Abstract
The aim of this study was to investigate the effect of the Coptis chinensis, Glycyrrhiza uralensis, and fermented Glycine max (3Hb) extract on lipid barrier recovery and the alleviation of atopic dermatitis (AD). The 3Hb extract was administered to lipid barrier-eliminated mice (3HbT) for 5 days. Subsequently, the effect of the 3Hb extract on general skin features and the regulation of filaggrin, inflammatory response, Th2 differentiation, and the skin micro-environment for defense, was evaluated. In the 3HbT, filaggrin was effectively recovered. The clinical skin score was significantly lower in the 3HbT compared with control groups. In addition, significant decreases in pH and TEWL as well as in the levels of kallikrein 7, PAR-2, TSLP, IL-4, Fc ε receptor, and phosphate-NF-κB p65 were observed in the 3HbT, compared with the other control groups. Further, compared with control groups, the 3HbT showed a significant increase in those of claudin, cathelicidin, TLR, and NHE-1. Our results indicated that the 3Hb extract effectively recovered filaggrin. Through the recovery of filaggrin, inflammation and the Th2 differentiation process can be regulated, and microenvironments for defense can be recovered. Therefore, we confirmed the potential of the 3Hb extract for use in the proactive therapy of AD.
1. Introduction
The skin acts as the primary barrier that separates the human body from the surrounding environment [1]. The skin is divided into the epidermis and the dermis. The epidermis protects the body from external threats, such as pathogens, toxins, and desiccation [2]. The epidermis is divided into the stratum corneum (SC), granular layer, spinous layer, and basal layer.
The barrier function of the epidermis is primarily executed through the SC, the outermost layer of the epidermis. The SC comprises a continuous layer of epidermal lipids that act create a layered structure, separating keratinocytes from other cells. Keratinocytes produce two membrane-circumscribed granules (keratohyalin granules and lamellar bodies) in the stratum granulosum and secrete them in the intercellular space. Keratohyalin granules are intracellular components of the SC. Filaggrin (FLG) is one such representative keratohyalin and is an intercellular component of the SC [3]. Epidermal lipids and keratohyalin granules determine the epidermal permeability.
Damage to the skin barrier results in an increase of transepidermal water loss (TEWL), pH, penetration by external antigens, and skin inflammation [4]. Consequently, damage to the skin barrier results in dry skin, an elevated pH, and itching. Itching further impairs the skin barrier, which increases TEWL and pH [4]. Damage to the skin barrier, dry skin, rise in skin pH and itching are therefore linked, and become the basis of atopic dermatitis (AD). Hence, skin barrier recovery is the most basic treatment for preventing the exacerbation of skin diseases.
Atopic dermatitis is a chronic inflammatory skin disease characterized by reduced skin barrier function. Decreased expression of FLG is observed in AD and is caused by a type 2 helper T cell (Th2)-polarized inflammatory reaction [5,6]. Therefore, AD is characterized by dry skin, itching, increased TEWL, and increased penetration by antigens. AD progresses more quickly in children with FLG-null mutations. Hence, recovery of FLG is key for the treatment of allergic diseases [7].
Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max (3Hb), that constitute the 3Hb extract used in this study, are herbs that are frequently used in traditional Korean medicine. In traditional Korean medicine, each of the three aforementioned herbs are applied not only in response to internal symptoms, but also in response to AD, and therefore have both external and internal applications [8,9,10]. Previous studies on these herbs have revealed the potential effects of the extract of 3Hb on the recovery of FLG and the regulation of inflammatory reactions in AD [10,11,12,13,14,15,16].
Rhizome of Coptis chinensis has wide pharmacological effects, such as antimicrobial, cardioprotective, antidiabetic, and anti-inflammatory effects [8]. Berberine, the main active component of Rhizome of Coptis chinensis, exerts anti-inflammatory effects by inhibiting the expression of inflammatory factors such as iNOS, COX-2, IL-1, IL-6, and NF-κB [17]. Palmatine, an active component of Rhizome of Coptis chinensis, has anti-inflammatory, antibacterial, and antiviral effects [18]. Rhizome of Glycyrrhiza uralensis has anti-inflammatory, antimicrobial, antiviral, and antioxidant activities. Liquiritin and liquiritigenin, flavonoids of Rhizome of Glycyrrhiza uralensis, have anti-inflammatory effects [19,20]. Fermented Glycine max contains isoflavones such as daidzein, glycitein, genistein, daidzin, glycitin, and genistin. Isoflavones have anti-inflammatory effects and are involved in the regulation of Th2-skewed conditions [10,21,22,23]. In our previous studies, we observed the synergistic effects of Rhizome of Coptis chinensis and Rhizome of Glycyrrhiza uralensis [11,12,24]. Therefore, in this study, we aimed to investigate the synergistic effect of the three constituents of the 3Hb extract.
Based on the above-mentioned findings of previous research, we hypothesized that the 3Hb extract has a positive effect on the recovery of the skin barrier, which can help prevent the exacerbation of AD. We performed high-performance liquid chromatography (HPLC) to confirm the properties of the herbs extracted from 3Hb. To evaluate the toxicity of the 3Hb extract and to determine the FLG recovery effect of the 3Hb extract in keratinocytes, an in vitro experiment using human keratinocyte (HaCaT) cells was performed. To test our hypothesis, we studied the effect of the 3Hb extract on the regulation of pH, inflammation, FLG, Th2 differentiation, and the skin microenvironment for the promotion of skin defense.
2. Materials and Methods
2.1. Preparation of the 3Hb Extract
Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max purchased from Omniherb (Yeongcheon, Korea). Each 80 g sample of 3Hb was boiled for 3 h in 2000 mL of distilled water and then filtered. The filtrate was vacuum concentrated to 50 mL using a rotary evaporator and then freeze-dried to obtain 52 g of the extract (yield: 21.7%).
2.2. Bioactive Marker Profiling
HPLC was performed to evaluate and compare the markers of the 3Hb extract. An Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) was used. Liquiritin and liquiritigenin were analyzed for Rhizome of Glycyrrhiza uralensis; berberine and palmatine were analyzed for Rhizome of Coptis chinensis, and daidzein, glycitein, genistein, daidzin, glycitin, and genistin were analyzed for fermented Semen of Glycine max.
2.3. FLG Generating Capacity
2.3.1. Cell Viability
XTT assays were used to determine the cytotoxic effects of the 3Hb extract on HaCaT cells (a human keratinocyte cell line, Korea Institute of Oriental Medicine, Korea). After treating the cells with 3Hb extract (10, 50, 100, 500, and 1000 µg/mL) for 24 h, 50 µL of XTT solution (Sigma—Aldrich, St. Louis, MO, USA) was added and the cells were incubated for 4 h. Absorbance was then measured at 450 nm (using a reference wavelength of 650 nm) using a microplate reader (Tecan, Männedorf, Switzerland).
2.3.2. Western Blot Analysis
HaCaT cells were lysed with RIPA lysis buffer containing protease and phosphatase inhibitors (Atto, Tokyo, Japan). After sonication, the cell lysates were centrifuged at 8000× g for 10 min and the supernatants were collected. Protein concentration was determined using the Bradford protein assay reagent (Bio—Rad, Hercules, CA, USA). Subsequently, 30 µg of total protein was separated by 10% Sodium Dodecyl Sulfate (SDS)–PolyAcrylamide Gel Electrophoresis and transferred to polyvinylidene difluoride membranes (Merck Millipore, Carrigtwohill, Ireland). After blocking for 2 h in 5% skim milk in 1× PBS at room temperature, the membranes were incubated with anti-filaggrin antibody (1:2500, Santa Cruz, CA, USA), followed by incubation with horseradish peroxidase-conjugated anti-IgG secondary antibody. All membranes were detected using enhanced chemiluminescence (Bio-Rad, Hercules, CA, USA).
2.4. Experimental Animals
After keeping 4-week-old male Balb/C mice purchased from JA BIO Inc. (Suwon, Korea) in an aseptic cage for 2 weeks, mice weighing 20 ± 1.5 g were selected and used for the study and there were no exclusions. The mice were randomly allocated to four groups (n = 10 per group) as follows: normal (Ctrl) group, lipid barrier-eliminated (LBE) group, palmitoylethanolamide (PEA)-treated after lipid barrier elimination (PEAT) group, and 3Hb-treated after lipid barrier elimination (3HbT) group. All animal experiments were approved by the Institutional Animal Care and Use Committee of Pusan National University (IACUC number: PNU-2020-2503) (Supplementary file 1). We followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals throughout this study. All animal experiments followed the ARRIVE guidelines (Supplementary file 2).
2.5. Lipid Barrier Elimination and Administration
To eliminate the lipid barrier, desquamation of the SC was carried out using tape (3M, Saint Paul, MN, USA) after shaving the dorsal skin of the mouse. After applying 500 μL of 10% sodium dodecyl sulfate (SDS; Sigma, St. Louis, MO, USA), it was rubbed 20 times with a cotton swab to remove the lipid lamellae from the SC. Then, 87 mg/kg of 3Hb extract was mixed with saline, and 0.2 mL of this solution was orally administered to 3HbT mice for 5 days. Meanwhile, 600 mg/kg of PEA was mixed with saline, and 0.2 mL of this solution was orally administered to PEAT group for 5 days.
2.6. Skin Score, TEWL, and pH Measurement in Skin
The morphological severity of dorsal skin damage was evaluated and compared with the baseline. Skin scores were evaluated as follows: (1) erythema/hemorrhage, (2) scarring/dryness, (3) edema, and (4) excoriation/erosion were scored as 0 (none), 1 (mild), 2 (moderate), or 3 (severe), and the sum of the individual scores was defined as the AD skin score [25]. TEWL was measured for 30 s using a Vapometer device (Delfin Technologies, Kuopio, Finland) in accordance with the manufacturer’s instructions. Changes in skin pH were measured using SKIN-O-MAT (Cosmomed, Wetter, Germany).
2.7. Histochemistry
The skin was fixed with a vascular rinse and 10% neutral-buffered formalin (NBF). After fixing the dorsal skin sample in 10% NBF for 24 h at room temperature, it was embedded in paraffin, and serial sections with a thickness of 5 µm were made. Serial sections were investigated for common morphological changes following hematoxylin and eosin staining.
2.8. Immunohistochemistry
Immunohistochemical staining using antibodies was conducted to study the immunohistochemical changes in the microenvironment caused by inflammation in the epithelium. Antibodies against the following were used for immunohistochemical staining: FLG, kallikrein (KLK) 7, protease-activated receptor (PAR)-2, thymic stromal lymphopoietin (TSLP), interleukin (IL)-4, Fcε receptor (FcεR), phosphate-nuclear factor-κB (p-NF-κB) p65, claudin, cathelicidin, toll-like receptors (TLR), and sodium hydrogen antiporter (NHE) 1. First, after 5 min of proteolysis, the skin samples were treated with proteinase K (20 μg/mL, Dako, Santa Clara, CA, USA) and then blocked for 1 h in 10% normal goat serum (Vector Lab, Burlingame, CA, USA) containing 1% total bovine serum (Sigma, St. Louiscity, MO, USA). The skin samples were then incubated with primary antibodies, including mouse anti-filaggrin, mouse anti-KLK7, mouse anti-PAR-2, mouse anti-IL-4, mouse anti-FcεR, mouse anti-p-NF-κB 65 (1:100, Santa Cruz Biotec, Dallas, TX, USA), mouse anti-TSLP mouse anti-claudin, mouse anti-cathelicidin, mouse anti-TLR, and mouse anti-NHE (1:100, Abcam, Cambridge, UK), for 72 h in a humidified chamber at 4 °C. Next, the samples were incubated with biotinylated rabbit anti-goat IgG (1:100) secondary antibody at room temperature (21–23 °C) for 24 h. An avidin-biotin complex kit (Vector Lab, Burlingame, CA, USA) was used at room temperature for 1 h. As a final step, the samples were treated with 0.05 M Tris-HCl buffer solution (pH 7.4) composed of 0.05% 3,3′-diaminobenzidine and 0.01% HCl, and counter-stained with hematoxylin.
2.9. Image Analysis and Statistical Analysis
The results of immunohistochemistry were quantified through imaging analysis using Image Pro Plus 7 (Media Cybernetics, Rockville, MD, USA). Data were presented as the means ± standard error. Ten skin samples from each group were randomly selected and imaged at 400× magnification, followed by the analysis of the images at positive pixels (intensity 80–100)/20,000,000 pixels.
Statistical analyses were performed using SPSS 25 software (SPSS Inc., Chicago, IL, USA). The significance (p < 0.05) was verified using the one-way analysis of variance test, and the post-validation was performed using the Tukey’s honestly significant difference. Statistically significant (p < 0.05) differences between the LBE, PEAT, and 3HbT groups are expressed by an asterisk (*) in the image analysis results, whereas statistically significant (p < 0.05) differences between the PEAT and 3HbT groups expressed as (#) in the image analysis results.
3. Results
3.1. HPLC Analysis of the Herbal Extracts
To identify the bioactive markers of the 3Hb extract in this study, we performed HPLC profiling. Upon comparison with the results of the standard compounds, 10 main molecules were identified in the HPLC analysis: liquiritin (0.25 mg/kg), liquiritigenin (0.49 mg/kg), berberine (7.19 mg/kg), palmatine (21.97 mg/kg), daidzein (2.96 mg/kg), glycitein (0.55 mg/kg), genistein (0.06 mg/kg), daidzin (2.19 mg/kg), glycitin (1.13 mg/kg), and geinstin (0.25 mg/kg). We also found that the 3Hb extract contains known bioactive markers of 3Hb. The results of HPLC analysis are shown in Table 1 and Figure 1.

Table 1.
Contents (mg/kg) of the markers of processed herbal medicine extracts evaluated by high-performance liquid chromatography.
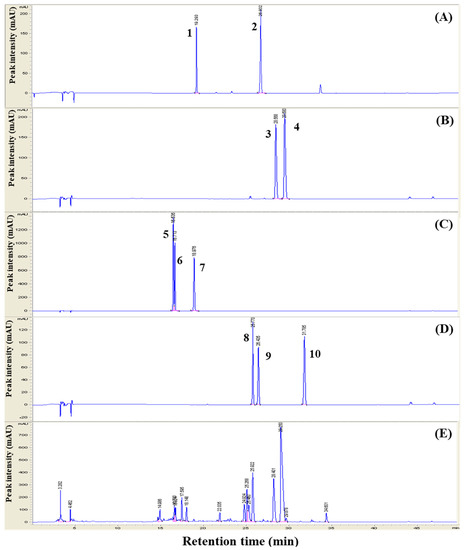
Figure 1.
The high-performance liquid chromatography profile of Three Herb (3Hb) and 3Hb extract 3Hb means Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max. Markers were eluted in the standard solution (Rhizome of Glycyrrhiza uralensis (A), Rhizome of Coptis chinensis (B), fermented Semen of Glycine max (C,D)), 3 Hb extract (E). Peak number: markers of liquiritin (1), liquiritigenin (2, berberine (3), palmatine (5) and isoflavones such as daidzein (5), glycitein (6), genistein (7), daidzin (8), glycitin (9), and genistin (10).
3.2. Regulation of Lipid Barrier
To determine the effect of the 3Hb extract on the regulation of the lipid barrier, we performed Western blotting and measured the expression of FLG in HaCaT cell. Treatment with 3Hb extract increased the expression of FLG in a concentration-dependent manner (<100 μg/mL) (Figure 2). Full length of Western blot was provided in Supplementary file 3 (Figure S1).
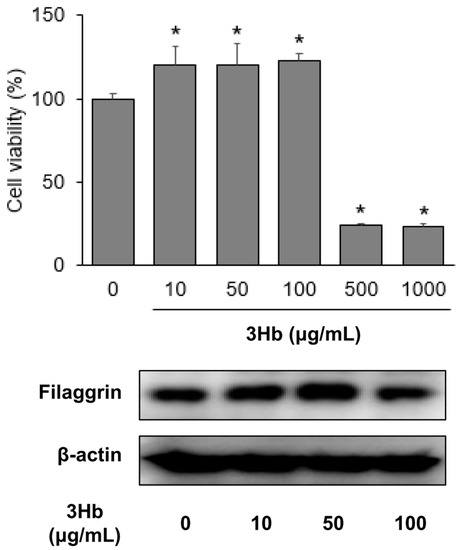
Figure 2.
Effect of Three Herb (3Hb) extract on viability and on expression of filaggrin in HaCaT cells. 3Hb means Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max. Cells were treated with the indicated concentration of 3Hb for 24 h. HaCaT, Human keratinocyte. The data were analyzed using a Student’s unpaired t-test. p < 0.05 were considered significant. * p < 0.05 compared to the untreated (0 μg/mL) group.
3.3. Changes in General Skin Features
Erythema, hemorrhage, scarring, and erosion were observed in the LBE group. In contrast, PEAT and 3HbT groups showed comparatively less skin damage (Figure 3A). Histochemical changes in the skin were observed after hematoxylin and eosin staining. In LBE group, epithelial cell formation, expansion of intercellular space in the spinous layer, increased lymphocyte erosion, and decay of the basal layer were observed in the stratum basale. In contrast, the epithelia of PEAT and 3HbT groups showed comparatively less damage (Figure 3A). The clinical skin scores for each group are shown in Figure 3B. The clinical skin scores of 3HbT mice were lower than those of PEAT and LBE groups (p < 0.01).
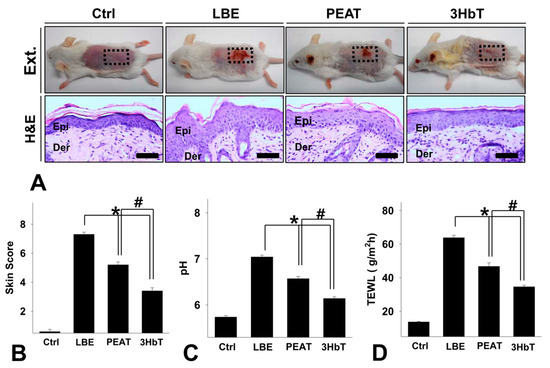
Figure 3.
The alleviation of symptoms of lipid barrier elimination using Three Herb (3Hb) extract. (A) The alleviation of symptoms of lipid barrier elimination. The lipid destruction-induced skin lesions were relieved in 3HbT compared to PEAT and LBE. Clinical skin score (B), pH (C) and TEWL (D) was significantly lower in 3HbT compared with PEAT and LBE (Immunohistochemistry; bar size, 50 µm). 3Hb, Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max; Ctrl, the normal group; LBE, lipid barrier elimination group; 3HbT, 3Hb treated group after lipid barrier elimination; Ext., External morphology; H&E, Hematoxylin and Eosin stain; Epi, epidermis; Der, dermis; * p < 0.05 compared to LBE; # p < 0.05 compared to PEAT.
The pH values of each group are shown in Figure 3C. FLG deficiency results in an increase in the pH of the SC due to the reduction of trans-urocanic acid (UCA) [26]. Therefore, the pH of the dorsal skin of the LBE group was higher than that of the dorsal skin of the Ctrl group. The pH values of the dorsal skin of the PEAT and 3HbT groups were lower than those observed in the LBE group (p < 0.05). Furthermore, the pH values of 3HbT mice were lower than that of PEAT group (p < 0.05).
The extent of TEWL in each group is shown in Figure 3D. FLG deficiency is known to weaken the formation of keratinous cells and reduce adhesion between keratinous cells, resulting in an increase in TEWL [26]. Therefore, the TEWL in LBE group was higher than that in the Ctrl group. Moreover, TEWL in the 3HbT group was lower than that in the PEAT group (p < 0.05).
These results indicate that lipid barrier elimination causes inflammation in the skin, causing conditions such as AD and an increase in TEWL and pH of the skin. The 3Hb extract effectively alleviated inflammation in the skin and decreased TEWL and pH in the skin compared to PEA.
3.4. Effect of Change in FLG-pH-Inflammation
The effect of change in FLG on pH and inflammation was investigated by measuring the extent of FLG-, KLK7-, PAR-2-, and TSLP-positive reactions in the immunohistochemical analysis (Figure 4). Decreased FLG results in increased skin pH. Additionally, increased skin pH results in allergic inflammation after a series of processes involving KLK7, PAR-2, and TSLP. Immunohistochemistry results showed that FLG-positive reaction was lower than in Ctrl and 3HbT groups. However, KLK7-, PAR-2-, and TSLP-positive reactions in LBE, PEAT, and 3HbT groups were stronger than those in the Ctrl group, and those in the 3HbT group were weaker than those in the LBE and PEAT groups (p < 0.05).
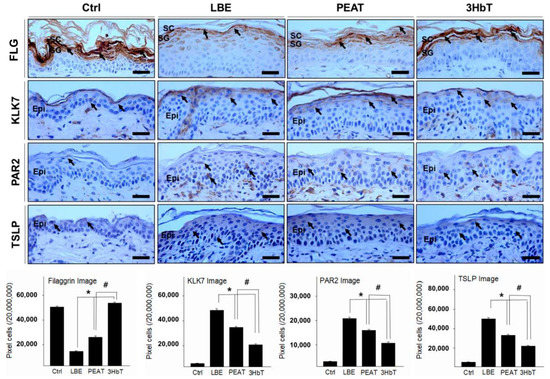
Figure 4.
The regulation of filaggrin and inflammation using Three Herb (3Hb) extract. The expression of FLG was significantly higher in 3HbT compared to PEAT and LBE. The expression of KLK7, PAR-2, TSLP (light brown particles indicated by arrows) was significantly lower in 3HbT compared to PEAT and LBE (immunohistochemistry; bar size, 50 µm). The data of image analysis for positive reaction showed the same results. 3Hb, Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max; FLG, Filaggrin; Ctrl, the normal group; LBE, lipid barrier elimination group; 3HbT, 3Hb treated group after lipid barrier elimination; KLK7, kallikrein7; PAR-2, protease-activated receptor-2; TSLP, thymic stromal lymphopoietin; Epi, epidermis; * p < 0.05 compared to LBE; # p < 0.05 compared to PEAT.
Lipid barrier elimination therefore causes a decrease in FLG level and an increase in KLK7, PAR-2, and TSLP levels. The 3Hb extract effectively recovers FLG and inhibits a series of processes involving KLK7, PAR-2, and TSLP (which are caused by lipid barrier elimination), compared to PEA.
3.5. Regulation of Th2 Differentiation
The regulation of Th2 differentiation was studied by measuring the extent of IL-4-, FcεR-, and p-NF-κB p65-positive reactions in the immunohistochemical analysis (Figure 5). TSLP overexpression results in allergic inflammation as well as Th2-skewed conditions after a series of processes involving IL-4, FcεR, and p-NF-κB p65 [27,28,29,30,31]. Immunohistochemistry results showed that IL-4-, FcεR-, and p-NF-κB p65-positive reactions in the LBE, PEAT, and 3HbT groups were higher than those in the Ctrl group, and those in the 3HbT group were lower than those in the LBE and PEAT groups (p < 0.05).
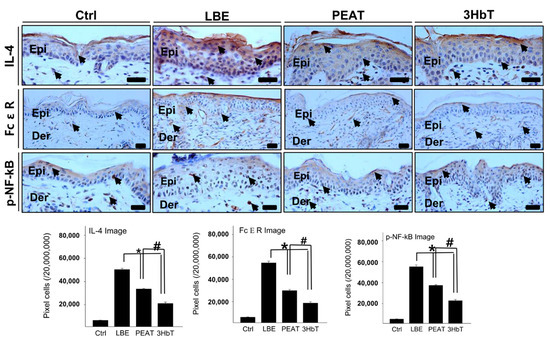
Figure 5.
The regulation of the Th2 skewed condition using Three Herb (3Hb) extract. The expression of IL-4, FcεR, p-NF-κB (light brown particles indicated by arrows) was significantly lower in 3HbT compared to PEAT and LBE (immunohistochemistry; bar size, 50 µm). The data of image analysis for positive reactions showed the same results. 3Hb, Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max; Ctrl, the normal group; LBE, lipid barrier elimination group; 3HbT, 3Hb treated group after lipid barrier elimination; IL-4, interleukin-4; FcεR, Fc ε receptor; p-NF-κB, phosphate-nuclear factor-κB; Epi, epidermis; Der, dermis; * p < 0.05 compared to LBE; # p < 0.05 compared to PEAT.
These results indicate that lipid barrier elimination causes an increase in IL-4, FcεR, and p-NF-κB p65 levels through TSLP overexpression. In addition, 3Hb extract effectively inhibits a series of processes involving IL-4, FcεR, and p-NF-κB p65 (caused by TSLP overexpression), compared to PEA.
3.6. Regulation of the Skin Microenvironment for Defense
Regulation of the skin microenvironment for defense was studied by measuring the extent of claudin-, cathelicidin-, TLR-, and NHE 1-positive reactions in the immunohistochemical analysis (Figure 6). Claudin, cathelicidin, TLR, and NHE 1 exist in the SC and act as supplementary barriers to external antigens [32,33,34,35]. The immunohistochemical results showed that the claudin-positive reactions in the LBE, PEAT, and 3HbT groups were lower than that in the Ctrl group, and the claudin-positive reaction in the 3HbT group was higher than those in the LBE and PEAT groups (p < 0.05).

Figure 6.
The regulation of the skin micro-environment for defense using Three Herb (3Hb) extract. The expression of claudin, cathelicidin, TLR, NHE (light brown particles indicated by arrows) was significantly lower in 3HbT compared to PEAT and LBE (immunohistochemistry; bar size, 50 µm). The data of image analysis for a positive reaction showed the same results. 3Hb, Rhizome of Coptis chinensis, Rhizome of Glycyrrhiza uralensis, and fermented Semen of Glycine max; Ctrl, the normal group; LBE, lipid barrier elimination group; 3HbT, 3Hb treated group after lipid barrier elimination; Cathe, cathelicidin; TLR, toll-like receptor; NHE, sodium hydrogen antiporter; Epi, epidermis; * p < 0.05 compared to LBE; # p < 0.05 compared to PEAT.
The elimination of the lipid barrier caused a decrease in the levels of claudin, cathelicidin, TLR, and NHE 1, as shown by the comparison of the LBE, PEAT, and 3HbT groups with the Ctrl group. The 3Hb extract restores the skin microenvironment for defense by increasing the expression of claudin, cathelicidin, TLR, and NHE 1, compared to PEA.
4. Discussion
The SC of the skin primarily acts as an external barrier for the human body and the lipid barrier of the SC plays an important role in this context. Two of its functions are to prevent excessive water loss through the skin and prevent the entry of harmful substances into the body [36]. Therefore, defects in the lipid barrier can facilitate sensitization by increasing antigen permeability, which can lead to AD [4,36,37,38]. FLG is one of the various components of SC and it functions in the aggregation of keratin filaments. FLG is being studied as a central component in the pathogenesis of AD and other allergic diseases [39,40], starting with a study in 1996 [41], which showed a decrease in the expression of FLG in the skin of patients with AD.
In traditional Korean medicine, Hataedock treatment is used to remove “fetal heat” [12] which is a cause of AD. 3Hb is the core ingredient used in the Hataedock treatment. Previous studies [10,11,12,13,14,15,16] have shown the effectiveness of alleviating AD by maintaining the lipid barrier through the use of 3Hb extract. Therefore, we assumed that the use of the 3Hb extract would work as a “proactive therapy” that could be administered prophylactically prior to the presentation of symptoms, and which could aid in suppressing a subsequent series of processes, by maintaining the lipid barrier through the recovery of FLG. Based on these assumptions, we used the lipid barrier-eliminated Balb/C mice to check the effectiveness of FLG and study its effect on general skin features, inflammatory reactions, the Th2 differentiation process, and skin microenvironment. We also used HPLC for quality control and the identification of the components of the 3Hb extract.
A decreased positive reaction and expression of FLG in LBE group indicates that damage to the lipid barrier causes reduced expression of FLG in the skin. As expected, the positive reaction and expression of FLG in 3HbT mice showed a substantial increase compared to that in the PEAT and LBE groups. The 3Hb extract has an effect on the recovery of FLG, which is superior to that of PEA.
FLG has important roles in skin acidification and hydration and exhibits antimicrobial functions [34]. Therefore, FLG deficiency negatively affects the formation of keratinocytes, decreases adhesion between keratinocytes, and increases TEWL, leading to a decrease in the overall skin barrier function. This increases the probability of sensitization and allergic reactions due to increased penetration of external allergens. In addition, the pH of the SC increases due to a decrease in trans-UCA (a degradation product of FLG), and a decrease in the contents of amino acids and pyrrolidone carboxylic acid causes dryness of the skin [26].
In this study, the pH and TEWL in the skin were significantly decreased in the 3HbT group compared to the LBE and PEAT groups. This suggests that the 3Hb extract can restore FLG, resulting in an increase in trans-UCA and recovery of FLG function. A previous study [33] showed that TEWL can act as a predictable indicator of the occurrence of early childhood AD, thus inferring the effectiveness of 3Hb extract administration as a proactive therapy for AD. In addition, we confirmed that the 3Hb extract can reduce observable and quantitative skin damage caused by lipid barrier elimination.
As the pH of the SC increases, the activity of LEKTI 1 decreases, leading to the induction of the activity of serine proteases such as KLK7 and an increase in keratinocyte dropout [42]. The activity of the serine proteases activates the G-protein-coupled receptor, which increases the activity of PAR-2 [43]. PAR-2 plays a role as an inflammatory mediator in the skin and is involved in itching and skin barrier homeostasis. The activation of PAR-2 decreases the secretion of lamellar bodies, weakens the lipid barrier, and overexpresses TSLP, which in turn induces Th2 differentiation mediators and allergic inflammatory responses [44]. It can be seen that the inflammatory response is activated through a series of processes that lead to an increase in the pH in the SC.
As described above, KLK7, PAR-2, and TSLP cause inflammation through a series of processes. In the 3HbT group, these processes were considerably inhibited, indicating alleviation of inflammation by the 3Hb extract. A previous study [45] suggested that normalization of pH in SC is effective in alleviating inflammatory reactions, and hence, the alleviation of inflammation by 3Hb extract is thought to be due to the normalization of pH in SC.
TSLP overexpression increases the secretion of cytokines such as IL-4. IL-4 secretion, in turn, increases the production of B cells, which produce allergen antibodies and induce Th2 differentiation to express CD40L. CD40L binds to CD40 on the surface of B cells, leading to the differentiation of B cells into plasma cells and the production of IgE [27]. IgE induces the activation of mast cells by binding to FcεR on the surface of mast cells [28,29]. The activation of FcεR induces NF-κB activity, and IL-4 induces NF-κB activity [24]. FcεR expression is increased at sites of inflammation [30]. NF-κB plays an important role in allergic inflammatory reactions by enhancing the production of inflammatory cytokines and chemokines. Therefore, the NF-κB transcription factor family, which includes p65, plays a central role in the progression and maintenance of AD [31].
Th2 cytokine activity is observed in acute AD lesions [46]. We observed that lipid barrier elimination enhanced Th2 differentiation reaction in LBE group. This leads to acute AD lesions on the skin. In the 3HbT mice, the decrease in Th2 differentiation could be inferred from the reduction in the number of acute lesions of AD after the administration of the 3Hb extract.
Through a series of processes described earlier, FLG deficiency can cause skin diseases such as AD by inducing inflammatory reactions and Th2 differentiation. We showed that the 3Hb extract restored FLG. Therefore, 3Hb extract can prevent a series of processes (inflammatory reactions and Th2 differentiation processes) caused by FLG deficiency. In addition, 3Hb extract also decreased skin damage in AD.
The skin in patients with AD is known to show reduced expression of the components of the skin microenvironment required for maintaining adequate defense. In AD, a decrease in claudin-1 was observed [32], and this decrease caused skin dryness due to an increase in TEWL. The expression of cathelicidin is decreased by Th2 cytokines [33]. TLR is a receptor that is activated when a pathogen is identified and acts as a secondary line of defense against pathogens. Defects in TLR are thought to be a factor leading to AD by potentially allowing bacterial colonization [34]. NHE-1 helps to maintain physiological pH. NHE-1 expression was also found to be decreased in AD [35]. Since FLG gene mutations cause increased skin permeability, it is thought that there is a correlation between FLG deficiency and the skin microenvironment.
This study found that in the LBE group, levels of claudin, cathelicidin, TLR, and NHE were lower than those in the Ctrl group. This suggests that the lipid barrier is also involved in the regulation of the skin microenvironment. In 3HbT mice, a considerable increase in claudin, cathelicidin, TLR, and NHE levels was observed compared to the levels in the PEA and LBE group, even in the skin microenvironment where a decrease in AD was observed. It can be seen that the 3Hb extract regulates the skin microenvironment through routes such as the recovery of tight junctions, antibacterial effects, and skin homeostasis. The process involves the recovery of the lipid barrier through the recovery of FLG.
In this study, we found that the 3Hb extract normalized the levels of FLG to form a physical support system for the lipid barrier. Therefore, the 3Hb extract restores the skin barrier functions, such as the acidification of the skin and prevention of TEWL. The 3Hb extract prevents continuous damage to the lipid barrier by regulating the inflammatory response and Th2 differentiation. In addition, secondary infection can also be prevented by controlling the microenvironment for defense. These results confirm that the 3Hb extract administration can be used as a proactive therapy, which effectively prevents AD through internal and external environmental control by FLG recovery. This study is also significant in that, not only were the immunological and histological differences of each group visually confirmed, but statistically significant quantitative differences were also confirmed by comparison of the protein expression level. We investigated the lipid barrier recovery effect and effect of modulating inflammatory response and Th2 differentiation response of 3Hb. However, additional studies on the role of each herb will be needed, and further studies will be needed to see if there is a synergistic effect of the 3Hb. We sought to confirm whether 3Hb extract can act as a proactive therapy in AD through this animal experiment. However, study will be needed on whether 3Hb extract works the same way in human skin. In future studies, the clinical stability of the 3Hb extract should be investigated for its application in clinical skin disease treatment.
5. Conclusions
In conclusion, this study demonstrated that the 3Hb extract restored FLG. As we initially assumed, the restorative effect of FLG of the 3Hb extract restored general skin features such as pH, TEWL, and clinical skin score. In addition, levels of inflammation mediators (KLK7, PAR-2, and TSLP) and Th2 differentiation mediators (IL-4, FcεR, and p-NF-κB p65) were decreased by the 3Hb extract. In addition, the 3Hb extract showed an increase in levels of the indicators of the skin microenvironment for defense (claudin, cathelicidin, TLR, and NHE 1). This suggests that the 3Hb extract is effective as a proactive therapy for AD by reducing AD pathogenesis and strengthening the defense barrier function.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11188380/s1, Supplementary file 1: IACUC Protocol Approval; Supplementary file 2: ARRIVE guideline checklist; Supplementary file 3: Figure S1 (full length of Western blot in Figure 2).
Author Contributions
B.-C.P. played a role in investigation, methodology, and writing—original draft. S.-H.A. played a role in conceptualization, formal analysis, investigation, methodology, and writing—original draft. I.-J.Y. played a role in formal analysis. K.-B.K. played a role in conceptualization, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF2019R1A2C1002443 to K. Kim).
Institutional Review Board Statement
All animal experiments were approved by the Institutional Animal Care and Use Committee of Pusan National University (IACUC number: PNU-2020-2503).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors assert no conflict of interest associated with this project.
References
- Kim, K.H.; Shim, J.S.; Kim, H.J.; Son, E.D. Penta-O-galloyl-β-D-glucose from Paeonia lactiflora Pall. root extract enhances the expression of skin barrier genes via EGR3. J. Ethnopharmacol. 2020, 10, 112337. [Google Scholar] [CrossRef]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Egawa, G.; Kabashima, K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018, 67, 3–11. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Ständer, S.; Schmelz, M. Skin Barrier Damage and Itch: Review of Mechanisms, Topical Management and Future Directions. Acta Derm. Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces fermentation filtrate prevents T helper2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015, 40, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef]
- Tenn, M.W.; Ellis, A.K. The clinical relevance of filaggrin mutations: Effect on allergic disease. Ann. Allergy Asthma Immunol. 2016, 117, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Lou, G.H.; Zeng, H.R.; Hu, J.; Huang, Q.W.; Peng, W.; Yang, X.B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.R.; Ahn, S.H.; Park, I.S.; Park, S.Y.; Jeong, S.I.; Cheon, J.H.; Kim, K.B. Douchi (fermented Glycine max Merr.) alleviates atopic dermatitis-like skin lesions in NC/Nga mice by regulation of PKC and IL-4. BMC Complement. Altern. Med. 2016, 16, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Ahn, S.H.; Yang, I.J.; Park, S.Y.; Kim, K.B. Effect of Hataedock Treatment on Epidermal Structure Maintenance through Intervention in the Endocannabinoid System. Evid. Based Complement. Altern. Med. 2020, 2020, 3605153. [Google Scholar] [CrossRef]
- Cha, H.Y.; Ahn, S.H.; Cheon, J.H.; Park, S.Y.; Kim, K.B. Hataedock treatment has preventive therapeutic effects for atopic dermatitis through skin barrier protection in Dermatophagoides farinae-induced NC/Nga mice. J. Ethnopharmacol. 2017, 206, 327–336. [Google Scholar] [CrossRef]
- Man, M.Q.; Hu, L.Z.; Elias, P.M. Herbal Medicines Prevent the Development of Atopic Dermatitis by Multiple Mechanisms. Chin. J. Integr. Med. 2019, 25, 151–160. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Kesharwani, P.; Khan, S.; Hussain, F. Phytotherapeutic potential of natural herbal medicines for the treatment of mild-to-severe atopic dermatitis: A review of human clinical studies. Biomed. Pharmacother. 2017, 93, 596–608. [Google Scholar] [CrossRef]
- Wu, S.; Yu, D.; Liu, W.; Zhang, J.; Liu, X.; Wang, J.; Yu, M.; Li, Z.; Chen, Q.; Li, X.; et al. Magnoflorine from Coptis chinese has the potential to treat DNCB-induced Atopic dermatits by inhibiting apoptosis of keratinocyte. Bioorganic Med. Chem. 2020, 28, 115093. [Google Scholar] [CrossRef]
- Kim, K.J.; Xuan, S.H.; Park, S.N. Licoricidin, an isoflavonoid isolated from Glycyrrhiza uralensis Fisher, prevents UVA-induced photoaging of human dermal fibroblasts. Int. J. Cosmet. Sci. 2017, 39, 133–140. [Google Scholar] [CrossRef]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Zhao, R.J.; Park, S.J.; Lee, J.R.; Cho, I.J.; Yang, C.H.; Kim, S.J.; Kim, S.C. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-κB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 2008, 154, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef]
- Zhang, J.H.; Tatsumi, E.; Ding, C.H.; Li, L.T. Angiotensin I-converting enzyme inhibitory peptides in Douchi, a Chinese traditional fermented soybean product. Food Chem. 2006, 98, 551–557. [Google Scholar] [CrossRef]
- Lin, J.; Xu, Y.; Zhao, T.; Sun, L.; Yang, M.; Liu, T.; Sun, H.; Zhang, L. Genistein suppresses smooth muscle cell-derived foam cell formation through tyrosine kinase pathway. Biochem. Biophys. Res. Commun. 2015, 463, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.S.; Hong, L.; Guan, Y.; Dong, X.W.; Zheng, H.S.; Tan, G.L.; Xie, Q.X. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int. Immunopharmacol. 2011, 11, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.Y.; Ahn, S.H.; Cheon, J.H.; Park, I.S.; Kim, J.T.; Kim, K.B. Hataedock Treatment Has Preventive Therapeutic Effects in Atopic Dermatitis-Induced NC/Nga Mice under High-Fat Diet Conditions. Evid. Based Complement. Altern. Med. 2016, 2016, 1739760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.H.; Kim, T.H.; Kang, M.S.; Ahn, J.O.; Choi, J.H.; Chung, J.Y. Comparison of the presentation of atopic dermatitis induced by trinitrochlorobenzene and house dust mite in NC/Nga mice. J. Vet. Sci. 2020, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Norval, M. The Multiple Roles of Urocanic Acid in Health and Disease. J. Investig. Dermatol. 2021, 141, 496–502. [Google Scholar] [CrossRef]
- Brown, M.A.; Hural, J. Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 2017, 37, 181–212. [Google Scholar] [CrossRef]
- Schwartz, C.; Eberle, J.U.; Voehringer, D. Basophils in inflammation. Eur. J. Pharmacol. 2016, 778, 90–95. [Google Scholar] [CrossRef]
- Blank, U.; Charles, N.; Benhamou, M. The high-affinity immunoglobulin E receptor as pharmacological target. Eur. J. Pharmacol. 2016, 778, 24–32. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Fan, H.J.; Zhao, X.S.; Tan, Z.B.; Liu, B.; Xu, H.L.; Wu, Y.T.; Xie, L.P.; Bi, Y.M.; Lai, Y.G.; Liang, H.F.; et al. Effects and mechanism of action of Huang-Lian-Jie-Du-Tang in atopic dermatitis-like skin dysfunction in vivo and in vitro. J. Ethnopharmacol. 2019, 240, 111937. [Google Scholar] [CrossRef]
- Gruber, R.; Börnchen, C.; Rose, K.; Daubmann, A.; Volksdorf, T.; Wladykowski, E.; Vidal-Y-Sy, S.; Peters, E.M.; Danso, M.; Bouwstra, J.A.; et al. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am. J. Pathol. 2015, 185, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Gallo, R.L. The Critical and Multifunctional Roles of Antimicrobial Peptides in Dermatology. Dermatol. Clin. 2017, 35, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, W.; Zhang, L.J. The Role of Toll-Like Receptors in Skin Host Defense, Psoriasis, and Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1824624. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Matsuda, A.; Jung, K.; Karasawa, K.; Matsuda, K.; Oida, K.; Ishizaka, S.; Ahn, G.; Amagai, Y.; Moon, C.; et al. Skin pH Is the Master Switch of Kallikrein 5-Mediated Skin Barrier Destruction in a Murine Atopic Dermatitis Model. J. Investig. Dermatol. 2016, 136, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Rawlings, A.V. The chemistry, function and (patho)physiology of stratum corneum barrier ceramides. Int. J. Cosmet. Sci. 2017, 39, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Lowe, A.J.; Leung, D.Y.M.; Tang, M.L.K.; Su, J.C.; Allen, K.J. The skin as a target for prevention of the atopic march. Ann. Allergy Asthma Immunol. 2018, 120, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Čepelak, I.; Dodig, S.; Pavić, I. Filaggrin and atopic march. Biochem. Med. (Zagreb) 2019, 29, 020501. [Google Scholar] [CrossRef]
- Seguchi, T.; Cui, C.Y.; Kusuda, S.; Takahashi, M.; Aisu, K.; Tezuka, T. Decreased expression of filaggrin in atopic skin. Arch. Dermatol. Res. 1996, 288, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Kishibe, M. Physiological and pathological roles of kallikrein-related peptidases in the epidermis. J. Dermatol. Sci. 2019, 95, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redhu, D.; Franke, K.; Kumari, V.; Francuzik, W.; Babina, M.; Worm, M. Thymic stromal lymphopoietin production induced by skin irritation results from concomitant activation of protease-activated receptor 2 and interleukin 1 pathways. Br. J. Dermatol. 2020, 182, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Peters, N.; Peters, A.T. Atopic dermatitis. Allergy Asthma Proc. 2019, 40, 433–436. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).