Generating Mechanism of Catalytic Effect for Hydrogen Absorption/Desorption Reactions in NaAlH4–TiCl3
Abstract
1. Introduction
2. Materials and Methods
3. Results
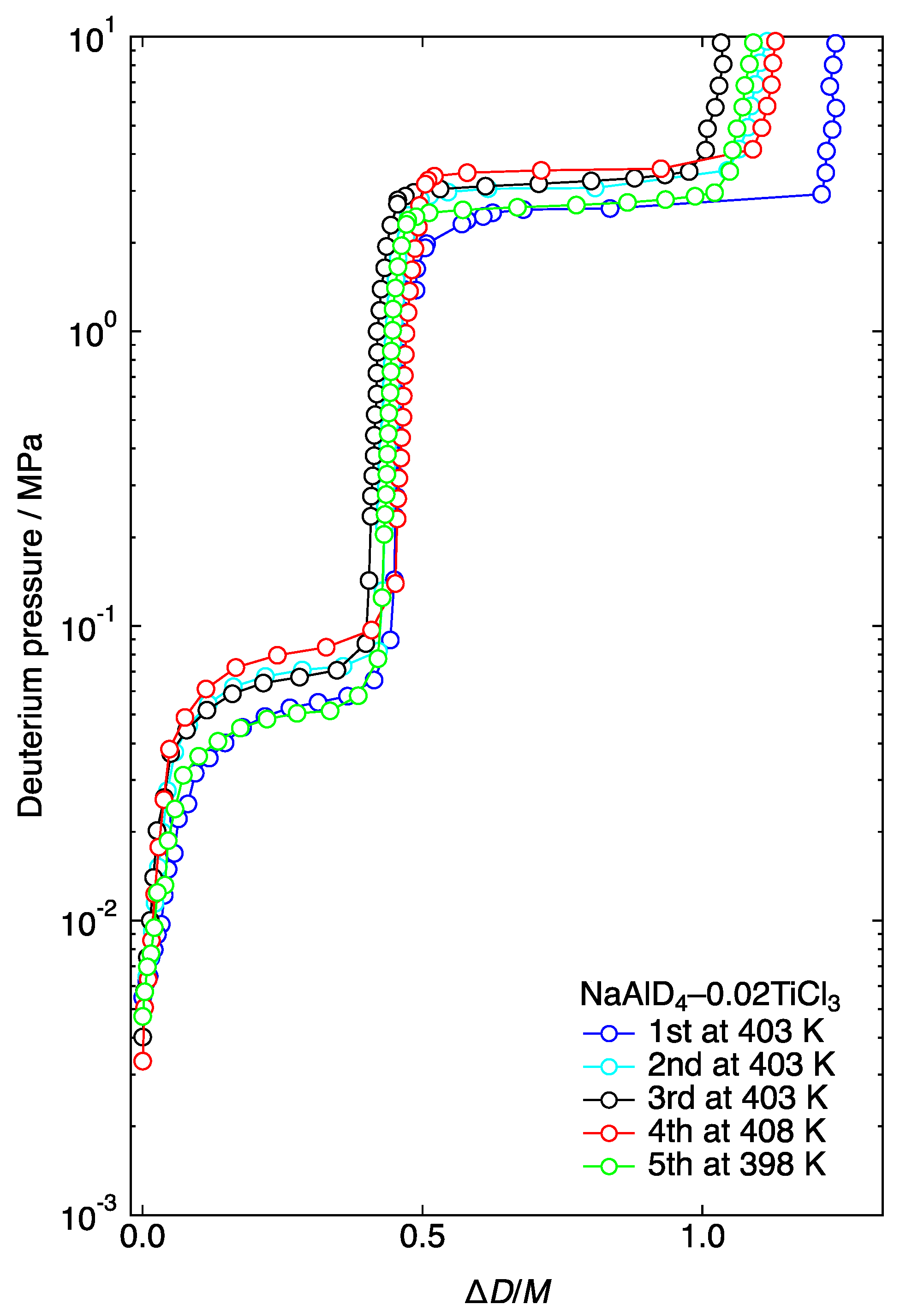
3.1. Pressure–Composition Isotherm (PCI)
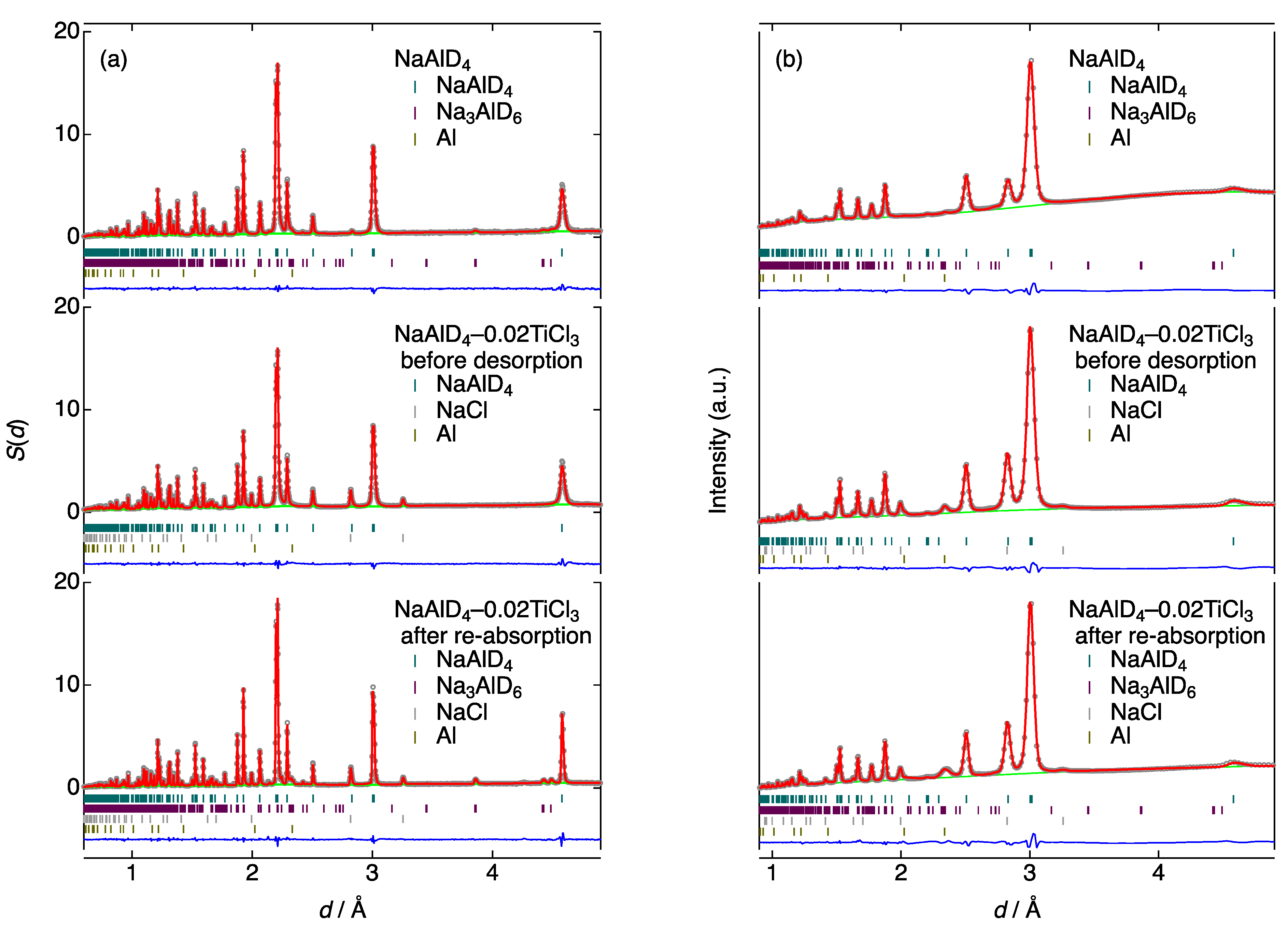
3.2. Neutron/X-ray Diffraction (ND/XRD)
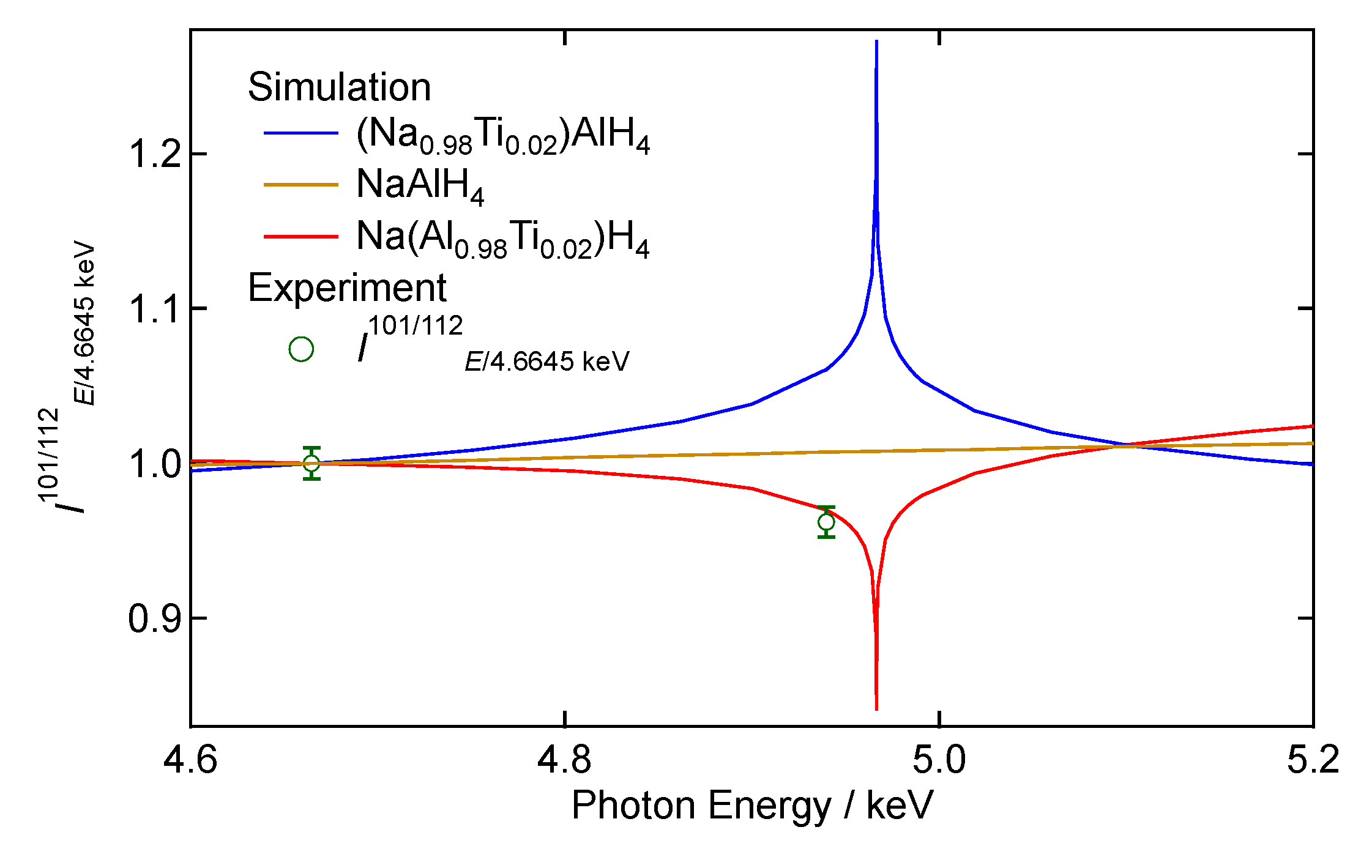
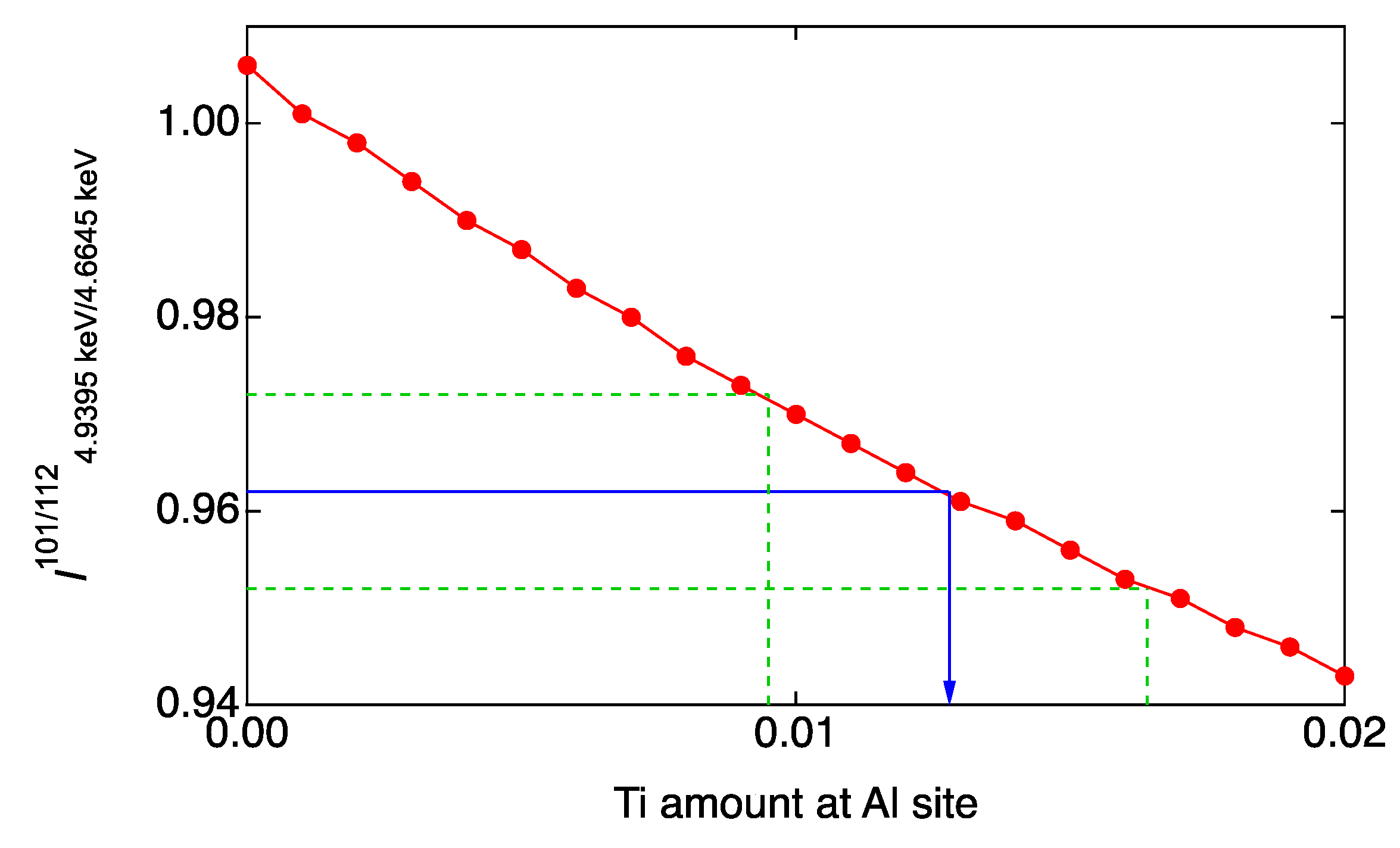
3.3. Anomalous X-ray Scattering (AXS)
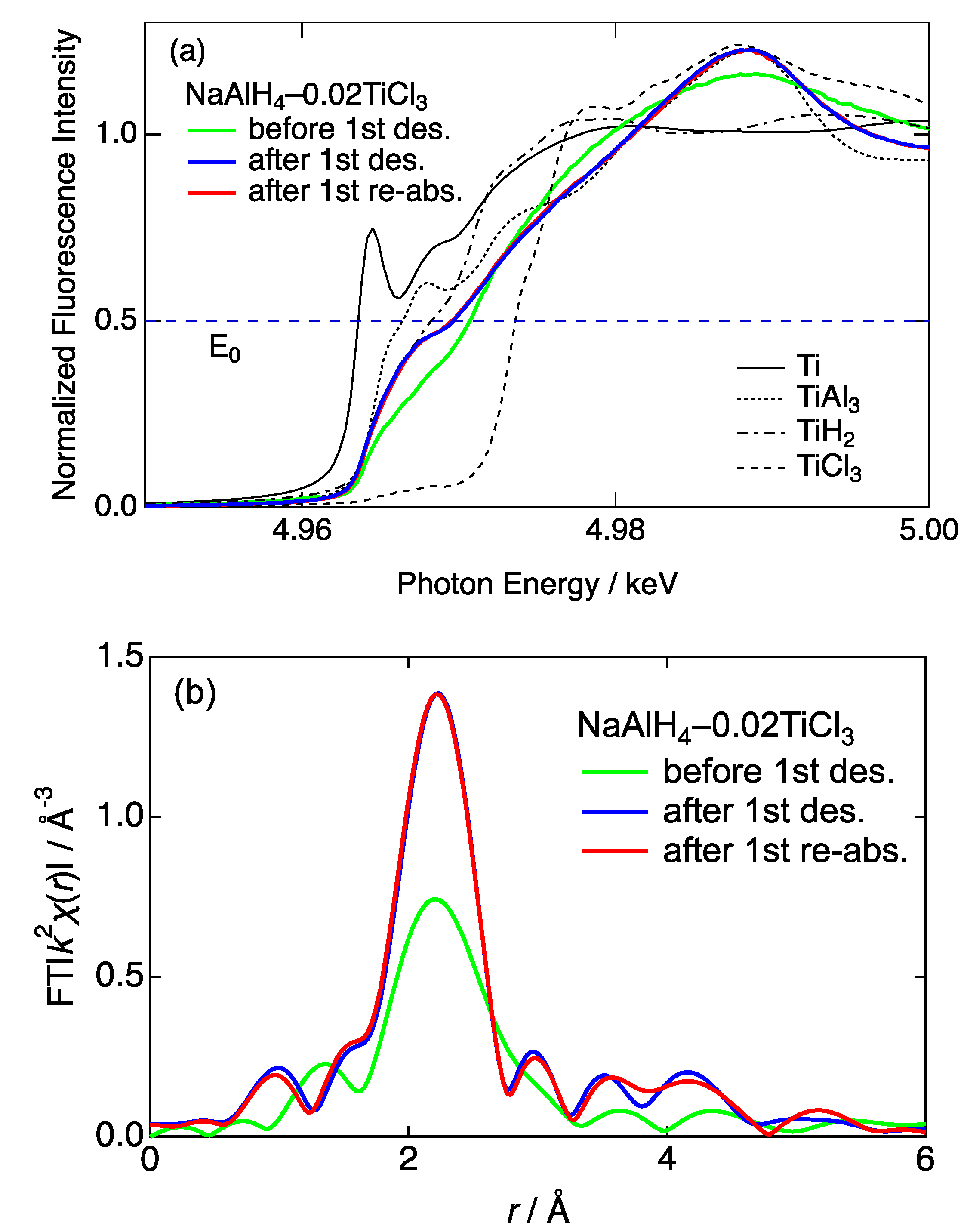
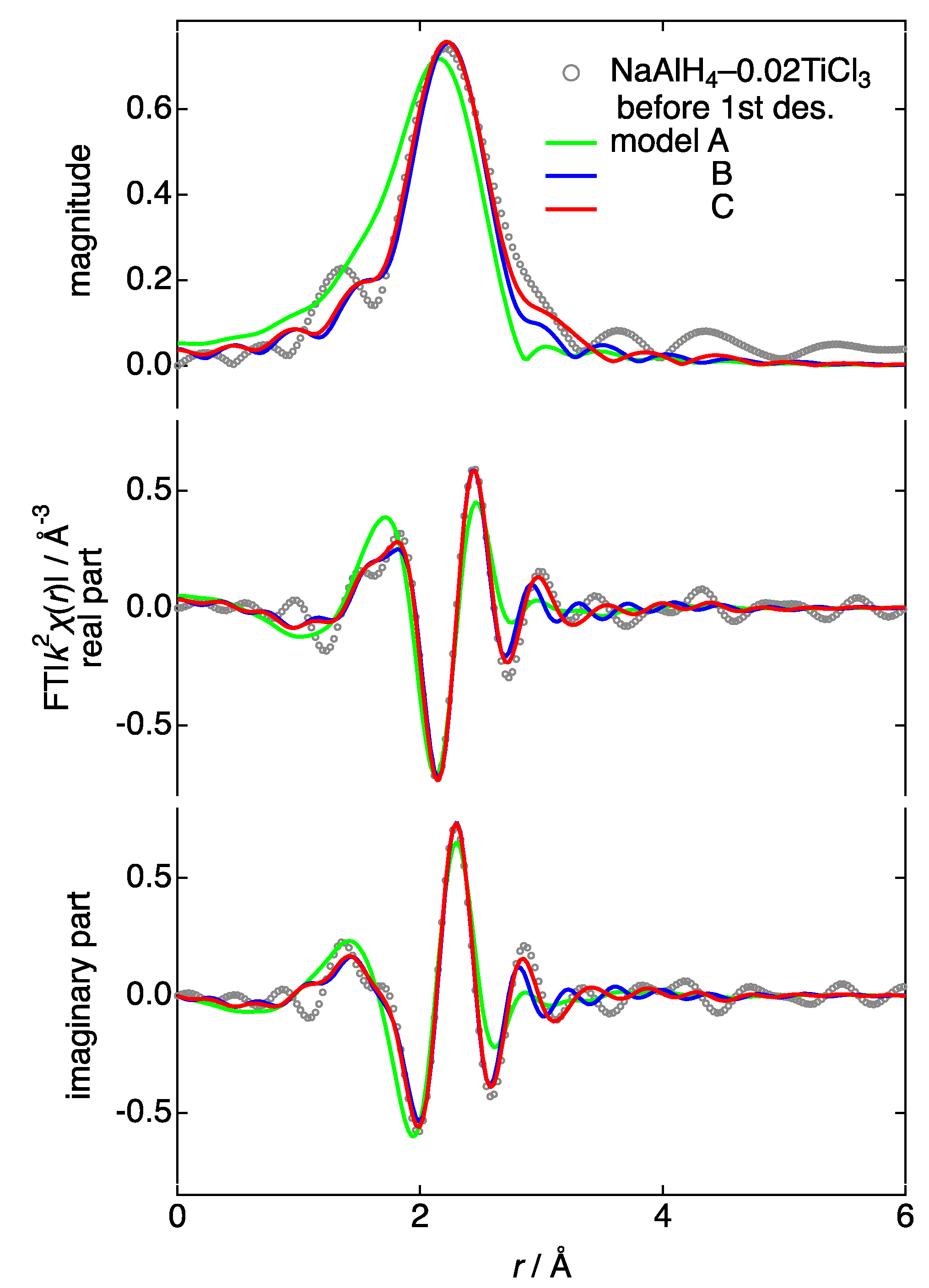
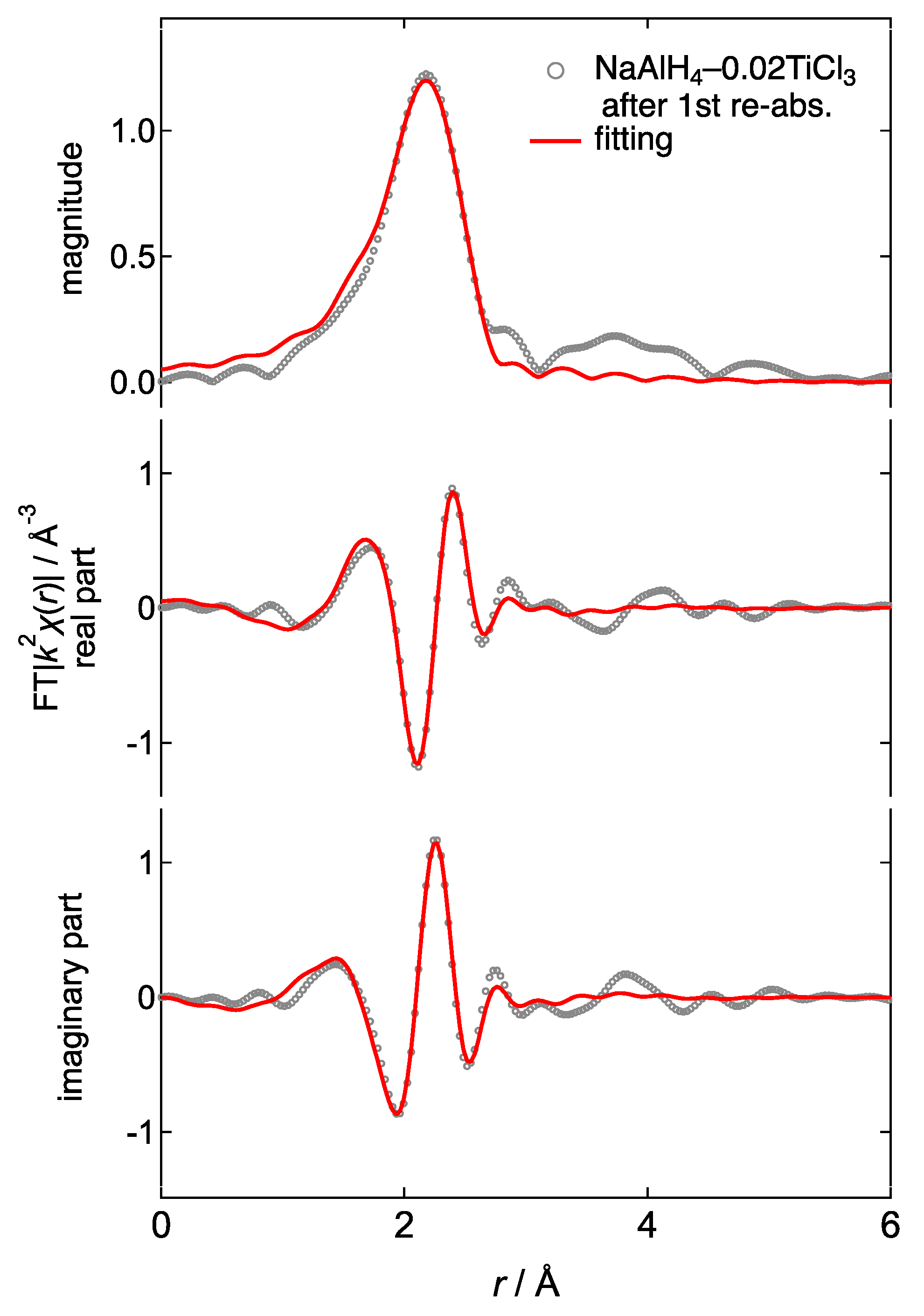
3.4. Ti K-Edge X-ray Absorption Fine Structure (XAFS)
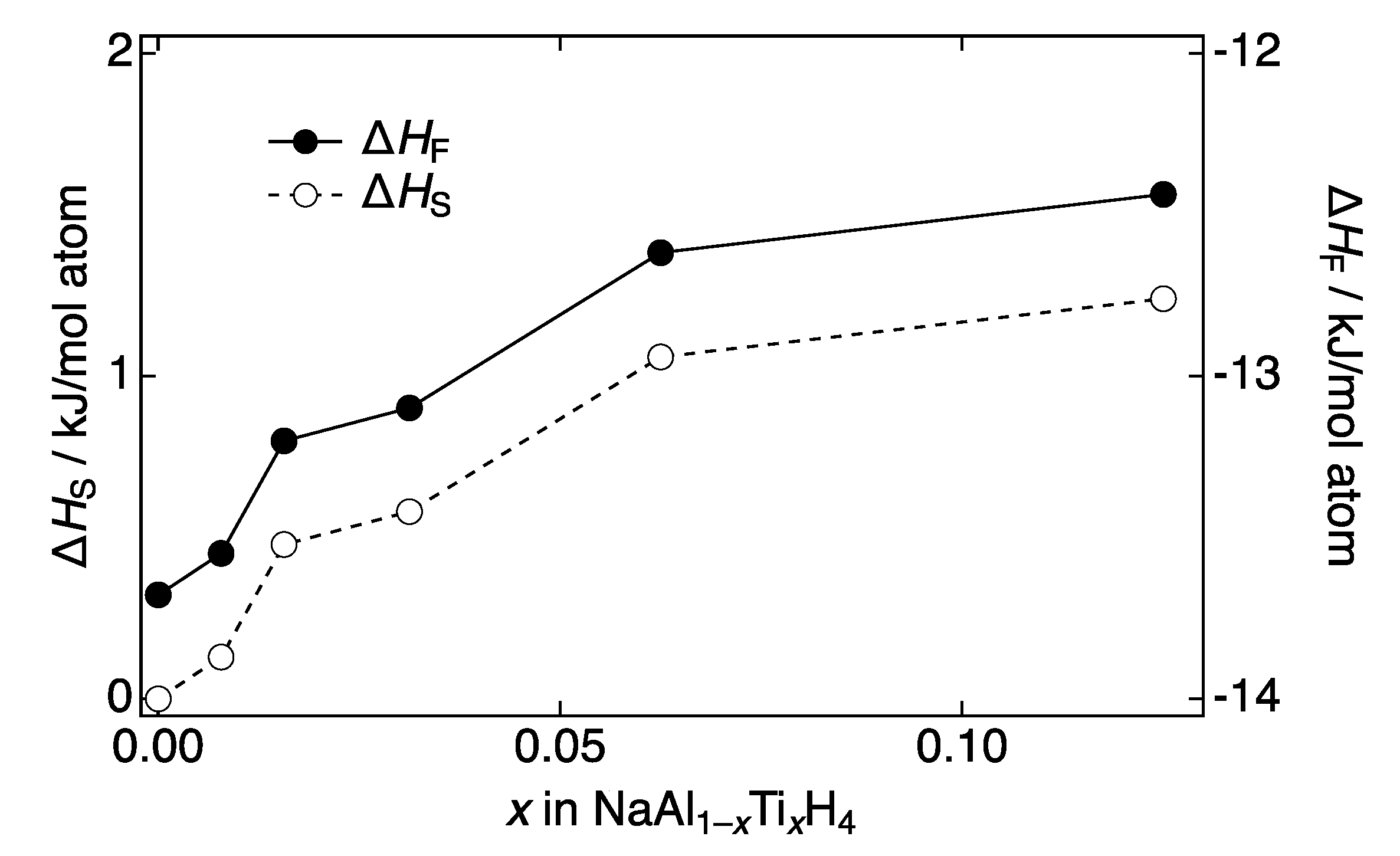
3.5. Density Functional Theory (DFT) Calculations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022–1–11. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Ashby, E.C. Simple and complex metal hydrides of the main group metals. A personal account of the early days. J. Organomet. Chem. 1980, 200, 1–6. [Google Scholar] [CrossRef]
- Lauher, J.W.; Dougherty, D.; Herley, P.J. Sodium tetrahydroaluminate. Acta Crystallogr. Sect. B Struct. Sci. 1979, 35, 1454–1456. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Jensen, C.M.; Murphy, K.; Maeland, A.J. Neutron diffraction structure determination of NaAlD4. J. Alloys Compd. 2003, 358, 142–145. [Google Scholar] [CrossRef]
- Isobe, S.; Yao, H.; Wang, Y.; Kawasaki, H.; Hashimoto, N.; Ohnuki, S. Study on decomposition process of NaAlH4 by in-situ TEM. Int. J. Hydrogen Energy 2010, 35, 7563–7567. [Google Scholar] [CrossRef]
- Dymova, T.N.; Dergachev, Y.M.; Sokolov, V.A.; Grechanaya, N.A. Dissociation pressure of NaAlH4 and Na3AlH6. Dokl. Akad. Nauk SSSR 1975, 224, 556–557. [Google Scholar]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Bogdanović, B.; Brand, R.A.; Marjanović, A.; Schwickardi, M.; Tölle, J. Metal-doped sodium aluminium hydrides as potential new hydrogen storage materials. J. Alloys Compd. 2000, 302, 36–58. [Google Scholar] [CrossRef]
- Rangsunvigit, P.; Suttisawat, Y.; Kitiyanan, B.; Kulprathipanja, S. Effects of Different Ti-compounds on the Reversibility of NaAlH4. Int. J. Energy Res. 2013, 37, 713–719. [Google Scholar] [CrossRef]
- Sandrock, G.; Gross, K.; Thomas, G. Effect of Ti-catalyst content on the reversible hydrogen storage properties of the sodium alanates. J. Alloys Compd. 2002, 339, 299–308. [Google Scholar] [CrossRef]
- Sandrock, G.; Gross, K.; Thomas, G.; Jensen, C.M.; Meeker, D.; Takara, S. Engineering considerations in the use of catalyzed sodium alanates for hydrogen storage. J. Alloys Compd. 2002, 330–332, 696–701. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.; Lomness, J.; Slattery, D. Effect of catalysts on hydrogen storage properties of LiAlH4 and NaAlH4. Int. J. Hydrogen Energy 2005, 30, 1417–1421. [Google Scholar] [CrossRef]
- Wang, P.; Kang, X.D.; Cheng, H.M. Direct formation of Na3AlH6 by mechanical milling NaH∕Al with TiF3. Appl. Phys. Lett. 2005, 87, 071911. [Google Scholar] [CrossRef]
- Baldé, C.P.; van der Eerden, A.M.J.; Stil, H.A.; de Groot, F.M.F.; de Jong, K.P.; Bitter, J.H. On the local structure of Ti during in situ desorption of Ti(OBu)4 and TiCl3 doped NaAlH4. J. Alloys Compd. 2007, 446–447, 232–236. [Google Scholar] [CrossRef][Green Version]
- Lee, G.; Shim, J.; Cho, Y.; Lee, K. Improvement in desorption kinetics of NaAlH4 catalyzed with TiO2 nanopowder. Int. J. Hydrogen Energy 2008, 33, 3748–3753. [Google Scholar] [CrossRef]
- Frankcombe, T.J. Proposed mechanisms for the catalytic activity of Ti in NaAlH4. Chem. Rev. 2012, 112, 2164–2178. [Google Scholar] [CrossRef]
- Xiong, R.; Sang, G.; Yan, X.; Zhang, G.; Xu, Q.; Zhang, H. Separation and characterization of the active species in Ti-doped NaAlH4. Chem. Commun. 2013, 49, 2046–2048. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Jiao, L.; Yuan, H. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst. J. Alloys Compd. 2013, 566, 137–141. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, K.; Gao, M.; Pan, H. Remarkably improved hydrogen storage properties of nanocrystalline TiO2-modified NaAlH4 and evolution of Ti-containing species during dehydrogenation/hydrogenation. Nano Res. 2015, 8, 533–545. [Google Scholar] [CrossRef]
- Ali, N.A.; Ismail, M. Modification of NaAlH4 properties using catalysts for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2021, 46, 766–782. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Opalka, S.M. Density functional calculations of Ti-enhancedNaAlH4. Phys. Rev. B Condens. Matter 2005, 71, 054103. [Google Scholar] [CrossRef]
- Qiu, C.; Opalka, S.M.; Løvvik, O.M.; Olson, G.B. Thermodynamic modeling of the Na–Al–Ti–H system and Ti dissolution in sodium alanates. CALPHAD 2008, 32, 624–636. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Germann, M.; Härtel, M.; Pommerin, A.; Schüth, F.; Weidenthaler, C.; Zibrowius, B. Investigation of hydrogen discharging and recharging processes of Ti-doped NaAlH4 by X-ray diffraction analysis (XRD) and solid-state NMR spectroscopy. J. Alloys Compd. 2003, 350, 246–255. [Google Scholar] [CrossRef]
- Brinks, H.W.; Jensen, C.M.; Srinivasan, S.S.; Hauback, B.C.; Blanchard, D.; Murphy, K. Synchrotron X-ray and neutron diffraction studies of NaAlH4 containing Ti additives. J. Alloys Compd. 2004, 376, 215–221. [Google Scholar] [CrossRef]
- Haiduc, A.G.; Stil, H.A.; Schwarz, M.A.; Paulus, P.; Geerlings, J.J.C. On the fate of the Ti catalyst during hydrogen cycling of sodium alanate. J. Alloys Compd. 2005, 393, 252–263. [Google Scholar] [CrossRef]
- Majzoub, E.H.; Herberg, J.L.; Stumpf, R.; Spangler, S.; Maxwell, R.S. XRD and NMR investigation of Ti-compound formation in solution-doping of sodium aluminum hydrides: Solubility of Ti in NaAlH4 crystals grown in THF. J. Alloys Compd. 2005, 394, 265–270. [Google Scholar] [CrossRef]
- Pitt, M.P.; Vullum, P.E.; Sørby, M.H.; Emerich, H.; Paskevicius, M.; Buckley, C.E.; Gray, E.M.; Walmsley, J.C.; Holmestad, R.; Hauback, B.C. Crystalline Al1–xTix phases in the hydrogen cycled NaAlH4 + 0.02TiCl3 system. Philos. Mag. 2013, 93, 1080–1094. [Google Scholar] [CrossRef]
- Sun, D.; Kiyobayashi, T.; Takeshita, H.T.; Kuriyama, N.; Jensen, C.M. X-ray diffraction studies of titanium and zirconium doped NaAlH4: Elucidation of doping induced structural changes and their relationship to enhanced hydrogen storage properties. J. Alloys Compd. 2002, 2002, L8–L11. [Google Scholar] [CrossRef]
- Canton, P.; Fichtner, M.; Frommen, C.; Léon, A. Synchrotron X-Ray Studies of Ti-Doped NaAlH4. J. Phys. Chem. B 2006, 110, 3051–3054. [Google Scholar] [CrossRef]
- Majzoub, E.H.; Gross, K.J. Titanium–halide catalyst-precursors in sodium aluminum hydrides. J. Alloys Compd. 2003, 356–357, 363–367. [Google Scholar] [CrossRef]
- Kang, X.D.; Wang, P.; Song, X.P.; Yao, X.D.; Lu, G.Q.; Cheng, H.M. Catalytic effect of Al3Ti on the reversible dehydrogenation of NaAlH4. J. Alloys Compd. 2006, 424, 365–369. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Brinks, H.W.; Hauback, B.C.; Sun, D.; Jensen, C.M. Long term cycling behavior of titanium doped NaAlH4 prepared through solvent mediated milling of NaH and Al with titanium dopant precursors. J. Alloys Compd. 2004, 377, 283–289. [Google Scholar] [CrossRef]
- Brinks, H.W.; Hauback, B.C.; Srinivasan, S.S.; Jensen, C.M. Synchrotron X-ray Studies of Al1-yTiy Formation and Re-hydriding Inhibition in Ti-Enhanced NaAlH4. J. Phys. Chem. B 2005, 109, 15780–15785. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Vullum, P.E.; Sørby, M.H.; Blanchard, D.; Sulic, M.P.; Emerich, H.; Paskevicius, M.; Buckley, C.E.; Walmsley, J.; Holmestad, R.; et al. The location of Ti containing phases after the completion of the NaAlH4+xTiCl3 milling process. J. Alloys Compd. 2012, 513, 597–605. [Google Scholar] [CrossRef]
- Léon, A.; Kircher, O.; Rothe, J.; Fichtner, M. Chemical State and Local Structure around Titanium Atoms in NaAlH4 Doped with TiCl3 Using X-ray Absorption Spectroscopy. J. Phys. Chem. B 2004, 108, 16372–16376. [Google Scholar] [CrossRef]
- Léon, A.; Yalovega, G.; Soldatov, A.; Fichtner, M. Investigation of the Nature of a Ti-Al Cluster Formed upon Cycling under Hydrogen in Na Alanate Doped with a Ti-Based Precursor. J. Phys. Chem. C 2008, 112, 12545–12549. [Google Scholar] [CrossRef]
- Gross, K.J.; Majzoub, E.H.; Spangler, S.W. The effects of titanium precursors on hydriding properties of alanates. J. Alloys Compd. 2003, 356–357, 423–428. [Google Scholar] [CrossRef]
- Fichtner, M.; Canton, P.; Kircher, O.; Léon, A. Nanocrystalline alanates—Phase transformations, and catalysts. J. Alloys Compd. 2005, 404–406, 732–737. [Google Scholar] [CrossRef]
- Humphries, T.D.; Makepeace, J.W.; Hino, S.; David, W.I.F.; Hauback, B.C. Regeneration of sodium alanate studied by powder in situ neutron and synchrotron X-ray diffraction. J. Mater. Chem. A 2014, 2, 16594–16600. [Google Scholar] [CrossRef]
- Sato, T.; Ikeda, K.; Li, H.W.; Yukawa, H.; Morinaga, M.; Orimo, S. Direct Dry Syntheses and Thermal Analyses of a Series of Aluminum Complex Hydrides. Mater. Trans. 2009, 50, 182–186. [Google Scholar] [CrossRef]
- Ikeda, K.; Muto, S.; Tatsumi, K.; Menjo, M.; Kato, S.; Bielmann, M.; Zuttel, A.; Jensen, C.M.; Orimo, S. Dehydriding reaction of AlH3: In situ microscopic observations combined with thermal and surface analyses. Nanotechnology 2009, 20, 204004. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ohshita, H.; Kaneko, N.; Zhang, J.; Yonemura, M.; Otomo, T.; Suzuya, K.; Yukawa, H.; Morinaga, M.; Li, H.-W.; et al. Structural and Hydrogen Desorption Properties of Aluminum Hydride. Mater. Trans. 2011, 52, 598–601. [Google Scholar] [CrossRef]
- Oishi, R.; Yonemura, M.; Nishimaki, Y.; Torii, S.; Hoshikawa, A.; Ishigaki, T.; Morishima, T.; Mori, K.; Kamiyama, T. Rietveld analysis software for J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 600, 94–96. [Google Scholar] [CrossRef]
- Oishi-Tomiyasu, R. Rapid Bravais-lattice determination algorithm for lattice parameters containing large observation errors. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.T.; Liberman, D. Relativistic Calculation of Anomalous Scattering Factors for X Rays. J. Chem. Phys. 1970, 53, 1891–1898. [Google Scholar] [CrossRef]
- Waseda, Y.; Shinoda, K.; Sugiyama, K. Cation Distribution of ZnFe2O4 and CoFe2O4 Spinels from Anomalous X-ray Scattering. Z. Naturforsch. A Phys. Sci. 1995, 50, 1199–1204. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B: Condens. Matter 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter 1999, 59, 1758–1770. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Takeshita, T. Some applications of hydrogenation-decomposition-desorption-recombination (HDDR) and hydrogen-decrepitation (HD) in metals processing. J. Alloys Compd. 1995, 231, 51–59. [Google Scholar] [CrossRef]
| Comp. | Condition | Method | Unit Cell Volume/Å3 | Occupancy at Al | Phase Ratio | Reliability | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ti | NaAlD4 | Na3AlD6 | NaCl | Al | Rwp/% | ||||
| NaAlD4 | before dope | neutron | 285.608 (4) | 1.0 | 0 | 0.9723 (10) | 0.0276 (3) | - | 0.0000 (19) | 2.36 |
| X-ray | 284.864 (9) | 1.0 | 0 | 0.9753 (13) | 0.0063 (4) | - | 0.0183 (14) | 2.51 | ||
| NaAlD4– 0.02TiCl3 | before 1st des. | neutron X-ray | 285.640 (3) | 0.9786 (5) | 0.0213 (5) | 0.9019 (5) | - | 0.0665 (19) | 0.0314 (12) | 4.24 |
| 284.385 (5) | 0.97 (2) | 0.02 (2) | 0.862 (4) | - | 0.061 (2) | 0.076 (3) | 1.98 | |||
| after 1st re-abs. | neutron | 285.629 (16) | 0.9945 (5) | 0.0054 (5) | 0.8663 (4) | 0.03931 (16) | 0.07328 (18) | 0.0210 (8) | 5.64 | |
| X-ray | 284.703 (6) | 0.99 (2) | 0.00 (2) | 0.777 (3) | 0.00179 (18) | 0.0581 (13) | 0.162 (3) | 2.34 | ||
| Condition | Model | Phase | Symmetry | Shell | Bond Length | Debye–Waller | Edge Shift | Intrinsic Loss | Reliability |
|---|---|---|---|---|---|---|---|---|---|
| r/Å | σ2/Å2 | ΔE/eV | R/% | ||||||
| before des. | A | Al–Ti | Fmm | Al | 2.83 (5) | 0.023 (5) | −0.6 (20) | 0.61 | 17.8 |
| B | Al–Ti | Fmm | Al | 2.83 (2) | 0.007 (4) | 0.2 (10) | 0.29 (6) | 4.9 | |
| C | Al–Ti | Fmm | Al | 2.81 (1) | 0.0090 (8) | −1.3 (9) | 0.26 (35) | 1.1 | |
| Na(Al, Ti)H4 | I41/a | Na | 3.15 (5) | 0.05 (2) | −4.0 (36) | 0.23 (23) | |||
| Al | 3.37 (5) | 0.007 (2) | |||||||
| Na | 3.37 (5) | 0.05 (2) | |||||||
| after re-abs. | - | Al–Ti | Fmm | Al | 2.780 (15) | 0.0140 (14) | −0.8 (8) | 0.61 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, K.; Fujisaki, F.; Otomo, T.; Ohshita, H.; Honda, T.; Kawamata, T.; Arima, H.; Sugiyama, K.; Abe, H.; Kim, H.; et al. Generating Mechanism of Catalytic Effect for Hydrogen Absorption/Desorption Reactions in NaAlH4–TiCl3. Appl. Sci. 2021, 11, 8349. https://doi.org/10.3390/app11188349
Ikeda K, Fujisaki F, Otomo T, Ohshita H, Honda T, Kawamata T, Arima H, Sugiyama K, Abe H, Kim H, et al. Generating Mechanism of Catalytic Effect for Hydrogen Absorption/Desorption Reactions in NaAlH4–TiCl3. Applied Sciences. 2021; 11(18):8349. https://doi.org/10.3390/app11188349
Chicago/Turabian StyleIkeda, Kazutaka, Fumika Fujisaki, Toshiya Otomo, Hidetoshi Ohshita, Takashi Honda, Toru Kawamata, Hiroshi Arima, Kazumasa Sugiyama, Hitoshi Abe, Hyunjeong Kim, and et al. 2021. "Generating Mechanism of Catalytic Effect for Hydrogen Absorption/Desorption Reactions in NaAlH4–TiCl3" Applied Sciences 11, no. 18: 8349. https://doi.org/10.3390/app11188349
APA StyleIkeda, K., Fujisaki, F., Otomo, T., Ohshita, H., Honda, T., Kawamata, T., Arima, H., Sugiyama, K., Abe, H., Kim, H., Sakaki, K., Nakamura, Y., Machida, A., Sato, T., Takagi, S., & Orimo, S.-i. (2021). Generating Mechanism of Catalytic Effect for Hydrogen Absorption/Desorption Reactions in NaAlH4–TiCl3. Applied Sciences, 11(18), 8349. https://doi.org/10.3390/app11188349






