Pressure–Temperature Phase Diagram of Ta-H System up to 9 GPa and 600 °C
Abstract
:1. Introduction
2. Materials and Methods
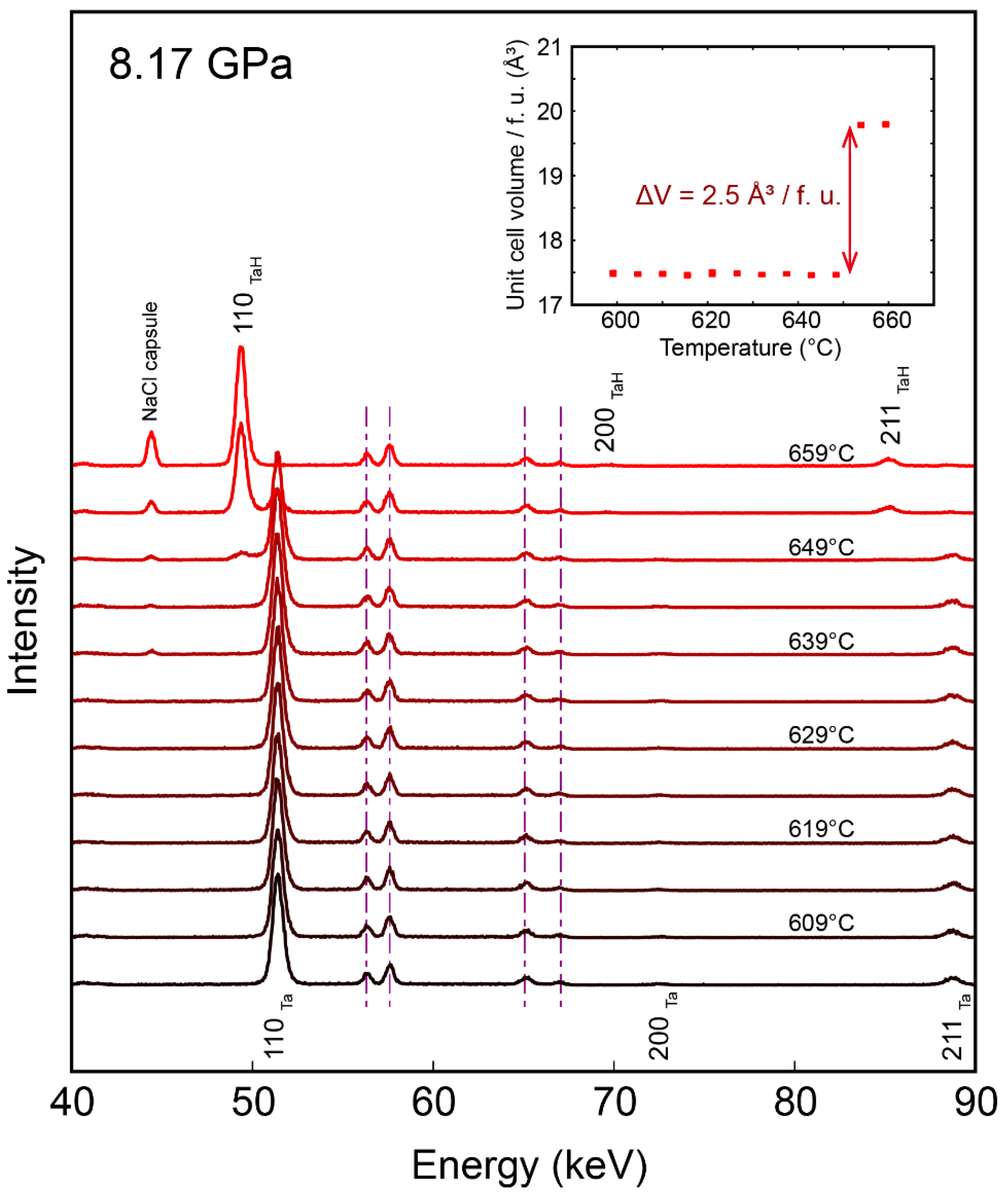
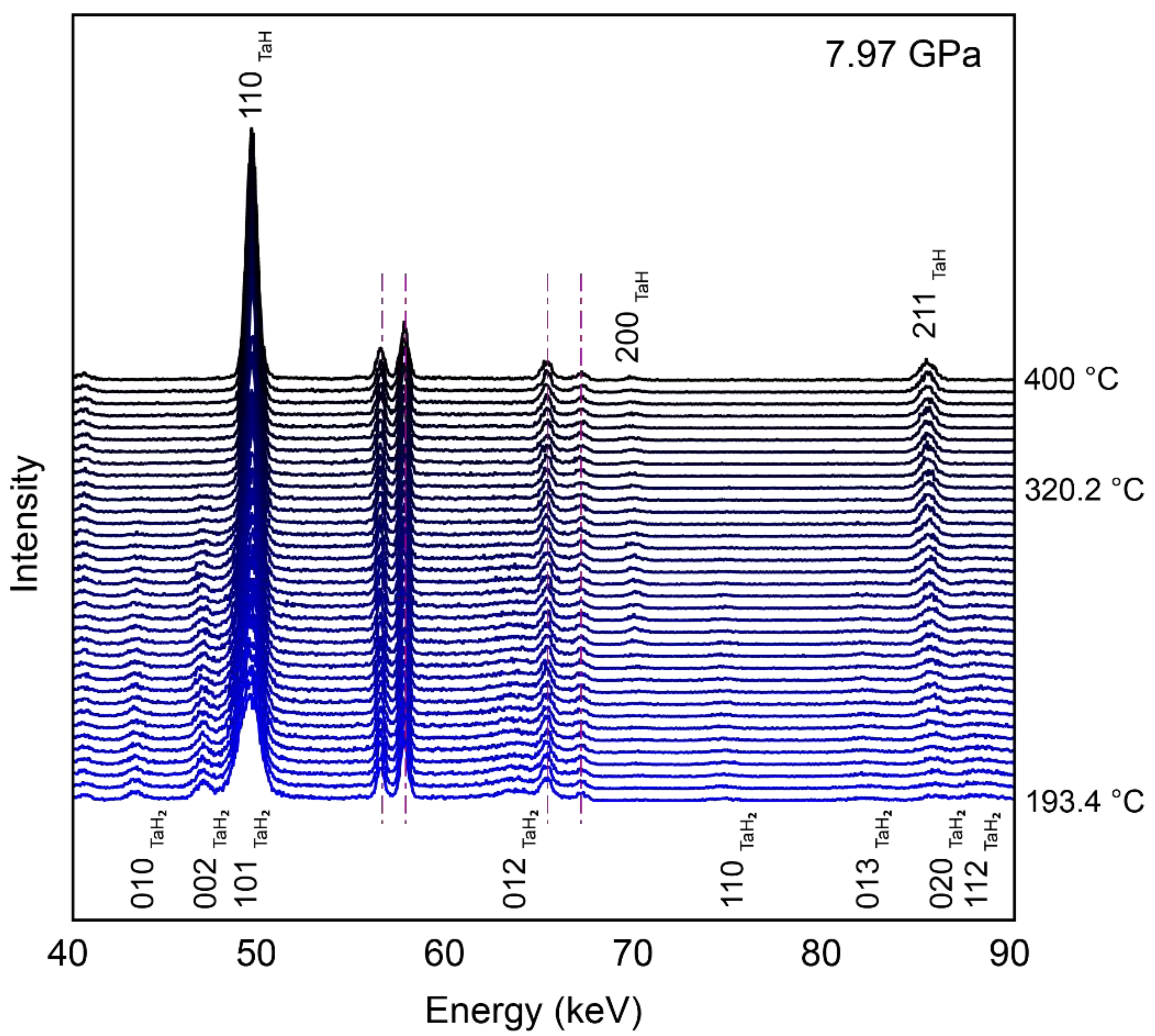
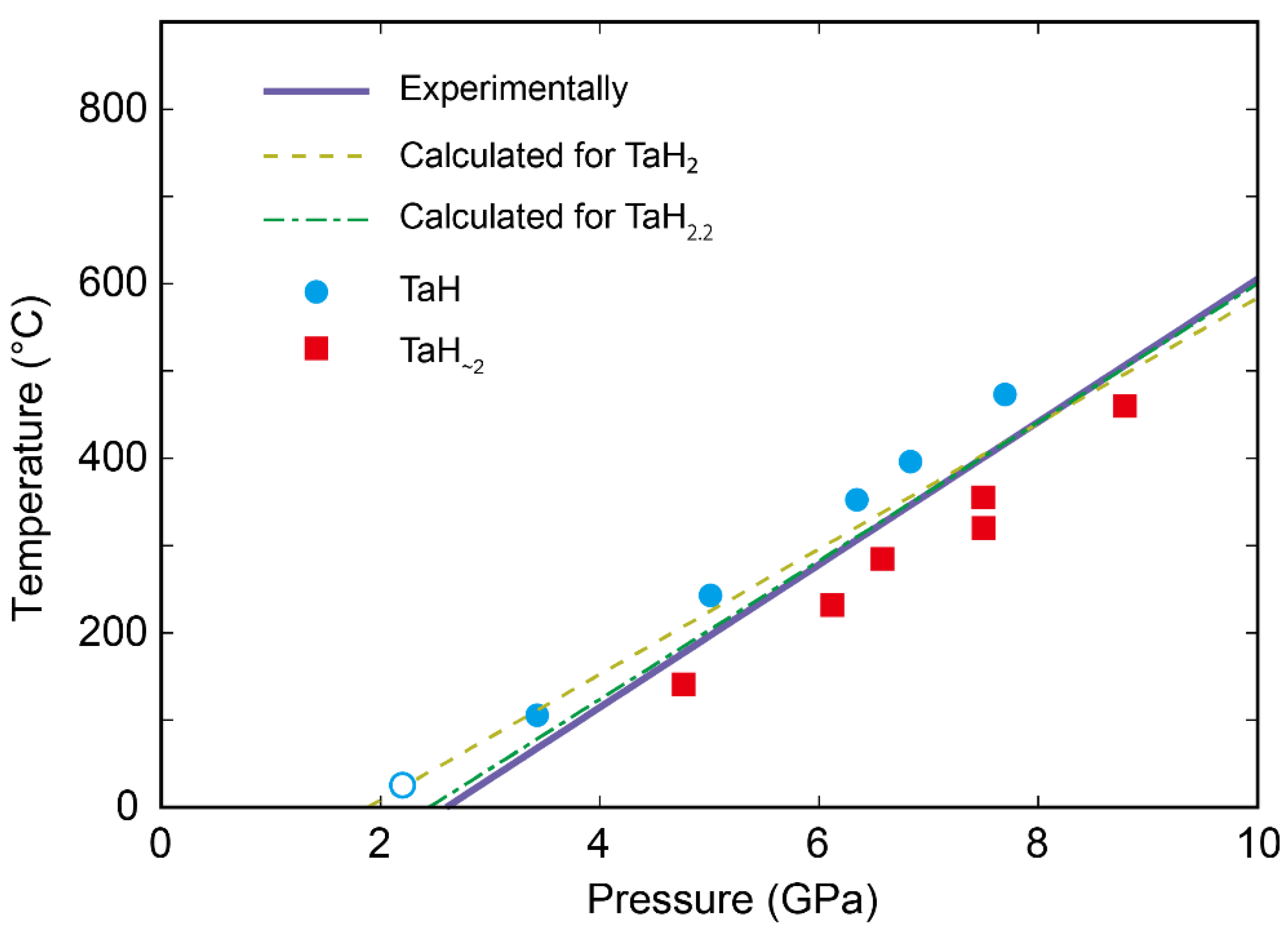
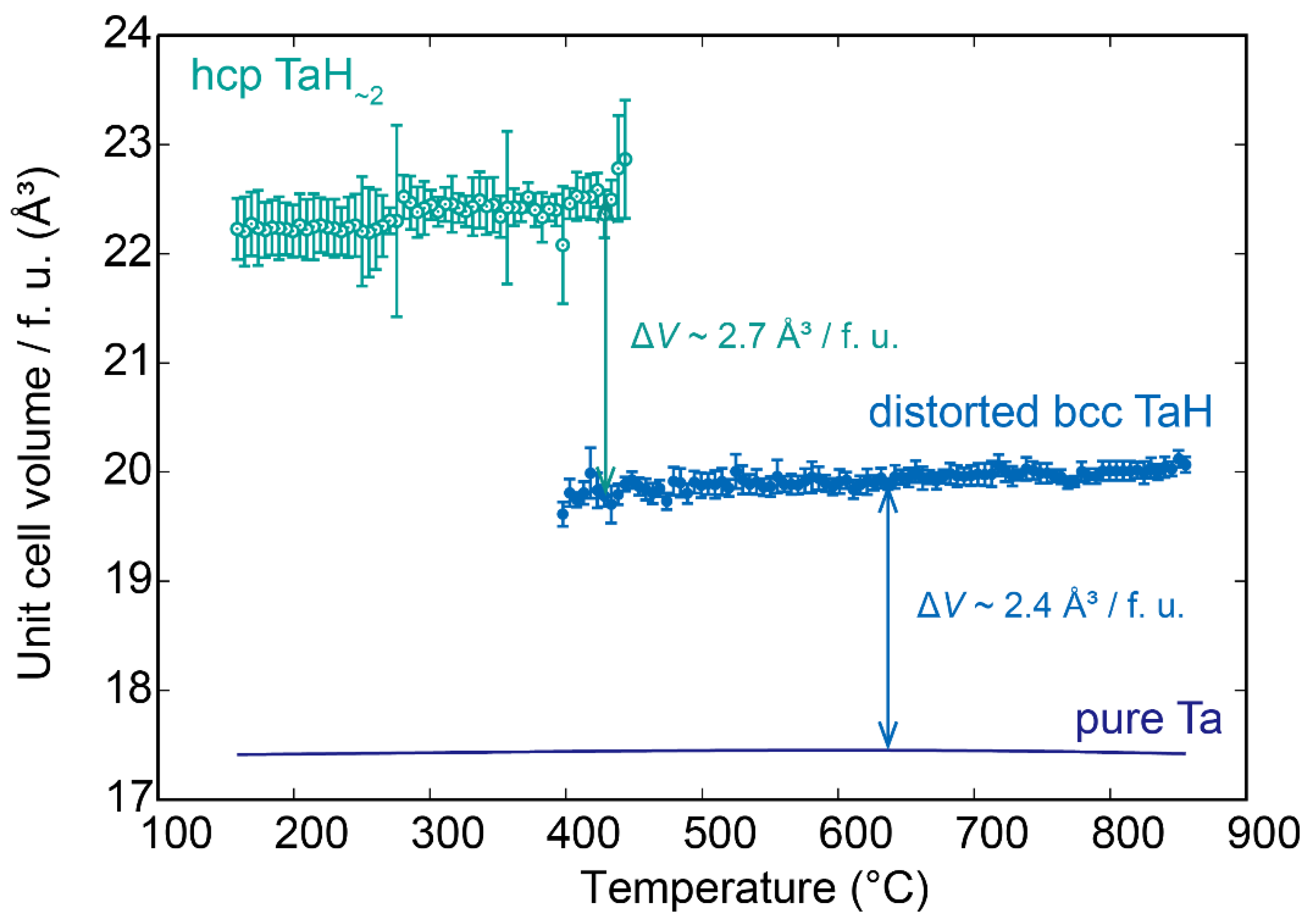
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drozdov, A.P.; Eremets, M.I.; Troyan, I.A.; Ksenofontov, V.; Shylin, S.I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef]
- Drozdov, A.P.; Kong, P.P.; Minkov, V.S.; Besedin, S.P.; Kuzovnikov, M.A.; Mozaffari, S.; Balicas, L.; Balakirev, F.F.; Graf, D.E.; Prakapenka, V.B.; et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 2019, 569, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somayazulu, M.; Ahart, M.; Mishra, A.K.; Geballe, Z.M.; Baldini, M.; Meng, Y.; Struzhkin, V.V.; Hemley, R.J. Evidence for Superconductivity above 260 K in Lanthanum Superhydride at Megabar Pressures. Phys. Rev. Lett. 2019, 122, 27001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, M.; Nakamori, Y.; Orimo, S.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 224103. [Google Scholar] [CrossRef]
- Udovic, T.; Matsuo, M.; Tang, W.S.; Wu, H.; Stavila, V.; Soloninin, A.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Rush, J.J.; et al. Exceptional Superionic Conductivity in Disordered Sodium Decahydro-closo-decaborate. Adv. Mater. 2014, 26, 7622–7626. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, G.; Hinuma, Y.; Matsuoka, S.; Watanabe, A.; Iqbal, M.; Hirayama, M.; Yonemura, M.; Kamiyama, T.; Tanaka, I.; Kanno, R. Pure H- conduction in oxyhydrides. Science 2016, 351, 1314–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, K.; Iimura, S.; Tada, T.; Fujitsu, S.; Sasase, M.; Tamatsukuri, H.; Honda, T.; Ikeda, K.; Otomo, T.; Hosono, H. Characteristic fast H− ion conduction in oxygen-substituted lanthanum hydride. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, R.; Orimo, S. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2016, 2, 16091. [Google Scholar] [CrossRef]
- Kuzovnikov, M.A.; Antonov, V.E.; Ivanov, A.S.; Hansen, T.; Savvin, S.; Kulakov, V.I.; Tkacz, M.; Kolesnikov, A.I. Neutron scattering study of tantalum monohydride and monodeuteride. Int. J. Hydrogen Energy 2021, 46, 20630–20639. [Google Scholar] [CrossRef]
- Kuzovnikov, M.A.; Tkacz, M.; Meng, H.; Kapustin, D.I.; Kulakov, V.I. High-pressure synthesis of tantalum dihydride. Phys. Rev. B 2017, 96, 134120. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Q.; Jin, X.; Cui, T.; Ma, Y.; Lv, Q.; Li, Y.; Zhang, H.; Meng, X.; Bao, K. Pressure-Stabilized Superconductive Ionic Tantalum Hydrides. Inorg. Chem. 2017, 56, 3901–3908. [Google Scholar] [CrossRef]
- Fukai, Y.; Okuma, N. Evidence of Copious Vacancy Formation in Ni and Pd under a High Hydrogen Pressure. Jpn. J. Appl. Phys. 1993, 32, L1256–L1259. [Google Scholar] [CrossRef]
- Decker, D.L. High-Pressure Equation of State for NaCl, KCl, and CsCl. J. Appl. Phys. 1971, 42, 3239–3244. [Google Scholar] [CrossRef] [Green Version]
- Graetz, J.; Reilly, J.J.; Yartys, V.A.; Maehlen, J.P.; Bulychev, B.M.; Antonov, V.E.; Tarasov, B.P.; Gabis, I.E. Aluminum hydride as a hydrogen and energy storage material: Past, present and future. J. Alloy. Compd. 2011, 509, S517–S528. [Google Scholar] [CrossRef]
- Sugimoto, H.; Fukai, Y. Solubility of hydrogen in metals under high hydrogen pressures: Thermodynamical calculations. Acta Met. Mater. 1992, 40, 2327–2336. [Google Scholar] [CrossRef]
- Takagi, S.; Iijima, Y.; Sato, T.; Saitoh, H.; Ikeda, K.; Otomo, T.; Miwa, K.; Ikeshoji, T.; Orimo, S. Formation of novel transition metal hydride complexes with ninefold hydrogen coordination. Sci. Rep. 2017, 7, 44253. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitoh, H.; Takagi, S.; Sato, T.; Orimo, S.-i. Pressure–Temperature Phase Diagram of Ta-H System up to 9 GPa and 600 °C. Appl. Sci. 2021, 11, 6719. https://doi.org/10.3390/app11156719
Saitoh H, Takagi S, Sato T, Orimo S-i. Pressure–Temperature Phase Diagram of Ta-H System up to 9 GPa and 600 °C. Applied Sciences. 2021; 11(15):6719. https://doi.org/10.3390/app11156719
Chicago/Turabian StyleSaitoh, Hiroyuki, Shigeyuki Takagi, Toyoto Sato, and Shin-ichi Orimo. 2021. "Pressure–Temperature Phase Diagram of Ta-H System up to 9 GPa and 600 °C" Applied Sciences 11, no. 15: 6719. https://doi.org/10.3390/app11156719
APA StyleSaitoh, H., Takagi, S., Sato, T., & Orimo, S.-i. (2021). Pressure–Temperature Phase Diagram of Ta-H System up to 9 GPa and 600 °C. Applied Sciences, 11(15), 6719. https://doi.org/10.3390/app11156719





