Abstract
Psoriasis is not considered a strictly skin condition, but a complex disease with multisystem involvement due to the frequent associated comorbidities. We conducted a retrospective database study of 10,986 patients admitted in the interval January 2008–January 2019 to the Dermatology Clinic of the Iasi County “St. Spiridon” Emergency Hospital. Of the 10,986 patients admitted, 1288 were diagnosed with psoriasis. The association of malignancies was found in 40 of the psoriasis group cases and 399 of the control group cases that included various dermatological conditions. The calculation of Odds Ratios allowed us to determine if the patients with psoriasis could be at risk for certain malignancies. Thus, an association was suggested between psoriasis and central nervous system (CNS), upper aerodigestive tract cancer, endocrine cancer, bladder cancer, lung cancer, prostate cancer, breast cancer, or colorectal cancer. It is the first study of its kind in the northeastern region of Romania and can be the starting point for future long-term prospective cohort studies that will allow a more accurate data collection and a better understanding of the psoriasis–cancer relationship.
1. Introduction
Psoriasis is a chronic immune-mediated inflammatory disease that affects 2–3% of the world population; in Europe the prevalence rates range from 0.73% (in Scotland) to 2.9% (in Italy) [1]. Currently, it is no longer considered strictly a skin condition but a complex disease with multisystem involvement due to the frequent associated comorbidities. Additionally, as psoriasis affects patients’ physical appearance, the disease often has a psychological impact.
Comorbidities of psoriasis are a topical issue of modern dermatology, the literature suggesting the association of the severe forms with an increased incidence of cardiovascular diseases, metabolic syndrome, diabetes, depression, and cancer [2,3].
Two meta-analyses including several studies [4,5] were aimed at estimating the risk of cancer in patients with psoriasis. The first meta-analysis [4] showed that psoriasis patients are at slightly increased risk for developing solid organ cancer (upper aerodigestive tract, liver, lung, pancreas, and urinary tract), increased risk for squamous cell carcinoma and basal cell carcinoma, without increasing the risk for melanoma, and with regard to lymphoma, the misdiagnosis of primary cutaneous lymphoma as psoriasis would have overestimated the risk. In the most recent meta-analysis [5], the results partially coincide with those reported by the first meta-analysis, suggesting a possible risk for colorectal, upper aerodigestive tract, kidney cancers, lymphomas, keratinocyte, esophageal, and pancreatic cancers. Additionally, the overall risk of cancer mortality was higher in patients with severe psoriasis.
Previous studies evaluating the risk of developing cancer in patients with psoriasis are contradictory. A study conducted in the United States reported a 78% increase in total cancer risk for patients with severe psoriasis (treated with systemic drugs) and by 13% for those with mild disease compared to the control group including hypertensive patients without psoriasis [6]. Another perspective was opened by Brauchli et al. [7] who investigated an association between psoriasis and cancer after adjusting for risk factors. In this study, 55% of the psoriasis patients were younger than 50 years at diagnosis of psoriasis and had a slight increase in the total cancer rate.
The biological mechanism that explains the relationship between psoriasis and cancer is under investigation. The hypotheses underlying the possible risk of malignancy among the psoriasis population are the disease itself due to its inflammatory nature, the use of immunosuppressive medication, biologic therapy, exposure to ultraviolet (UV) therapies, or the association of lifestyle factors (smoking, alcohol, diet) [8]. However, so far there is no consensus whether these patients need a cancer screening or prevention program.
The present study aims to:
- -
- Provide descriptive epidemiological data on the association of psoriasis with malignancies in Northeastern Romania;
- -
- Quantify the risk of developing different forms of cancer in patients with psoriasis compared to the general population;
- -
- Analyze a possible benefit of implementing a cancer screening/prevention program in patients with psoriasis.
2. Materials and Methods
This 10-year retrospective study included 10,986 patients admitted to the Dermatology Clinic of the Iasi County “Sf. Spiridon” Emergency Hospital. Patient data were collected from the hospital database. The diagnoses of psoriasis and malignancy were based on the national diagnosis codes L40.0-L40.9 for psoriasis and C00-96 and D00-D09 for malignancies, respectively. Of a total of 10,986 unique in-patients admitted to our clinic, 1288 were diagnosed with psoriasis (group 1) and 9698 with other skin diseases (group 2).
3. Results
The association of malignancies was found in 40 cases in the psoriasis group and 399 cases in the control group that included patients with various dermatological conditions. We mention that in the two groups of patients with malignancies, non-melanocytic skin cancers (NMSC) were not included (Figure 1).
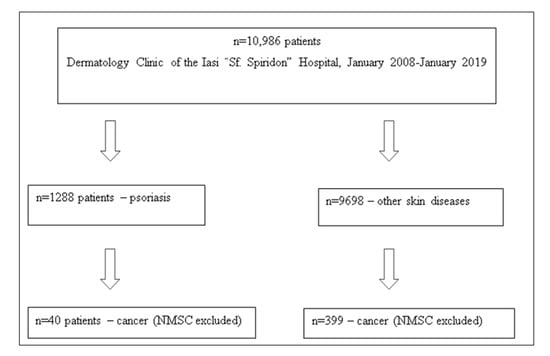
Figure 1.
Patients associating the diagnosis of psoriasis with various types of cancer at the Dermatology Clinic of the Iasi “Sf. Spiridon” Emergency Hospital in the interval January 2008–January 2019.
Males predominated in both study groups, 65% in the psoriasis group and 55.39% in the control group, and there was a positive association with urban residence, 62.50% and 55.14%, respectively. Interestingly, the mean age in the psoriasis group was significantly lower (60.87 vs. 65.93 years), Table 1.

Table 1.
Demographic data.
Based on OR calculation we could determine if the patients with psoriasis could be at risk for certain malignancies (Table 2). Thus, a psoriasis-cancer association was suggested in the case of CNS cancer OR = 16.09 (95% CI: 2.61–99.4), upper aerodigestive tract cancer OR = 4.81 (95% CI: 1.41–16.41), endocrine cancer OR = 3.15 (95% CI: 0.8–11.97), bladder cancer OR = 3.15 (95% CI: 0.83–11.97), lung cancer OR = 2.61 (95% CI: 0.71–9.68), prostate cancer OR = 1.46 (95% CI: 0.42–5.12), breast cancer OR = 1.42 (95% CI: 0.47–4.26, or colorectal cancer OR = 1.28 (95% CI: 0.48–3.46). Due to the small number of liver and cervical cancer cases in the psoriasis group, we could not decide on a possible association between psoriasis and these two. Of the cancers for which a possible association with psoriasis could not be demonstrated in our study are blood cancers OR = 0.9 (95% CI: 0.34–2.38), cutaneous lymphomas OR = 0.46 (95% CI: 0.11–1.98), melanoma OR = 0.17 (95% CI: 0.002–1.29), and Kaposi’s sarcoma OR = 0.16 (95% CI: 0.002–1.14).

Table 2.
Association of psoriasis with some cancer types.
The psoriasis patients received the following treatment in descending order: topical = 18 (45%) patients, systemic = 11 (27.5%) patients, and biological = 3 (7.5%) patients. Eight (20%) of the patients were treatment naive.
Also, regarding the prevalence of NMSC, the database included 5 patients with NMSC and psoriasis and 577 with NMSC and other dermatoses.
4. Discussion
Although the risk of developing certain malignancies among patients with psoriasis has been studied in several European countries, to our knowledge this is the first study conducted in Romania (Northeastern Romania).
Interestingly, our results showed that the risk of CNS cancers in patients with psoriasis ranked first (RR = 15.0; 95% CI: 2.58–86.90; p = 0.001). Studies that have shown a possible association between CNS cancer and psoriasis are not very conclusive and most of them are geographically limited to Sweden and Finland [9,10,11,12]. In meta-analyses [4,5] the significant risk of developing CNS cancer among patients with psoriasis was eliminated. A similar situation as in our study was presented in a prospective U.S. cohort study from the time when methotrexate was the only systemic medication used in psoriasis [13].
Upper aerodigestive tract cancers (RR = 4.43; 95% CI: 1.43–13.75; p = 0.006) ranked second, and their association with psoriasis is known in the literature. [9,10,11,12,14,15,16,17,18]. Moreover, it is the only type of cancer for which the risk is found in both meta-analyzes [4,5] and in our study. Only in the study by Brauchli et al. [7] were adjustments made for the associated risk factors (alcohol and smoking), but the risk remained but was insignificant. In the first meta-analysis [4], almost half of the patients were under 50 years of age, and the follow-up period was short (4.6 years), supporting the presence of the risk of aerodigestive tract cancer risk among patients with psoriasis.
Regarding the risk of thyroid cancer (RR = 2.99; 95% CI: 0.86–10.43; p = 0.076) in patients with psoriasis, the studies are contradictory. Boffetta et al. [19] found no significant risk, and other studies reported a possible risk [20,21]. Moreover, interesting is the reported complete resolution of psoriasis in a patient after excision of thyroid cancer [22], which could suggest common pathogenic aspects, but also new therapeutic options.
The positive association of psoriasis with the risk of bladder cancer (RR = 2.99; 95% CI: 0.86–10.43; p = 0.076) in patients with psoriasis is not at all surprising, previous studies demonstrating the same thing [10,11,12,13,16,17]. In this respect, our results are in agreement with those of the first meta-analysis [4]. However, in the study by Brauchli et al. [7] where adjustments were made for smoking and alcohol consumption, no significant association was found between the two.
As in the case of urinary tract cancer, the risk of lung cancer (RR = 2.49; 95% CI: 0.73–8.47; p = 0.136) was assessed among the population with psoriasis, sources that support this association being cited in the literature [11,12,13,16]. A large cohort study of 198,366 patients with psoriasis the risk was present especially among those with moderate to severe disease. [23] On the other hand, the strong association between smoking and lung cancer is well known. Thus, the question is whether or not psoriasis is an independent risk factor for lung cancer. In two studies in which adjustment was made for smoking, the risk for lung cancer was not reported [7,9].
The correlation of psoriasis with prostate cancer (RR = 1.43; 95% CI: 0.44–4.57; p = 0.351), although present in our study, is rarely found in the literature. This is found in a recent South Korean cohort study that included about 900,000 people [20].
The association between psoriasis and breast cancer is ambiguous (RR = 1.38; 95% CI: 0.51–3.72; p = 0.533), with previous studies reporting contradictory results [6,8,9,10,11,12,13,14,15,20]. In a prospective study analyzing the risk of cancer among women with psoriasis aged over 65 years, the result was negative [9]. Another study on the incidence of psoriasis among women with breast cancer showed a slight increase [24]. Further studies are needed to clarify whether or not there is a relationship between psoriasis and the risk of breast cancer.
The risk of colorectal cancer (RR = 1.25; 95% CI: 0.52–2.98; p = 0.242) is not new, being frequently associated with inflammatory diseases [9,14,16,25]. This risk was also high in the most recent meta-analysis [5]. A study of Iowa women that included over 32,000 women [9] found a significant association between psoriasis and colorectal cancer, especially proximal colorectal cancer. An argument in favor of the fact that inflammation has a role in colorectal tumorogenesis could be the proven protective effect of an anti-inflammatory drug—aspirin [26]. In addition, the correlation of increased C-reactive protein (CRP) level with colorectal cancer risk has been reported [27].
The risk of cervical cancer in people with systemic inflammatory diseases such as inflammatory bowel disease, rheumatoid arthritis, and lupus erythematosus has been documented [28,29,30,31]. The psoriasis–cervical cancer association is not supported by the literature data [12,13,32,33].
Possibly due to the insufficient number of cases, we failed to investigate the association between psoriasis and other cancers cited in the literature, such as NMSCs [10,17,33,34,35,36], lymphomas [7,20], pancreas [19], liver and genital cancers [19,20].
Although our study did not identify a risk for lymphohematopoietic cancers, previous studies have reported a risk for their development in patients with psoriasis [6,20,37,38,39,40]. Additionally, in a retrospective cohort study that included biologic-naive children with psoriasis and a control group of children without psoriasis, a risk for developing lymphoma was found only in the former [41].
Surprisingly, in our study no correlation was found between NMSC and psoriasis. One possible explanation is that the wide range of systemic medications and effective biologic therapy currently available have significantly reduced the use of 8-methoxypsoralen-ultraviolet-A (PUVA), especially since the study is relatively recent. Previous studies have shown a linear increase in the risk of NMSC with the number of PUVA sessions [21,34]. A systematic review of 46 studies has identified that squamous cell carcinoma (SCC) can develop following relatively low exposure to UVA, and the risk increases linearly with the number of treatments [42]. Worryingly, the increased risk of SCC persists after discontinuation of PUVA therapy. Despite the increased risk of NMSC in patients with psoriasis, there is little evidence that such skin cancers are either more aggressive or associated with increased mortality [43].
Regarding the risk of developing melanoma, our results are in agreement with the meta-analyzes, only one such patient in the psoriasis group being recorded. Previous studies have contradictory results [7,15,16,17,33,44,45,46]. In addition, a retrospective Italian study including 72,739 patients showed a low frequency of melanoma among the psoriasis patients attributed to the network of proinflammatory cytokines that would have a protective role against melanoma [47].
The role of various classes of drugs used in the treatment of psoriasis (biological therapy included) in carcinogenesis has been under scrutiny. They inhibit inflammation by suppressing the activity and proliferation of immune cells and the production of cytokines involved in innate and adaptive immune responses. A meta-analysis conducted in that respect [48] indicates an increased risk of lymphoma, but it could not specify whether it is due to the disease itself, the immunosuppressive effect of the medication, or both. Thus, in patients with severe disease and multiple comorbidities, the choice of immunomodulatory therapies may be confusing due to the increased risk of cancer. On the other hand, early and effective control by immunosuppressive therapies significantly reduces inflammation which in its turn is an independent risk factor for tumorogenesis.
Of the immunosuppressive therapies, we mention Cyclosporine and Methotrexate. Cyclosporine is a calcineurin inhibitor that inhibits inflammation by blocking the production of IL-2 by activated CD4+ cells. It has been shown to significantly increase the risk of NMSC in patients with psoriasis and rheumatoid arthritis (RA) [15,49]. However, a review of 60 studies including over 1700 cyclosporine-treated psoriasis patients did not document skin cancer when cyclosporine was used continuously for less than 6 months or less than 2 years if used intermittently [50]. Methotrexate is an affordable and effective drug and is considered the gold standard for RA and psoriasis treatment. The consequences of its systemic immunosuppression in the development of cancer have been debated, and the results are contradictory. Although the association with melanoma and Epstein–Barr virus-associated lymphomas has been reported [51], a subsequent meta-analysis did not indicate an increased risk of lymphoma or other malignancy in patients treated with methotrexate [52]. However, a 2010 systematic review by Krathen et al. suggested that methotrexate in combination with anti-TNF may increase the risk of melanoma and double the risk for NMSC [53].
As acitretin does not increase the risk of solid organ cancer and is beneficial in treating NMSC and cutaneous T-cell lymphoma, some authors advocate prescribing it alone or in combination with biologics, phototherapy, or systemic therapies [43].
The introduction of effective biological therapies for psoriasis has revolutionized the therapeutic approach by obtaining fast, satisfactory and persistent results. The problem of their association with various types of cancers has been raised. A recent meta-analysis [54] that included databases from Israel, Spain, Italy, U.K. and Ireland has shown that cumulative exposure to various biological therapies in psoriasis patients does not increase the risk of cancer. In another meta-analysis aimed strictly at anti-TNF therapy, it does not appear to be linked to a higher risk of cancer [55]. A review of eight cohort studies on TNF inhibitors, except for one study on ustekinumab, showed an increase in NMSC, especially SCC, in the TNF inhibitor group compared with both a general US population and a population of rheumatoid arthritis treated with TNF inhibitors, without increasing the risk of developing other cancers [56]. For the state-of-the-art biological molecules, such as IL-12/23, IL17, data are insufficient and the time since their introduction is relatively short for analyzing the risk of developing malignancies
The prognosis of patients with psoriasis was a topic of interest. A cohort study in the United Kingdom showed a slightly increased risk of cancer deaths, but this increase was modest compared to the risk of cardiovascular death among patients with severe psoriasis [57]. Another cohort study showed a worse prognosis for psoriasis patients diagnosed with cancer before the age of 65 and for those who received treatment for conditions related to ethanol consumption. Additionally, those with more than one hospitalization for psoriasis were more likely to be associated with an increased risk for cancer-specific mortality [58]. A retrospective case–control study aimed at determining the risk of melanoma and blood cancers in patients with psoriasis compared with a control group without psoriasis did not show differences in prognosis [59].
Smoking, alcohol consumption and obesity are independent risk factors for psoriasis, and they are also cancer-enhancing factors. In addition, previous exposure to immunosuppressive medication and ultraviolet radiation appears to increase the risk of cancer. Thus, for dermatologists’ risk–benefit assessment of systemic immunosuppressive treatment and PUVA is a dynamic process [60].
There is still no consensus on the therapeutic management of psoriasis patients with a history of neoplasia, and a multidisciplinary approach, with the initiation of a personalized treatment that takes into account the risk of cancer recurrence and its susceptibility to immunosuppression, as well as other associated comorbidities is needed [61]. Even the data on sporadic cancer development in psoriasis patients are not very clear. Thus, we wonder whether a screening and prevention program for neoplasms should be included in psoriasis management Such a recommendation comes from a group of researchers in Australia/New Zealand [43] who argue that reducing common risk factors (obesity, smoking, and alcohol consumption) in patients with psoriasis has a more beneficial effect on cancer risk reduction than stopping or avoiding an immunosuppressive agent, and in patients who received >100 PUVA treatments, and especially if they had also received cyclosporine, they recommend regular additional monitoring of skin cancer. In a psoriasis patient with significant sun exposure but without previous skin cancer, they suggest photoprotection, skin cancer screening, weight loss (if BMI> 25), topical retinoid, and possibly oral nicotinamide. Additionally, by extrapolating data from high-risk organ transplant patients, they recommend chemoprevention of skin cancer in patients who have received >200 PUVA treatments, >6 months of continuous cyclosporine, or >1 year of anti-TNF-α by administration of low-dose nicotinamide and systemic retinoids.
Although useful, our study has some limitations. An inconvenience in many of the cited studies, including ours, is that of adjustment for cancer-enhancing factors such as smoking, alcohol consumption, hormonal treatments, diet or obesity is impossible. Additionally, the possible coding errors, demographic variations could not be excluded, and the exact severity of psoriasis could not be assessed. Last but not least, the date of psoriasis or cancer onset could not be objectively estimated.
5. Conclusions
Psoriasis patients may be at increased risk for developing certain types of cancer. This is the first study of its kind in the Northeastern Romania and can be the starting point for future long-term prospective cohort studies that will allow a more accurate data collection and help us understand the psoriasis–cancer relationship and the long-term safety of modern treatments. It may also be useful for implementing a cancer screening and/or prevention program in these patients.
Author Contributions
A.C. and D.V. researched the data and wrote the manuscript. A.I.H. and A.P., performed the analysis. L.G.-S. and E.P.-A. revised the manuscript and had primary responsibility for final content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials supporting the results of the present study are available in the published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parisi, R.; Symmons, D.; Griffiths, C.; Ashcroft, D.M. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Kimball, A.B.; Gladman, D.; Gelfand, J.; Gordon, K.; Horn, E.J.; Korman, N.J.; Korver, G.; Krueger, G.G.; Strober, B.E.; Lebwohl, M.G. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J. Am. Acad. Dermatol. 2008, 58, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Pouplard, C.; Brenaut, E.; Horreau, C.; Barnetche, T.; Misery, L.; Richard, M.-A.; Aractingi, S.; Aubin, F.; Cribier, B.; Joly, P.; et al. Risk of cancer in psoriasis: A systematic review and meta-analysis of epidemiological studies. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Trafford, A.; Parisi, R.; Kontopantelis, E.; Griffiths, C.E.M.; Ashcroft, D. Association of Psoriasis With the Risk of Developing or Dying of Cancer: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.; Bilker, W.; Hennessy, S.; Vittorio, C.; Santanna, J.; Strom, B.L. The risk of malignancy associated with psoriasis. Arch. Dermatol. 2001, 137, 778–783. [Google Scholar] [PubMed]
- Brauchli, Y.B.; Jick, S.; Miret, M.; Meier, C.R. Psoriasis and Risk of Incident Cancer: An Inception Cohort Study with a Nested Case–Control Analysis. J. Investig. Dermatol. 2009, 129, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.F.; Gourraud, P.-A. Cancer Risk Evaluation in Psoriasis: In Search of the Holy Grail? J. Investig. Dermatol. 2009, 129, 2547–2549. [Google Scholar] [CrossRef]
- Prizment, A.E.; Alonso, A.; Folsom, A.R.; Ahmed, R.L.; Virnig, B.A.; Warshaw, E.M.; Anderson, K.E. Association between psoriasis and incident cancer: The Iowa’s Women’s Health Study. Cancer Causes Control. 2011, 22, 1003–1010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Svahn, H.; Sigurgeirsson, B.; Pukkala, E.; Lindelöf, B.; Berne, B.; Hannuksela, M.; Poikolainen, K.; Karvonen, J. Trioxsalen bath PUVA did not increase the risk of squamous cell skin carcinoma and cutaneous malignant melanoma in a joint analysis of 944 Swedish and Finnish patients with psoriasis. Br. J. Dermatol. 1999, 141, 497–501. [Google Scholar] [CrossRef]
- Hannuksela-Svahn, A.; Pukkala, E.; Läärä, E.; Poikolainen, K.; Karvonen, J. Psoriasis, its Treatment, and Cancer in a Cohort of Finnish Patients. J. Investig. Dermatol. 2000, 114, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, X.; Sundquist, J.; Hemminki, K. Cancer risk in hospitalised psoriasis patients: A follow-up study in Sweden. Br. J. Cancer 2009, 100, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Lindelöf, B.; Eklund, G.; Lidén, S.; Stern, R. The prevalence of malignant tumors in patients with psoriasis. J. Am. Acad. Dermatol. 1990, 22, 1056–1060. [Google Scholar] [CrossRef]
- Vaengebjerg, S.; Skov, L.; Egeberg, A.; Loft, N.D. Prevalence, Incidence, and Risk of Cancer in Patients With Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.F.; Ho, V.C.; McGeown, C.; Christophers, E.; Schmidtmann, B.; Guillaume, J.-C.; Lamarque, V.; Dubertret, L. Risk of Malignancies in Psoriasis Patients Treated with Cyclosporine: A 5 y Cohort Study. J. Investig. Dermatol. 2003, 120, 211–216. [Google Scholar] [CrossRef]
- Geller, S.; Xu, H.; Lebwohl, M.; Nardone, B.; Lacouture, M.E.; Kheterpal, M. Malignancy Risk and Recurrence with Psoriasis and Its Treatments: A Concise Update. Am. J. Clin. Dermatol. 2017, 19, 363–375. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, C.-Y.; Chen, T.-J.; Shen, J.-L.; Chu, S.-Y.; Wang, C.-B.; Chang, Y.-T. The risk of cancer in patients with psoriasis: A population-based cohort study in Taiwan. J. Am. Acad. Dermatol. 2011, 65, 84–91. [Google Scholar] [CrossRef]
- Tsai, T.-F.; Wang, T.-S.; Hung, S.-T.; Tsai, P.I.-C.; Schenkel, B.; Zhang, M.; Tang, C.-H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 2011, 63, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Gridley, G.; Lindelöf, B. Cancer Risk in a Population-Based Cohort of Patients Hospitalized for Psoriasis in Sweden. J. Investig. Dermatol. 2001, 117, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.; Han, K.D.; Kim, H.-N.; Park, Y.M.; Lee, J.Y.; Park, Y.-G.; Lee, Y.B. Cancer risk in 892 089 patients with psoriasis in Korea: A nationwide population-based cohort study. J. Dermatol. 2018, 46, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S.; Väkevä, L.H.; PUVA Follow-up Study. Noncutaneous malignant tumors in the PUVA follow-up study: 1975–1996. J. Investig. Dermatol. 1997, 108, 897–900. [Google Scholar] [CrossRef]
- Kano, Y.; Chiba, M.; Yagita, A.; Shiohara, T. Complete resolution of psoriasis vulgaris after excision of thyroid cancer. Int. J. Dermatol. 2008, 36, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Fuxench, Z.C.C.; Shin, D.B.; Beatty, A.O.; Gelfand, J. The Risk of Cancer in Patients With Psoriasis: A Population-Based Cohort Study in the Health Improvement Network. JAMA Dermatol. 2016, 152, 282–290. [Google Scholar] [CrossRef]
- Yang, H.; Brand, J.S.; Li, J.; Ludvigsson, J.F.; Ugalde-Morales, E.; Chiesa, F.; Hall, P.; Czene, K. Risk and predictors of psoriasis in patients with breast cancer: A Swedish population-based cohort study. BMC Med. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.H.; Moller, H.; Frentz, G. Malignant tumors in patients with psoriasis. J. Am. Acad. Dermatol. 1992, 27, 716–722. [Google Scholar] [CrossRef]
- Pasche, B. Differential Effects of Aspirin before and after Diagnosis of Colorectal Cancer. JAMA 2013, 309, 2598–2599. [Google Scholar] [CrossRef] [PubMed]
- Erlinger, T.P.; Platz, E.A.; Rifai, N.; Helzlsouer, K.J. C-Reactive Protein and the Risk of Incident Colorectal Cancer. JAMA 2004, 291, 585–590. [Google Scholar] [CrossRef]
- Kane, S.; Khatibi, B.; Reddy, D. Higher Incidence of Abnormal Pap Smears in Women with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2008, 103, 631–636. [Google Scholar] [CrossRef]
- Contreras, W.R.; Fuentes, H.M.; Nava, J.I.G.; Rincón, A.E.S.; Salcedo, J.V.; Rosas, M.P.; Rodríguez, L.M.B.; Trujillo, X.; Flores, M.R.; Hernández, B.T.; et al. Prevalence and cervical human papilloma virus associated factors in patients with rheumatoid arthritis. Ginecol. Obstet. México 2008, 76, 9–17. [Google Scholar]
- Santana, I.U.; Gomes, A.D.N.; Lyrio, L.D.; Grassi, M.F.; Santiago, M.B. Systemic lupus erythematosus, human papillomavirus infection, cervical pre-malignant and malignant lesions: A systematic review. Clin. Rheumatol. 2010, 30, 665–672. [Google Scholar] [CrossRef]
- Rojo-Contreras, W.; Olivas-Flores, E.M.; Gamez-Nava, J.; Montoya-Fuentes, H.; Trujillo-Hernandez, B.; Trujillo, X.; Suarez-Rincon, A.; Baltazar-Rodriguez, L.; Sanchez-Hernandez, J.; Ramirez-Flores, M.; et al. Cervical human papillomavirus infection in Mexican women with systemic lupus erythematosus or rheumatoid arthritis. Lupus 2011, 21, 365–372. [Google Scholar] [CrossRef]
- Kim, S.C.; Glynn, R.J.; Giovannucci, E.; Hernández-Díaz, S.; Liu, J.; Feldman, S.; Karlson, E.W.; Schneeweiss, S.; Solomon, D.H. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: A population-based cohort study. Ann. Rheum. Dis. 2014, 74, 1360–1367. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Implications for management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef]
- Nijsten, T.E.; Stern, R.S. The Increased Risk of Skin Cancer Is Persistent after Discontinuation of Psoralen+Ultraviolet A: A Cohort Study. J. Investig. Dermatol. 2003, 121, 252–258. [Google Scholar] [CrossRef]
- Stern, R.S.; Study, P.F. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef]
- Bruynzeel, I.; Bergman, W.; Hartevelt, H.; Kenter, C.; Van De Velde, E.A.; Schothorst, A.; Suurmond, D. High single-dose European PUVA regimen also causes an excess of non-melanoma skin cancer. Br. J. Dermatol. 1991, 124, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M.; Shin, D.B.; Neimann, A.L.; Wang, X.; Margolis, D.J.; Troxel, A.B. The Risk of Lymphoma in Patients with Psoriasis. J. Investig. Dermatol. 2006, 126, 2194–2201. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Berlin, J.; Van Voorhees, A.; Margolis, D.J. Lymphoma Rates Are Low but Increased in Patients With Psoriasis: Results from a population-based cohort study in the United Kingdom. Arch. Dermatol. 2003, 139, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Lymphoma Risk in Psoriasis: Results of the PUVA follow-up study. Arch. Dermatol. 2006, 142, 1132–1135. [Google Scholar] [CrossRef]
- Ekström, K.; Hjalgrim, H.; Brandt, L.; Baecklund, E.; Klareskog, L.; Ekbom, A.; Askling, J. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003, 48, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Nordstrom, B.L. The risk of malignancy among biologic-naïve pediatric psoriasis patients: A retrospective cohort study in a US claims database. J. Am. Acad. Dermatol. 2017, 77, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Archier, E.; Devaux, S.; Castela, E.; Gallini, A.; Aubin, F.; Le Maître, M.; Aractingi, S.; Bachelez, H.; Cribier, B.; Joly, P.; et al. Carcinogenic risks of Psoralen UV-A therapy and Narrowband UV-B therapy in chronic plaque psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 22–31. [Google Scholar] [CrossRef]
- Rademaker, M.; Rubel, D.M.; Agnew, K.; Andrews, M.; Armour, K.S.; Baker, C.; Foley, P.; Gebauer, K.; Goh, M.S.; Gupta, M.; et al. An Australian/New Zealand Narrative. Australas. J. Dermatol. 2018, 60, 12–18. [Google Scholar] [CrossRef]
- Maiorino, A.; De Simone, C.; Perino, F.; Caldarola, G.; Peris, K. Melanoma and non-melanoma skin cancer in psoriatic patients treated with high-dose phototherapy. J. Dermatol. Treat. 2016, 27, 443–447. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.; Patterson, C.; Handley, J.; McGinn, S.; Allen, G. Cutaneous neoplasia following PUVA therapy for psoriasis. Br. J. Dermatol. 1996, 134, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, A.; Tabolli, S.; Didona, B.; Sobrino, L.; Russo, N.; Abeni, D. Reduced frequency of melanoma in 72,739 patients with psoriasis: A retrospective study. Eur. J. Dermatol. 2015, 25, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Kandiel, A.; Fraser, A.G.; Korelitz, B.I.; Brensinger, C.; Lewis, J.D. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005, 54, 1121–1125. [Google Scholar] [CrossRef]
- Masunaga, Y.; Ohno, K.; Ogawa, R.; Hashiguchi, M.; Echizen, H.; Ogata, H. Meta-Analysis of Risk of Malignancy with Immunosuppressive Drugs in Inflammatory Bowel Disease. Ann. Pharmacother. 2007, 41, 21–28. [Google Scholar] [CrossRef]
- Muellenhoff, M.W.; Koo, J.Y. Cyclosporine and skin cancer: An international dermatologic perspective over 25 years of experience. A comprehensive review and pursuit to define safe use of cyclosporine in dermatology. J. Dermatol. Treat. 2011, 23, 290–304. [Google Scholar] [CrossRef]
- Buchbinder, R.; Barber, M.; Heuzenroeder, L.; Wluka, A.; Giles, G.; Hall, S.; Harkness, A.; Lewis, D.; Littlejohn, G.; Miller, M.H.; et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008, 59, 794–799. [Google Scholar] [CrossRef]
- Salliot, C.; Van Der Heijde, D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: A systematic literature research. Ann. Rheum. Dis. 2008, 68, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Krathen, M.S.; Gottlieb, A.B.; Mease, P.J. Pharmacologic Immunomodulation and Cutaneous Malignancy in Rheumatoid Arthritis, Psoriasis, and Psoriatic Arthritis. J. Rheumatol. 2010, 37, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Doval, I.; Descalzo, M.; Mason, K.; Cohen, A.; Ormerod, A.; Gómez-García, F.; Cazzaniga, S.; Feldhamer, I.; Ali, H.; Herrera-Acosta, E.; et al. Cumulative exposure to biological therapy and risk of cancer in patients with psoriasis: A meta-analysis of Psonet studies from Israel, Italy, Spain, the U.K. and Republic of Ireland. Br. J. Dermatol. 2018, 179, 863–871. [Google Scholar] [CrossRef]
- Dommasch, E.; Abuabara, K.; Shin, D.B.; Nguyen, J.; Troxel, A.; Gelfand, J.M. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: A systematic review and meta-analysis of randomized controlled trials. J. Am. Acad. Dermatol. 2011, 64, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Peleva, E.; Exton, L.; Kelley, K.; Kleyn, C.; Mason, K.; Smith, C.H. Risk of cancer in patients with psoriasis on biological therapies: A systematic review. Br. J. Dermatol. 2017, 178, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Abuabara, K.; Azfar, R.; Shin, D.; Neimann, A.; Troxel, A.; Gelfand, J. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the U.K. Br. J. Dermatol. 2010, 163, 586–592. [Google Scholar] [CrossRef]
- Shu, X.; Ji, J.; Sundquist, J.; Sundquist, K.; Hemminki, K. Survival in cancer patients hospitalized for psoriasis: A population-based cohort study in Sweden. Br. J. Dermatol. 2011, 165, 129–136. [Google Scholar] [CrossRef]
- Reddy, S.; Martires, K.; Wu, J.J. The risk of melanoma and hematologic cancers in patients with psoriasis. J. Am. Acad. Dermatol. 2017, 76, 639–647. [Google Scholar] [CrossRef]
- Elder, J.T.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Stuart, P.E.; Tejasvi, T.; Voorhees, J.J.; Abecasis, G.; Nair, R.P. Molecular Dissection of Psoriasis: Integrating Genetics and Biology. J. Investig. Dermatol. 2010, 130, 1213–1226. [Google Scholar] [CrossRef]
- Beyaert, R.; Beaugerie, L.; Van Assche, G.; Brochez, L.; Renauld, J.-C.; Viguier, M.; Cocquyt, V.; Jerusalem, G.; Machiels, J.-P.; Prenen, H.; et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol. Cancer 2013, 12, 98. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).