Enhanced Heat Transfer Performance of the Tube Heat Exchangers Using Carbon-Based Nanofluids
Abstract
:1. Introduction
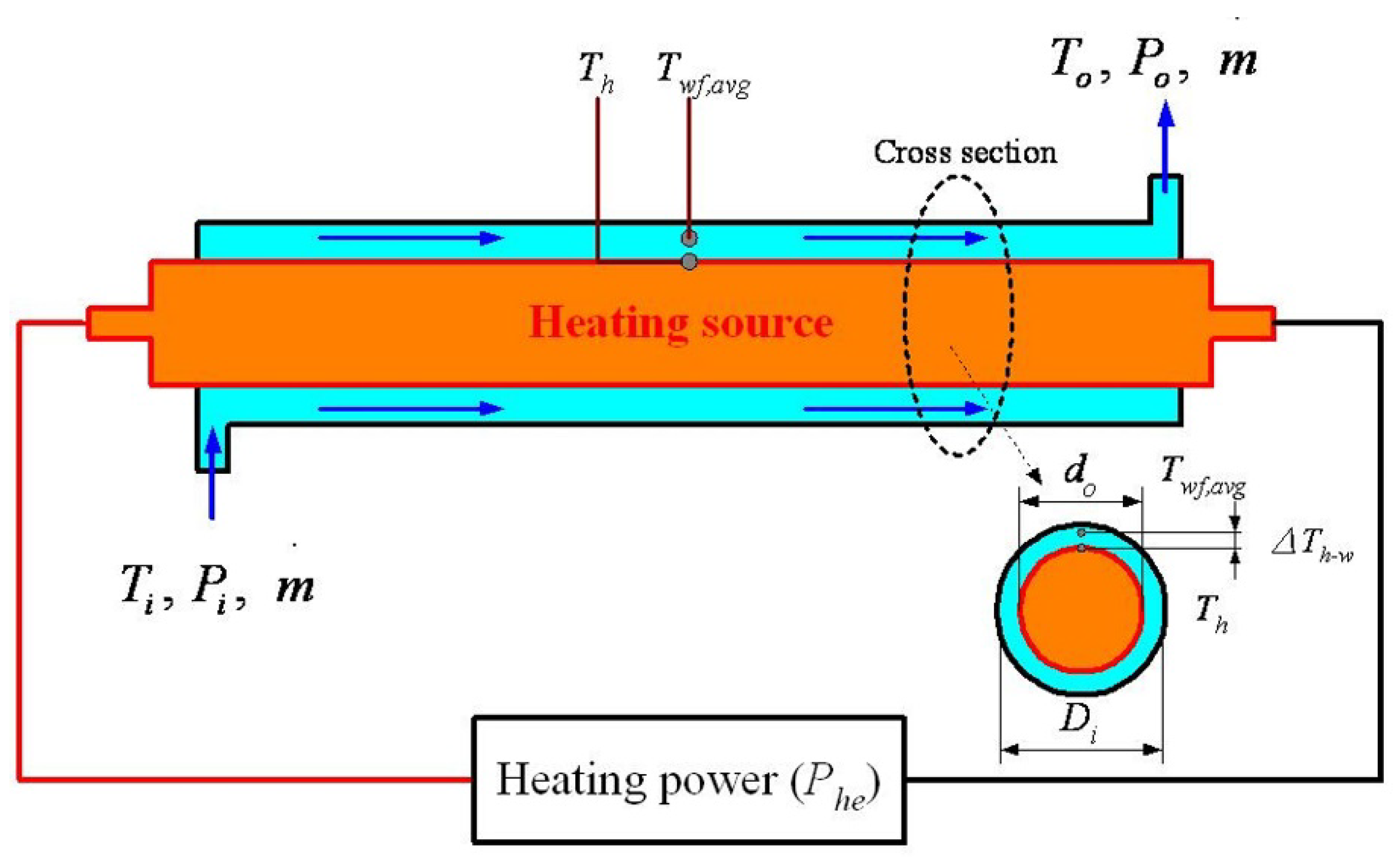
2. Performance Calculations of the Tube Heat Exchanger
3. Experiment
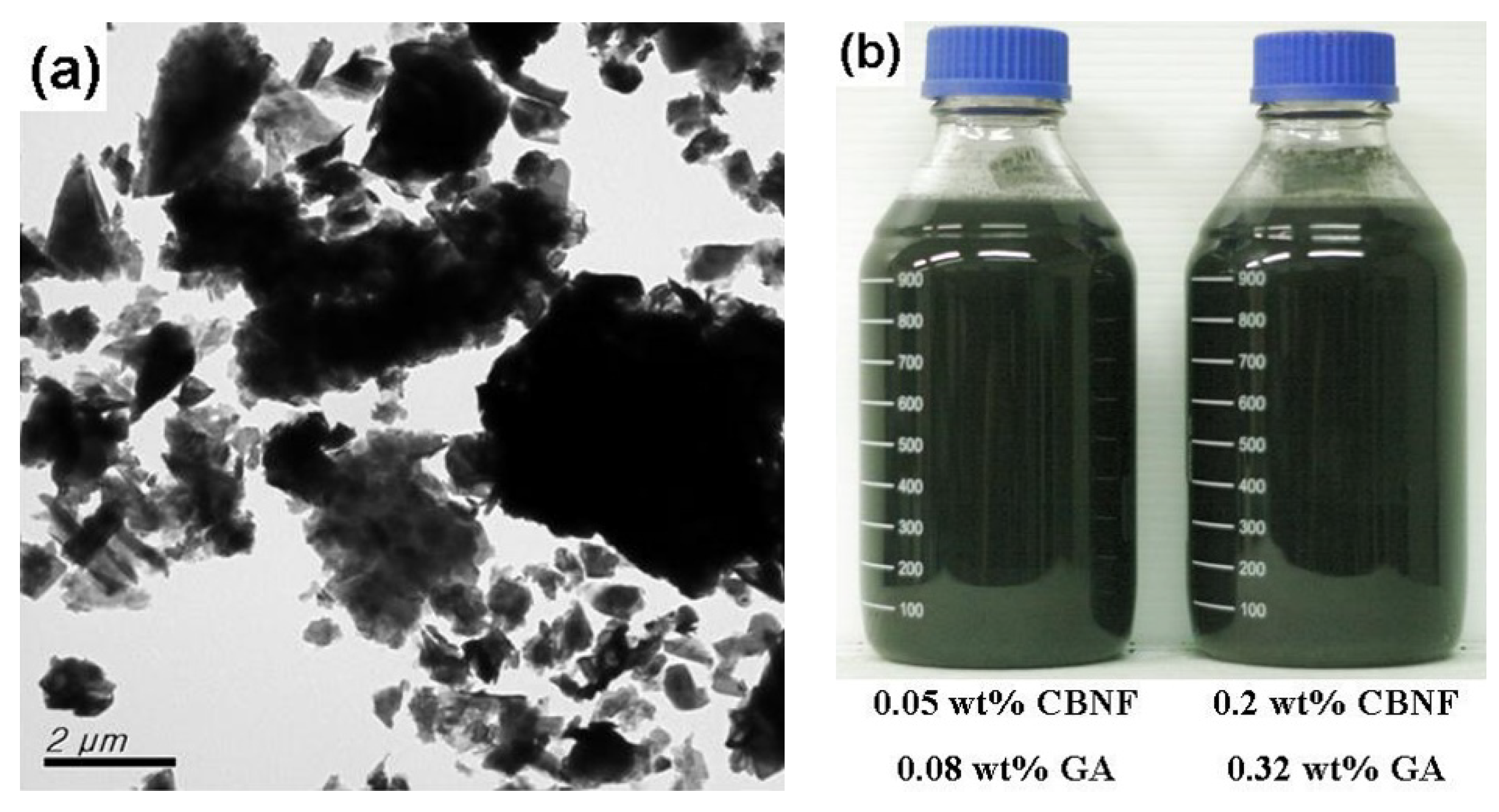
3.1. Preparation of CBNFs
3.2. Density and Viscosity Measurement
3.3. Heat Transfer Performance Experiments
3.4. Data Analysis and Relative Uncertainty
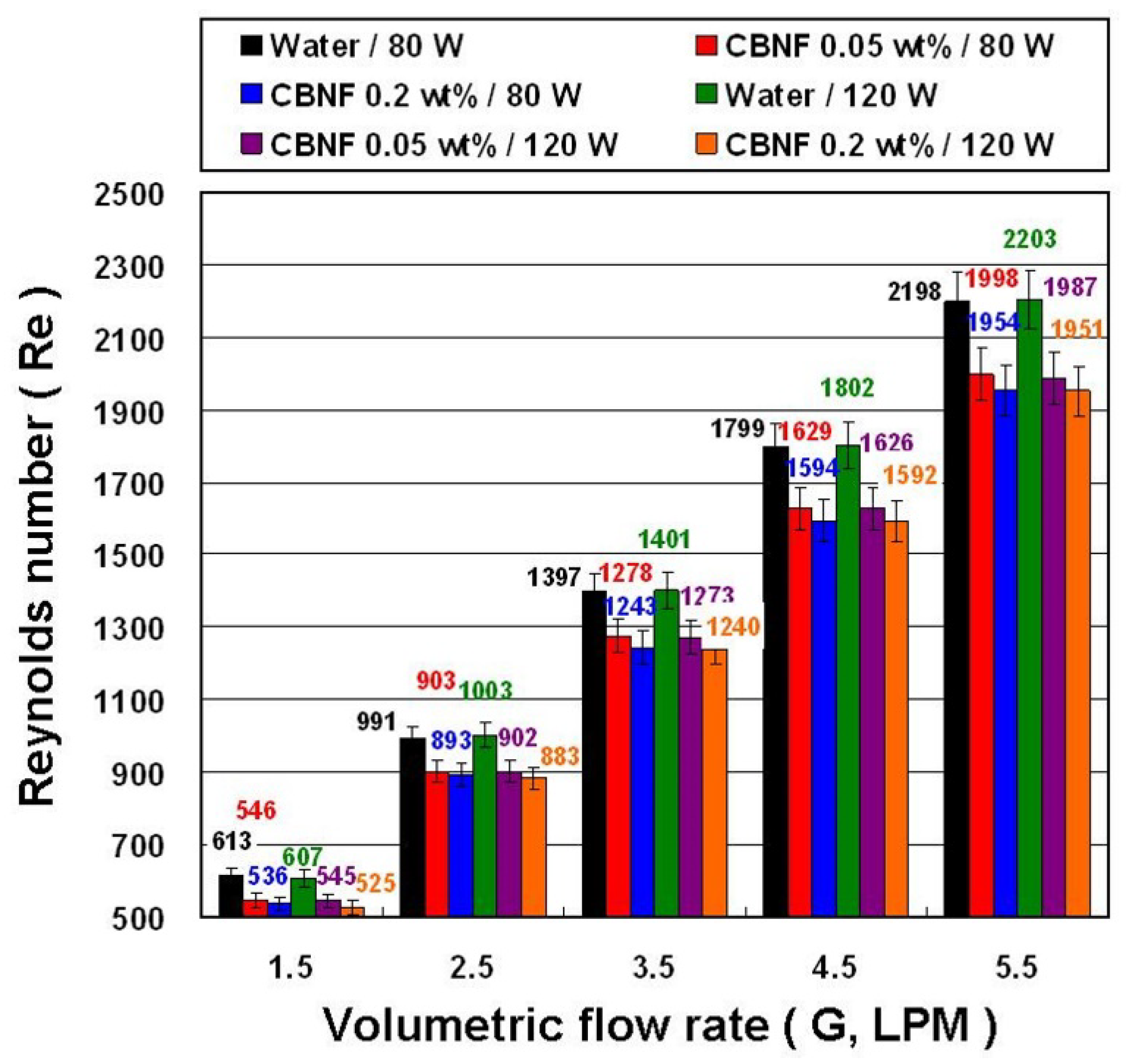
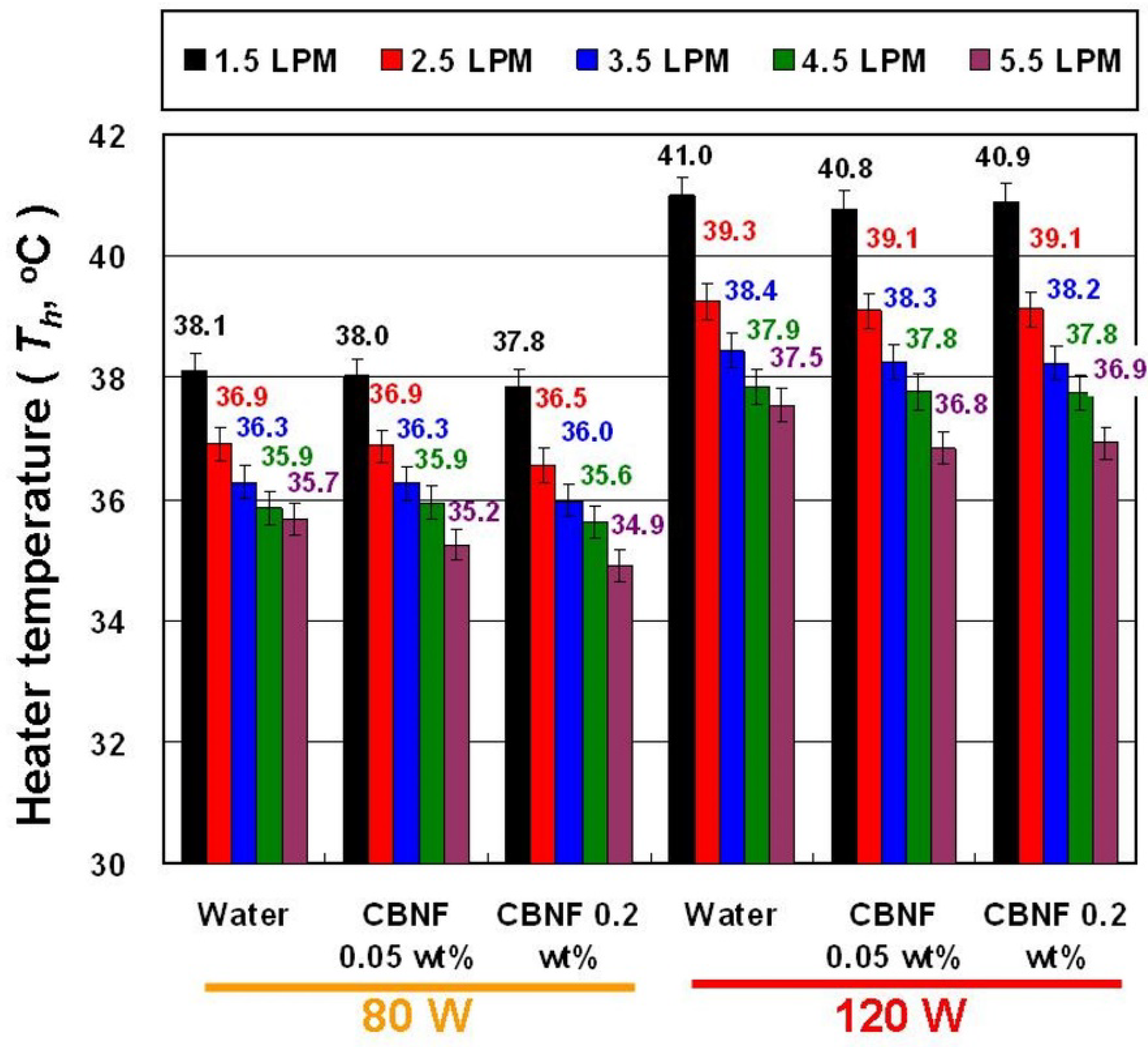
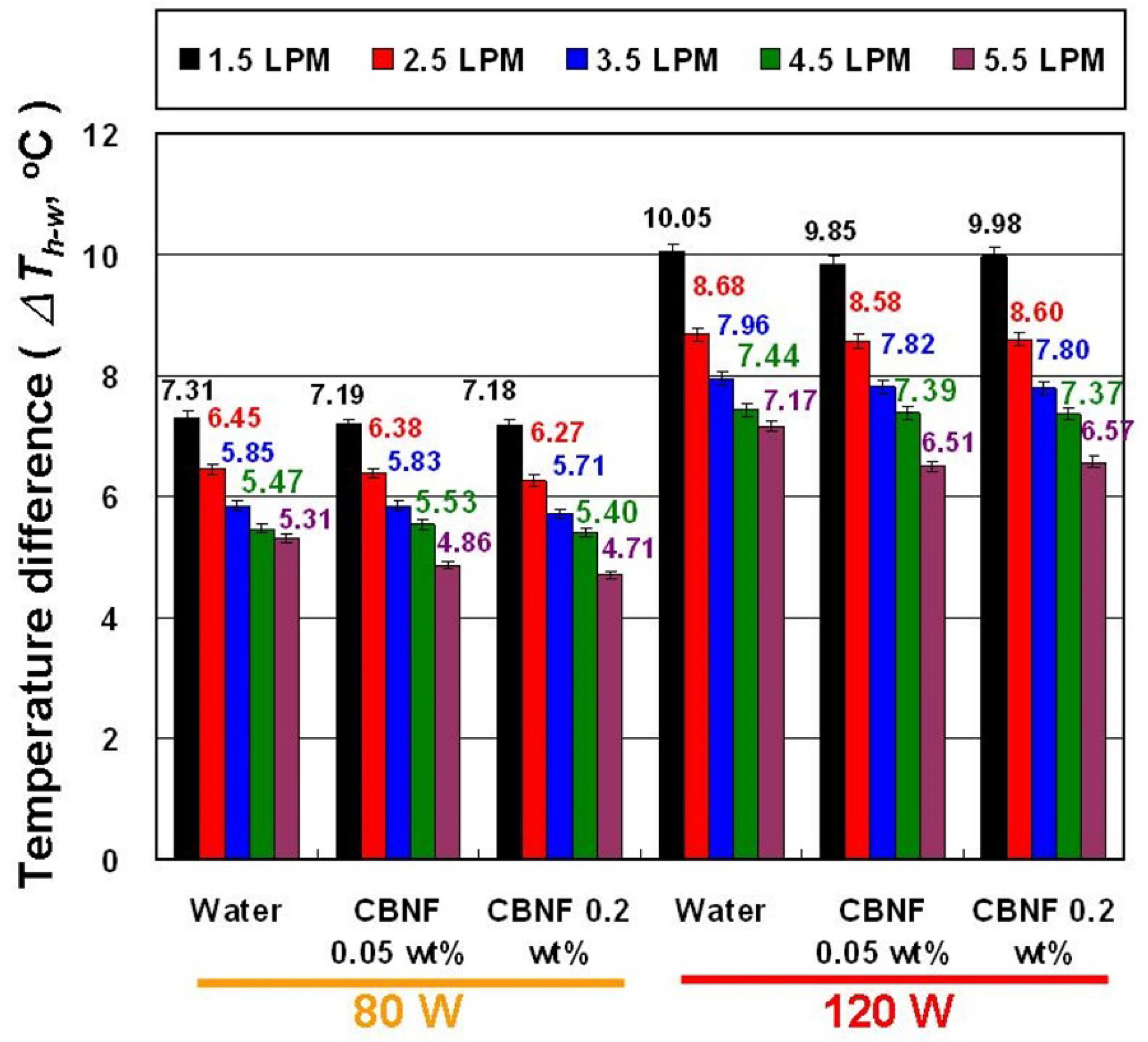
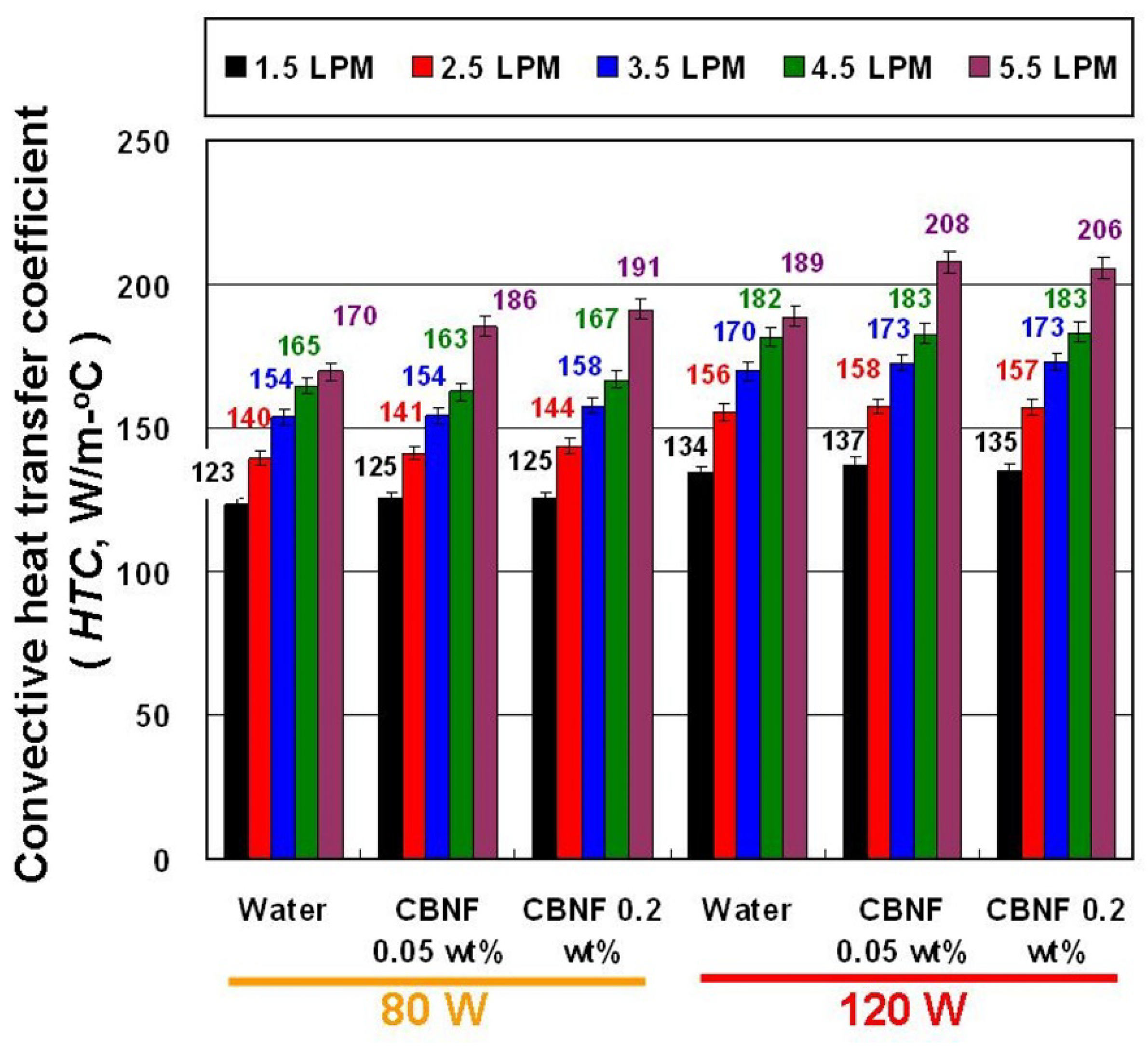
4. Results and Discussion
5. Conclusions
- The CBNFs have a lower Re than that of water because CBNFs have higher μ.
- The CBNFs flowing through the THE have a greater Pf than that of water under the same experimental parameters. This phenomenon is mainly because CBNFs have a higher μ, which results in a higher pressure drop when flowing through the THE.
- CBNFs as a working fluid in a THE system have little effect on Ppe compared with water under the same experimental parameters.
- CBNFs can reduce the Th under the same experimental parameters, and a lower heater temperature helps to extend the service life of the heater.
- The results cumulatively indicate that CBNFs in the THE can lead to a higher HTC than water. In the optimal condition, the average HTC of 0.05 wt% CBNFs at 120 W heating power was about 3.33% higher than that of water, and the average HTC of 0.2 wt% CBNFs at 80 W heating power was about 4.52% higher than that of water.
- Estimation of PIe indicates that 0.05 wt% CBNF has better system efficiency than 0.2 wt% CBNF and is thus more suitable for the THE system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ρ: density (kg/m3) | T: temperature (°C) |
| μ: viscosity (mPa s or Pa s) | uε: relative uncertainty (%) |
| A: area (m2) | WP: wet perimeter (m) |
| Ac: cross-sectional area (m2) | ΔP: pressure drop (Pa) |
| cp: specific heat (kJ/kg °C) | ΔT: temperature difference (°C) |
| Dh: hydraulic diameter (m) | ε: measurement deviations (%) |
| Di: inner diameter of the outer tube (m) | |
| do: outer diameter of internal tube (m) | |
| DT: experimental data | Subscripts |
| G: volumetric flow rate (m3/s or LPM) | avg: average value |
| HTC: convective heat transfer coefficient (W/m2 °C) | CBNFs: carbon-based nanofluids |
| Pf: flow loss (W) | cs: cross-sectional |
| Phe: heating power (W) | h: heater |
| PIe: performance index | i: inlet of THE |
| Ppe: power consumption of pump (W) | o: outlet of THE |
| R: percentage differences (%) | THE: tube heat exchanger |
| Re: Reynolds number | w: water |
| wf: working fluid |
References
- Abbas, N.; Awan, M.B.; Amer, M.; Ammar, S.M.; Sajjad, U.; Ali, H.M.; Zahra, N.; Hussain, M.; Badshah, M.A.; Jafry, A.T. Applications of nanofluids in photovoltaic thermal systems: A review of recent advances. Physica A 2019, 536, 122513. [Google Scholar] [CrossRef]
- Pordanjani, A.H.; Aghakhani, S.; Afrand, M.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Conv. Manag. 2019, 198, 111886. [Google Scholar] [CrossRef]
- Esfe, M.H.; Bahiraei, M.; Hajbarati, H.; Valadkhani, M. A comprehensive review on convective heat transfer of nanofluids in porous media: Energy-related and thermohydraulic characteristics. Appl. Therm. Eng. 2020, 178, 115487. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Mao, M.; Huang, J.-N. An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J. Clean Prod. 2020, 257, 120408. [Google Scholar] [CrossRef]
- Abbas, F.; Ali, H.M.; Shah, T.R.; Babar, H.; Janjua, M.M.; Sajjad, U.; Amer, M. Nanofluid: Potential evaluation in automotive radiator. J. Mol. Liq. 2020, 297, 112014. [Google Scholar] [CrossRef]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. A review of heat transfer application of carbon-based nanofluid in heat exchangers. Nano-Struct. Nano-Obj. 2019, 20, 100394. [Google Scholar] [CrossRef]
- Ghalandari, M.; Maleki, A.; Haghighi, A.; Shadloo, M.S.; Nazari, M.A.; Tlili, I. Applications of nanofluids containing carbon nanotubes in solar energy systems: A review. J. Mol. Liq. 2020, 313, 113476. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sust. Energ. Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Gao, Y.; An, J.; Xi, Y.; Yang, Z.; Liu, J.; Mujumdar, A.S.; Wang, L.; Sasmito, A.P. Thermal conductivity and stability of novel aqueous graphene oxide–Al2O3 hybrid nanofluids for cold energy storage. Appl. Sci. 2020, 10, 5768. [Google Scholar] [CrossRef]
- Sani, E.; Vallejo, J.P.; Mercatelli, L.; Martina, M.R.; Rosa, D.D.; Dell’Oro, A.; Lugo, L. A comprehensive physical profile for aqueous dispersions of carbon derivatives as solar working fluids. Appl. Sci. 2020, 10, 528. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, I.; Xu, S.; Mazouffre, S.; Lev, D.; Pedrini, D.; Goebel, D.; Garrigues, L.; Taccogna, F.; Bazak, K. Perspectives, frontiers, and new horizons for plasma-based space electric propulsion. Phys. Plasmas 2020, 27, 020601. [Google Scholar] [CrossRef] [Green Version]
- M’hamed, B.; Che Sidik, N.A.; Akhbar, M.F.A.; Mamat, R.; Najafi, G. Experimental study on thermal performance of MWCNT nanocoolant in Perodua Kelisa 1000cc radiator system. Int. Commun. Heat Mass Transf. 2016, 76, 156–161. [Google Scholar] [CrossRef]
- Selvam, C.; Mohan Lal, D.; Harish, S.; Lal, D.M.; Harish, S. Enhanced heat transfer performance of an automobile radiator with graphene based suspensions. Appl. Therm. Eng. 2017, 123, 50–60. [Google Scholar] [CrossRef]
- Martin, E.; Contreras, C.; Oliveira, A.; Pedone, E.; Filho, B. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar]
- Teng, T.-P.; Cheng, C.-M.; Yu, S.-P. Evaluation of heat-exchange performance of carbon-based nanofluids for air-cooled exchangers with different cross-section shapes. Appl. Therm. Eng. 2020, 179, 115725. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Rashidi, A.; Shariaty-Niassar, M. Convective heat transfer enhancement of graphene nanofluids in shell and tube heat exchanger. Exp. Therm. Fluid Sci. 2014, 53, 136–141. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M. Exergy analysis of a shell-and-tube heat exchanger using graphene oxide nanofluids. Exp. Therm. Fluid Sci. 2017, 83, 100–106. [Google Scholar] [CrossRef]
- Fares, M.; AL-Mayyahi, M.; AL-Saad, M. Heat transfer analysis of a shell and tube heat exchanger operated with graphene nanofluids. Case Stud. Therm. Eng. 2020, 18, 100584. [Google Scholar] [CrossRef]
- Goodarzi, M.; Amiri, A.; Goodarzi, M.S.; Safaei, M.R.; Karimipour, A.; Languri, E.M.; Dahari, M. Investigation of heat transfer and pressure drop of a counter flow corrugated plate heat exchanger using MWCNT based nanofluids. Int. Commun. Heat Mass Transf. 2015, 66, 172–179. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Effect of variable spacing on performance of plate heat exchanger using nanofluids. Energy 2016, 114, 1107–1119. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Cardenas Gomez, A.O.; Bandarra Filho, E.P.; Parise, J.A.R. Experimental evaluation of SWCNT-water nanofluid as a secondary fluid in a refrigeration system. Appl. Therm. Eng. 2017, 111, 1487–1492. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Sunden, B. Effects of graphene ethylene glycol/water nanofluids on the performance of a brazed plate heat exchanger. J. Nanofluids 2018, 7, 1069–1074. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Experimentation on effect of particle ratio on hydrothermal performance of plate heat exchanger using hybrid nanofluid. Appl. Therm. Eng. 2019, 162, 114309. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Mahian, O.; Wongwises, S. Heat transfer characteristics and pressure drop of COOH-functionalized DWCNTs/water nanofluid in turbulent flow at low concentrations. Int. J. Heat Mass Transf. 2014, 73, 186–194. [Google Scholar] [CrossRef]
- Arzani, H.K.; Amiri, A.; Kazi, S.N.; Chew, B.T.; Badarudin, A. Experimental investigation of thermophysical properties and heat transfer rate of covalently functionalized MWCNT in an annular heat exchanger. Int. Commun. Heat Mass Transf. 2016, 75, 67–77. [Google Scholar] [CrossRef]
- Poongavanam, G.K.; Panchabikesan, K.; Murugesan, R.; Duraisamy, S.; Ramalingam, V. Experimental investigation on heat transfer and pressure drop of MWCNT—Solar glycol based nanofluids in shot peened double pipe heat exchanger. Powder Technol. 2019, 345, 815–824. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Cardenas Contreras, E.M.; Bandarra Filho, E.P. Experimental study on the heat transfer of MWCNT/water nanofluid flowing in a car radiator. Appl. Therm. Eng. 2017, 111, 1450–1456. [Google Scholar] [CrossRef]
- Goshayeshi, H.R.; Safaei, M.R.; Goodarzi, M.; Dahari, M. Particle size and type effects on heat transfer enhancement of Ferro-nanofluids in a pulsating heat pipe. Powder Technol. 2016, 301, 1218–1226. [Google Scholar] [CrossRef]
- Bahiraei, M.; Jamshidmofid, M.; Goodarzi, M. Efficacy of a hybrid nanofluid in a new microchannel heat sink equipped with both secondary channels and ribs. J. Mol. Liq. 2019, 273, 88–98. [Google Scholar] [CrossRef]
- Teng, T.-P.; Hsiao, T.-C.; Chung, C.-C. Characteristics of carbon-based nanofluids and their application in a brazed plate heat exchanger under laminar flow. Appl. Therm. Eng. 2019, 146, 160–168. [Google Scholar] [CrossRef]
- Hung, Y.-H.; Wang, W.-P.; Hsu, Y.-C.; Teng, T.-P. Performance evaluation of an air-cooled heat exchange system for hybrid nanofluids. Exp. Therm. Fluid Sci. 2017, 81, 43–55. [Google Scholar] [CrossRef]
- Teng, T.-P.; Hsiao, T.-C.; Chung, C.-C. Preparation and experimental evaluation of phase-change characteristics in carbon-based suspensions. Materials 2018, 11, 1315. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-M.; Yu, S.-P.; Teng, T.-P. Fabrication and characterization of carbon-based nanfluids through water vortex trap method. J. Nanomater. 2018, 2018, 3264621. [Google Scholar] [CrossRef] [Green Version]
- Teng, T.-P.; Yu, S.-P.; Hsiao, T.-C.; Chung, C.-C. Study on the phase-change characteristics of carbon-based nanofluids. J. Nanomater. 2018, 2018, 8230120. [Google Scholar] [CrossRef] [Green Version]
- Teng, T.-P.; Hsu, Y.-C.; Wang, W.-P.; Fang, Y.-B. Performance assessment of an air-cooled heat exchanger for multiwalled carbon nanotubes-water nanofluids. Appl. Therm. Eng. 2015, 89, 346–355. [Google Scholar] [CrossRef]
- Goodarzi, M.; Toghraie, D.; Reiszadeh, M.; Afrand, M. Experimental evaluation of dynamic viscosity of ZnO–MWCNTs/engine oil hybrid nanolubricant based on changes in temperature and concentration. J. Therm. Anal. Calorim. 2019, 136, 513–525. [Google Scholar] [CrossRef]




| Materials/BFs | Concentrations | HE Types | Main Findings | Ref. |
|---|---|---|---|---|
| MWCNTs/EG-W (1:1, v/v) | 0.1, 0.25, and 0.5 vol% | Vehicle radiator | HTC was proportional to flow rate (G), Re, and MWCNTs concentration. Maximum enhancements of HTC was 196.3% at 0.5 vol% NF at Re of 1400. | [12] |
| GNP/EG-W (3:7, v/v) | 0.1–0.5 vol% | Automobile radiator | HTC was proportional to GNP concentration, inlet temperature (Ti), and mass flow rate (mwf). A maximum enhancement of HTC was 51% for 0.5 vol% NF at mwf of 100 g/s and Ti of 45 °C. Maximum pressure drop (ΔP) was 4.80 kPa for 0.5 vol% NF at Ti of 35 °C and mwf of 100 g/s. | [13] |
| GN/EG-W (1:1, v/v) | 0.01, 0.05 and 0.1 vol% | Automobile radiator | A maximum enhancement of heat exchange rate was 3.3% at 0.1 vol% NF and Ti of 85 °C. The heat exchange rate of 0.01 vol% and 0.05 vol% NF was lower than BF. | [14] |
| CBNMs/W | 0.01 and 0.05 wt% | Rectangular (R) and circular (C) tubes ACHE | A maximum improvement of heat exchange capacity was 8.17% at 0.05 wt% NF at G of 2.0 LPM and Ti of 40 °C for R-ACHE. A maximum enhancement of heat exchange capacity was 4.88% at 0.01 wt% NF at G of 2.5 LPM and Ti of 40 °C for C-ACHE. 0.01 wt% NF had higher system efficiency. | [15] |
| GN/W | 0.025–0.1 wt% | Shell and tube HE | The HTC increased at higher NF concentration and temperature. A maximum enhancement of HTC was 35.6% at 0.1 wt% NF at fluid temperature of 38 °C. | [16] |
| GO/W | 0.01 and 0.1 wt% | Shell and tube HE | Exergy loss of 0.01 wt% and 0.1 wt% NF were 22% and 109% lower than water. Exergy loss was proportional to the Ti of the NF. | [17] |
| GN/W | 0.01, 0.05, 0.1, and 0.2 wt% | Vertical shell and tube HE | A maximum enhancement of HTC was 29% at 0.2 wt% NF. Average thermal efficiency of the HE improved by 13.7%. | [18] |
| SWCNTs/W | 0–0.21 vol% | Brazed PHE in refrigeration system | The overall thermal performance and refrigerating capacity of the system using NF as the secondary fluid were higher than those of water at the same mwf (40–80 g/s) and Ti (30–40 °C). | [21] |
| GN/EG–W | 0.01–1.0 wt% | Brazed PHE in the hot fluid side | GN NF from 0.01 to 0.1 wt% significantly enhanced the HE performance and created a slight ΔP in the PHE. HTC improvement was about 4–7% for the different NF concentrations. | [22] |
| Al2O3-MWCNTs/ W | vol% Al2O3: MWCNTs (5:0, 4:1, 3:2, 2:3, 1:4, and 0:5, v/v) | PHE | The optimal overall performance is MWCNTs NF (0:5, v/v). HTC improvement was15.2%. Pump power elevated by 0.02%, and the performance index enhanced by 2.96%. | [23] |
| Functionaliz-ed DWCNTs/W | 0.01–0.4 wt% | Double-pipe HE | The enhancement of HTC, the average Nusselt number, and ΔP was 32%, 18%, and 20% for 0.4 wt% NF, respectively. | [24] |
| Functionaliz-ed MWCNTs/W | 0.025, 0.05, 0.075, and 0.1 wt% | Horizontal annular (tube) HE | HTC enhancement far exceeded the enhancement in k for NF. A maximum enhancement of HTC was 22.4% for 0.1 wt% NF at Re of 6807 and heating power of 1200 W. The mean ΔP was increased to 38% due to higher μ of NF. | [25] |
| MWCNTs/so-lar glycol | 0.2, 0.4 and, 0.6 vol% | Double pipe HE with a modified inner surface | A maximum enhancement of HTC was 115% at 0.6 vol% NF at mwf of 0.04 kg/s. ΔP increased 1.56 times at mwf of 0.08 kg/s. Modified inner surface (shot peening process) significantly affected on flow behavior and heat exchange rate. | [26] |
| Item | Temp. (°C) | Water | CBNF 0.05 wt% | CBNF 0.2 wt% |
|---|---|---|---|---|
| ρ (kg/m3) | 30 | 996.63 | 997.08 | 997.94 |
| 40 | 993.24 | 993.45 | 994.25 | |
| 50 | 989.02 | 988.81 | 989.99 | |
| 60 | 984.28 | 983.52 | 984.62 | |
| μ (mPa-s) | 30 | 0.80 | 0.88 | 0.90 |
| 40 | 0.70 | 0.78 | 0.82 | |
| 50 | 0.65 | 0.68 | 0.70 | |
| 60 | 0.55 | 0.58 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-P.; Lue, Y.-F.; Teng, T.-P.; Hsieh, H.-K.; Huang, C.-C. Enhanced Heat Transfer Performance of the Tube Heat Exchangers Using Carbon-Based Nanofluids. Appl. Sci. 2021, 11, 8139. https://doi.org/10.3390/app11178139
Yu S-P, Lue Y-F, Teng T-P, Hsieh H-K, Huang C-C. Enhanced Heat Transfer Performance of the Tube Heat Exchangers Using Carbon-Based Nanofluids. Applied Sciences. 2021; 11(17):8139. https://doi.org/10.3390/app11178139
Chicago/Turabian StyleYu, Shang-Pang, Yeou-Feng Lue, Tun-Ping Teng, Hsiang-Kai Hsieh, and Chia-Cing Huang. 2021. "Enhanced Heat Transfer Performance of the Tube Heat Exchangers Using Carbon-Based Nanofluids" Applied Sciences 11, no. 17: 8139. https://doi.org/10.3390/app11178139
APA StyleYu, S.-P., Lue, Y.-F., Teng, T.-P., Hsieh, H.-K., & Huang, C.-C. (2021). Enhanced Heat Transfer Performance of the Tube Heat Exchangers Using Carbon-Based Nanofluids. Applied Sciences, 11(17), 8139. https://doi.org/10.3390/app11178139





