parasympathetic and decreased sympathetic activity and that CO2 foot bathing is clinically effective on salvage of CLI (Fontaine stage IV) limbs [16]. Recently, patients with PAD risk factors (ABI in the normal range or ABI indicating some or moderate arterial disease, ABI > 0.5) were shown to benefit from CO2 therapy due to improvements in blood flow [51].
In our study, while data on usage of bespoke footwear, ability to walk barefoot at home, occurrence of amputation and use of antibiotics for the treatment of foot ulcer were collected, no differences were observed between control and treated groups (data not shown). It is also pointed out that in 2/7 hemodialysis subjects that were randomized into the treated group there was the occurrence of new pressure type ulcers in different region of the foot. Accordingly, in this North American population of subjects with foot ulcers and undergoing hemodialysis, there is potentially an increase in the risk (by about 28%) of new pressure-type ulcers forming and thus this negative effect from exposure to CO2 foot bathing is suggestive that foot bathing may be contraindicated in this population (data not shown). It should be mentioned that tap water contains various impurities and salts used for its purification. Salts dissolved in water, when exposed to CO2 can lead to the formation of substances that can cause chemical burns, it is thus possible that the high risk for new ulcer formation observed in some of the hemodialysis patients may be related to this. Therefore, in any follow up especially a large-scale study, use of distilled water may be indicated.
Several preclinical studies have examined the potential mechanisms for the beneficial actions of bathing of ischemic limbs in CO2-enriched water with respect to improved microcirculation. In this regard, Elimban et al. [52] have shown that CO2 bathing increased small blood vessel count, an index of vascular density. In addition, Xu et al. [53] have reported that CO2 water bath therapy of the ischemic hind limb of diabetic rat augments blood flow as well as evokes the development of angiogenesis in the skeletal muscle of diabetic ischemic animals. Taken together, such therapy may be some benefit for the treatment of PAD in diabetes that may also be due to a reduction in oxidative stress [54]. In this regard, a reduction of plasma free radical concentration, as well as an increase in antioxidant levels have been reported to be associated with an increase in microcirculation in patients with PAD (Fontaine stage III) [55].
In conclusion, although the sample size is small, the present pilot study is suggestive that foot bathing in CO2-enriched water may accelerate the healing of foot ulcers due to an improvement in microcirculation and adequate perfusion of the ischemic wound area. Thus, treatment of foot ulcers by immersing in CO2-enriched water may potentially serve as an adjunct to standard therapeutic approaches for diabetic foot wound healing.
Author Contributions
Conceptualization, P.S.T. and B.R.; clinical oversight, P.K., C.S. and A.S.A.; methodology and institutional review board approvals, P.S.T. and B.R.; validation and formal analysis, B.H. and R.S.; investigation, P.S.T. and B.R.; data curation, P.S.T. and B.H.; writing—original draft preparation, P.S.T.; writing—review and editing, P.S.T., B.H. and R.S.; project administration, B.R.; funding acquisition, P.S.T. and B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a research contract grant from Mitsubishi Rayon Cleansui, Tokyo, Japan.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The University of Manitoba (protocol code: B2014:037), St. Boniface Hospital (protocol code: RRC/2014/1402); date of approval, 7 August 2015, Seven Oaks Hospital (date of approval: 27 November 2014) and Health Sciences Centre (protocol code: RI2015:011; date of approval: 19 February 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions related to privacy and ethical considerations.
Acknowledgments
Infrastructural support was provided by the Albrechtsen Research Centre, St. Boniface Hospital. Carbothera device for the generation of CO2-enriched water at desired concentration was provided by Mitsubishi Rayon Cleansui, Tokyo, Japan.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Stratman, S.; Schneider, C.; Kirsner, R.S. New Therapies for the Treatment of Diabetic Foot Ulcers: Updated Review of Clinical Trials. Surg. Technol. Int. 2020, 37, 37–47. [Google Scholar]
- Abid, A.; Hosseinzadeh, S. Foot Ulcer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sorber, R.; Abularrage, C.J. Diabetic foot ulcers: Epidemiology and the role of multidisciplinary care teams. Semin. Vasc. Surg. 2021, 34, 47–53. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Petersen, B.J.; Rothenberg, G.M.; Armstrong, D.G. Lower extremity reamputation in people with diabetes: A systematic review and meta-analysis. BMJ Open Diabetes Res. Care 2021, 9, e002325. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Lavoie, H.M.; Ramsey, A.; Nguyen, M.; Singh, S. Diabetic Foot Infections. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fernández-Torres, R.; Ruiz-Muñoz, M.; Pérez-Belloso, A.; García-Romero, J.; Gónzalez-Sánchez, M. Is There an Association between Sleep Disorders and Diabetic Foot? A Scoping Review. J. Clin. Med. 2021, 10, 2530. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Syed, M.H.; Salata, K.; Hussain, M.A.; Zamzam, A.; De Mestral, C.; Wheatcroft, M.; Harlock, J.; Awartani, D.; Aljabri, B.; Verma, A.; et al. The economic burden of inpatient diabetic foot ulcers in Toronto, Canada. Vascular 2020, 28, 520–529. [Google Scholar] [CrossRef]
- Hopkins, R.B.; Burke, N.; Harlock, J.; Jegathisawaran, J.; Goeree, R. Economic burden of illness associated with diabetic foot ulcers in Canada. BMC Health Serv. Res. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, G.M.; Flanagin, E. How ESKD complicates the management of diabetic foot ulcers: The vital role of the dialysis team in prevention, early detection, and support of multidisciplinary treatment to reduce lower extremity amputations. Semin. Dial. 2020, 33, 245–253. [Google Scholar] [CrossRef]
- Game, F.L.; Chipchase, S.Y.; Hubbard, R.; Burden, R.P.; Jeffcoate, W.J. Temporal association between the incidence of foot ulceration and the start of dialysis in diabetes mellitus. Nephrol. Dial. Transplant. 2006, 21, 3207–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndip, A.; Rutter, M.; Vileikyte, L.; Vardhan, A.; Asari, A.; Jameel, M.; Tahir, H.A.; Lavery, L.; Boulton, A.J. Dialysis Treatment Is an Independent Risk Factor for Foot Ulceration in Patients with Diabetes and Stage 4 or 5 Chronic Kidney Disease. Diabetes Care 2010, 33, 1811–1816. [Google Scholar] [CrossRef] [Green Version]
- Beckert, S.; Sundermann, K.; Wolf, S.; Königsrainer, A.; Coerper, S. Haemodialysis is associated with changes in cutaneous microcirculation in diabetes mellitus. Diabet. Med. 2009, 26, 89–92. [Google Scholar] [CrossRef]
- Hartmann, B.R.; Bassenge, E.; Hartmann, M. Effects of Serial Percutaneous Application of Carbon Dioxide in Intermittent Claudication: Results of a Controlled Trial. Angiology 1997, 48, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.R.; Bassenge, E.; Pittler, M. Effect of Carbon Dioxide-Enriched Water and Fresh Water on the Cutaneous Microcirculation and Oxygen Tension in the Skin of the Foot. Angiology 1997, 48, 337–343. [Google Scholar] [CrossRef]
- Toriyama, T.; Kumada, Y.; Matsubara, T.; Murata, A.; Ogino, A.; Hayashi, H.; Nakashima, H.; Takahashi, H.; Matsuo, H.; Kawahara, H. Effect of artificial carbon dioxide foot bathing on critical limb ischemia (Fontaine IV) in peripheral arterial disease patients. Int. Angiol. 2002, 21, 367–373. [Google Scholar] [PubMed]
- Nishimura, N.; Sugenoya, J.; Matsumoto, T.; Kato, M.; Sakakibara, H.; Nishiyama, T.; Inukai, Y.; Okagawa, T.; Ogata, A. Effects of repeated carbon dioxide-rich water bathing on core temperature, cutaneous blood flow and thermal sensation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 87, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Yamada, S.; Kumada, Y.; Matsuo, H.; Nakashima, H.; Toriyama, T.; Kawahara, H. Short and long-term changes of the transcutaneous oxygen pressure (tcPO2) during carbon dioxide foot bathing in patients with ischemic limbs. Jpn. Coll. Angiol. 2006, 46, 411–416. [Google Scholar]
- Makita, S.; Ohira, A.; Naganuma, Y.; Abiko, A.; Nakamura, M. The effects on skin blood flow of immersing the ischemic legs of patients with peripheral arterial disease into artificially carbonated water. Int. J. Angiol. 2006, 15, 12–15. [Google Scholar] [CrossRef]
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Aldana, P.C.; Khachemoune, A. Diabetic Foot Ulcers: Appraising Standard of Care and Reviewing New Trends in Management. Am. J. Clin. Dermatol. 2019, 21, 255–264. [Google Scholar] [CrossRef]
- Barshes, N.R.; Grant, C.L. Advances in the Management of Peripheral Artery Disease. Curr. Diabetes Rep. 2019, 19, 36. [Google Scholar] [CrossRef]
- Hayashi, H.; Yamada, S.; Kumada, Y.; Matsuo, H.; Toriyama, T.; Kawahara, H. Immersing Feet in Carbon Dioxide-enriched Water Prevents Expansion and Formation of Ischemic Ulcers after Surgical Revascularization in Diabetic Patients with Critical Limb Ischemia. Ann. Vasc. Dis. 2008, 1, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhawaja, S. Carbal therapy for treatment of diabetic foot (CO2 water bath). Kufa Med. J. 2012, 15, 211–222. [Google Scholar]
- Game, F.L.; Apelqvist, J.; Attinger, C.; Hartemann, A.; Hinchliffe, R.J.; Löndahl, M.; Price, P.E.; Jeffcoate, W.J. International Working Group on the Diabetic Foot Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: A systematic review. Diabetes/Metab. Res. Rev. 2016, 32 (Suppl. 1), 154–168. [Google Scholar] [CrossRef] [Green Version]
- Margolis, D.J.; Kantor, J.; Berlin, J.A. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care 1999, 22, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; Chipchase, S.Y.; Ince, P.; Game, F.L. Assessing the Outcome of the Management of Diabetic Foot Ulcers Using Ulcer-Related and Person-Related Measures. Diabetes Care 2006, 29, 1784–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.C.; Bevilacqua, N.J.; Armstrong, D.G.; Andros, G. Digital Planimetry Results in More Accurate Wound Measurements: A Comparison to Standard Ruler Measurements. J. Diabetes Sci. Technol. 2010, 4, 799–802. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, J.; Zou, L.; Xu, Y.; Hu, D.; Pagoto, S.; Ma, Y. Sensitivity and specificity of the ankle—brachial index to diagnose peripheral artery disease: A structured review. Vasc. Med. 2010, 15, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; McCully, K.; Hong, L.; Nioka, S.; Chance, B. Regional Difference of Muscle Oxygen Saturation and Blood Volume during Exercise Determined by Near Infrared Imaging Device. Jpn. J. Physiol. 2001, 51, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Shuler, M.S.; Reisman, W.M.; Whitesides, T.E., Jr.; Kinsey, T.L.; Hammerberg, E.M.; Davila, M.G.; Moore, T.J. Near-Infrared Spectroscopy in Lower Extremity Trauma. J. Bone Jt. Surg. Am. 2009, 91, 1360–1368. [Google Scholar] [CrossRef]
- Wakimoto, M.; Kadosaki, M.; Nagata, H.; Suzuki, K.S. The usefulness of near-infrared spectroscopy in the anesthetic management of endovascular aortic aneurysm repair. J. Anesth. 2012, 26, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Fellahi, J.-L.; Butin, G.; Zamparini, G.; Fischer, M.-O.; Gerard, J.-L.; Hanouz, J.-L. Lower limb peripheral NIRS parameters during a vascular occlusion test: An experimental study in healthy volunteers. Ann. Fr. Anesth. Reanim. 2014, 33, e9–e14. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Babick, A.P.; Xu, Y.-J.; Takeda, N.; Rodriguez-Levya, D.; Dhalla, N.S. TNF-α-mediated signal transduction pathway is a major determinant of apoptosis in dilated cardiomyopathy. J. Cell. Mol. Med. 2010, 14, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The short-form McGill pain questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Tappia, P.S.; Pierce, G.N.; Ramjiawan, B. Evaluation of the clinical utility, efficacy and safety of a novel medical device for the treatment of foot ulcers: Rationale and design of the carbothera trial. Curr. Res. Cardiol. 2016, 3, 76. [Google Scholar] [CrossRef]
- Mohamad, M.; Pham, T.-T.; Jornayvaz, F.R.; Pignel, R.; Glauser, F.; Suva, D. Management of a foot ulcer in a patient with diabetes. Rev. Med. Suisse 2020, 16, 2446–2452. [Google Scholar]
- Lv, H.; Liu, J.; Zhen, C.; Wang, Y.; Wei, Y.; Ren, W.; Shang, P. Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif. 2021, 54, e12982. [Google Scholar] [CrossRef]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2020, 9, 1530–1546. [Google Scholar] [CrossRef]
- Dixon, D.; Edmonds, M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs 2020, 81, 29–56. [Google Scholar] [CrossRef]
- Thanigaimani, S.; Singh, T.; Golledge, J. Topical oxygen therapy for diabetes-related foot ulcers: A systematic review and meta-analysis. Diabet. Med. 2021, 38, e14585. [Google Scholar] [CrossRef]
- Bolton, L. Does hyperbaric oxygen improve lower extremity ulcer outcomes? Wounds 2020, 32, 291–293. [Google Scholar] [PubMed]
- Ji, S.; Liu, X.; Huang, J.; Bao, J.; Chen, Z.; Han, C.; Hao, D.; Hong, J.; Hu, D.; Jiang, Y.; et al. Consensus on the application of negative pressure wound therapy of diabetic foot wounds. Burn. Trauma 2021, 9, tkab018. [Google Scholar] [CrossRef]
- Kontopodis, N.; Tavlas, E.; Papadopoulos, G.; Pantidis, D.; Kafetzakis, A.; Chalkiadakis, G.; Ioannou, C. Effectiveness of Platelet-Rich Plasma to Enhance Healing of Diabetic Foot Ulcers in Patients with Concomitant Peripheral Arterial Disease and Critical Limb Ischemia. Int. J. Low. Extrem. Wounds 2015, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Tóth-Vajna, Z.; Tóth-Vajna, G.; Gombos, Z.; Szilágyi, B.; Járai, Z.; Berczeli, M.; Sótonyi, P. Screening of peripheral arterial disease in primary health care. Vasc. Health Risk Manag. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilly, S.M.; Qasim, A.N.; Mulvey, C.K.; Churchill, T.W.; Reilly, M.P.; Eraso, L.H. Non-compressible arterial disease and the risk of coronary calcification in type-2 diabetes. Atherosclerosis 2013, 230, 17–22. [Google Scholar] [CrossRef]
- Irie, H.; Tatsumi, T.; Takamiya, M.; Zen, K.; Takahashi, T.; Azuma, A.; Tateishi, K.; Nomura, T.; Hayashi, H.; Nakajima, N.; et al. Carbon Dioxide–Rich Water Bathing Enhances Collateral Blood Flow in Ischemic Hindlimb via Mobilization of Endothelial Progenitor Cells and Activation of NO-cGMP System. Circulation 2005, 111, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Moore, J.I.; Koss, M.C. Topical Application of CO2 Increases Skin Blood Flow. J. Investig. Dermatol. 1989, 93, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Zbroja, H.; Kowalski, M.; Lubkowska, A. The Effect of Dry Carbon Dioxide Bathing on Peripheral Blood Circulation Measured by Thermal Imaging among Patients with Risk Factors of PAD. Int. J. Environ. Res. Public Health 2021, 18, 1490. [Google Scholar] [CrossRef] [PubMed]
- Elimban, V.; Xu, Y.-J.; Bhullar, S.K.; Dhalla, N.S. Temperature-dependent effects on CO2 water bath therapy induced changes in blood flow and vascularity in hind limb ischemia. Can. J. Physiol. Pharmacol. 2020, 98, 228–235. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Elimban, V.; Bhullar, S.K.; Dhalla, N.S. Effects on CO2 water-bath treatment on blood flow and angiogenesis in ischemic hind limb of diabetic rat. Can. J. Physiol. Pharmacol. 2018, 96, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Elimban, V.; Dhalla, N.S. Carbon dioxide water-bath treatment augments peripheral blood flow through the development of angiogenesis. Can. J. Physiol. Pharmacol. 2017, 95, 938–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogliotti, G.; Galliera, E.; Lorio, E.; De Bernardi, M.; Valserra, D.; Solimene, U.; Corsi, M.M. Effect of immersion in CO2-enriched water on free radical release and total antioxidant status in peripheral arterial occlusive disease. Int. Angiol. 2011, 30, 12–17. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).