Adsorption Method for the Remediation of Brilliant Green Dye Using Halloysite Nanotube: Isotherm, Kinetic and Modeling Studies
Abstract
:1. Introduction
2. Constituent and Process Sequence
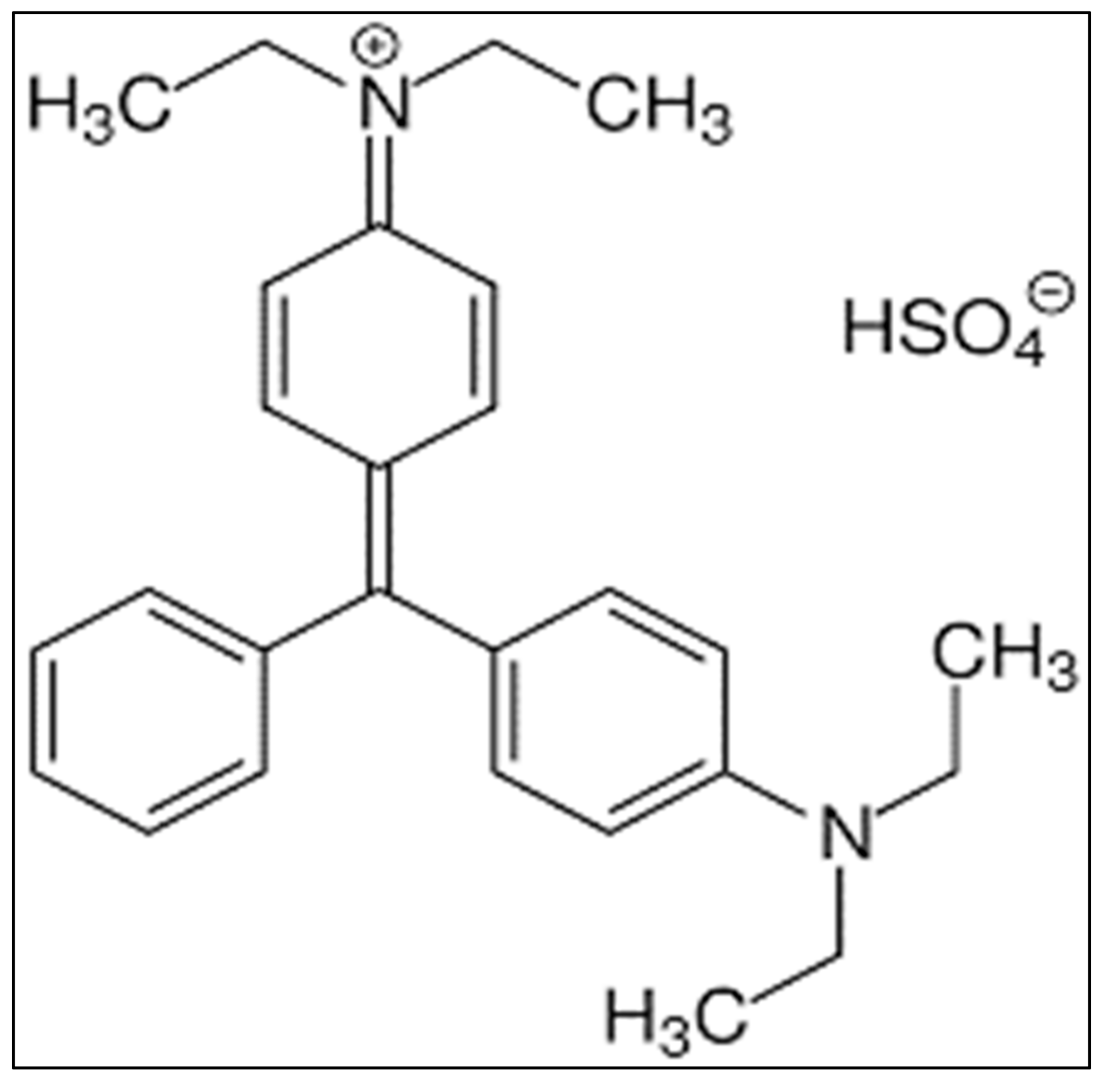
2.1. Constituents
2.2. Parametric Influence of BG on HNT
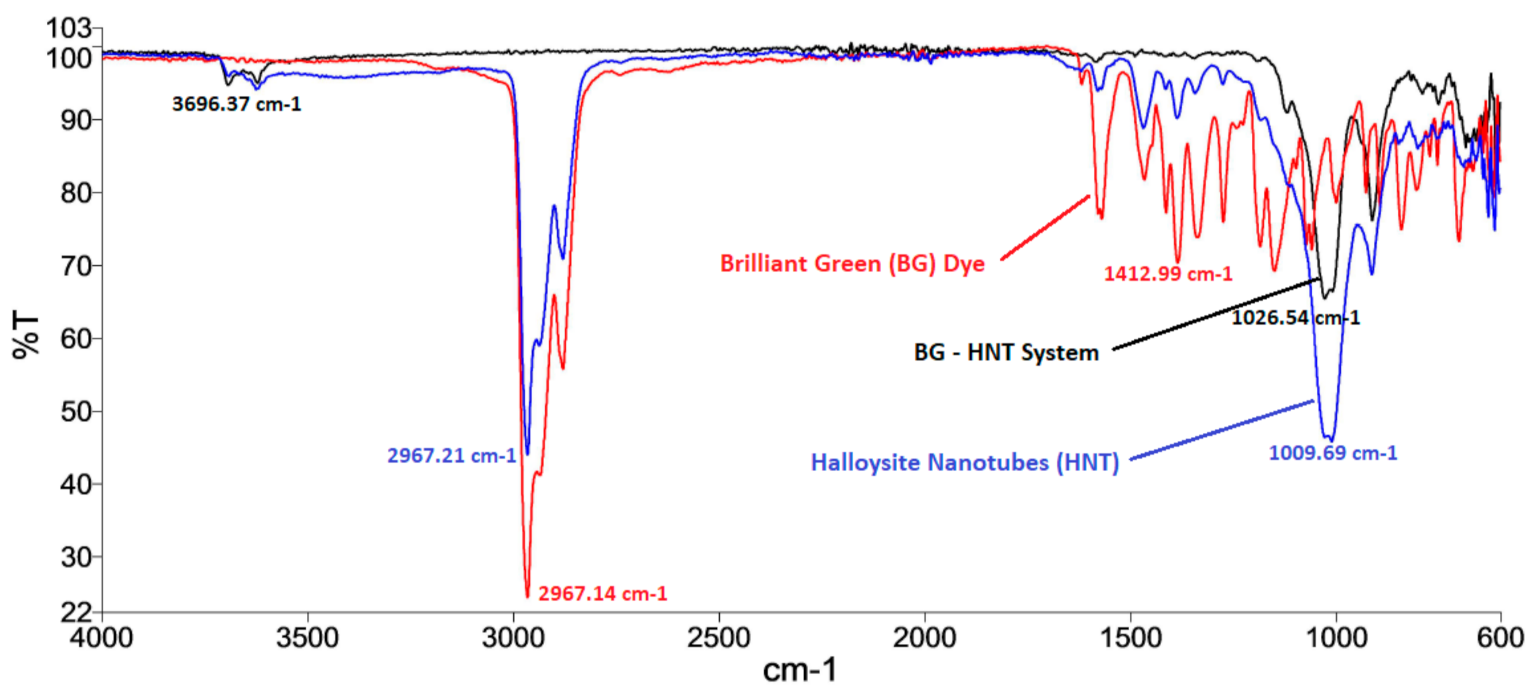
2.3. Characterization Methods
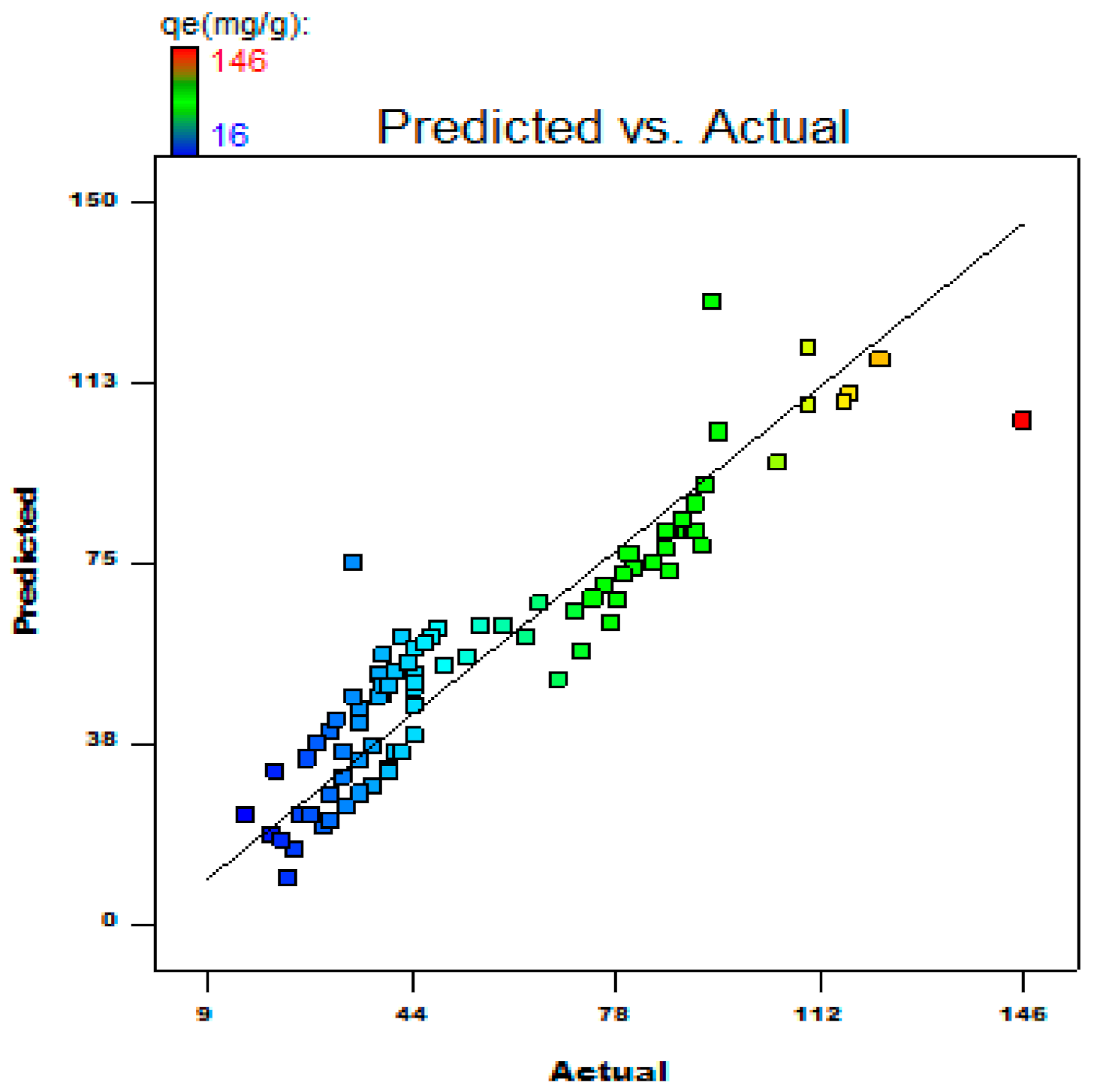
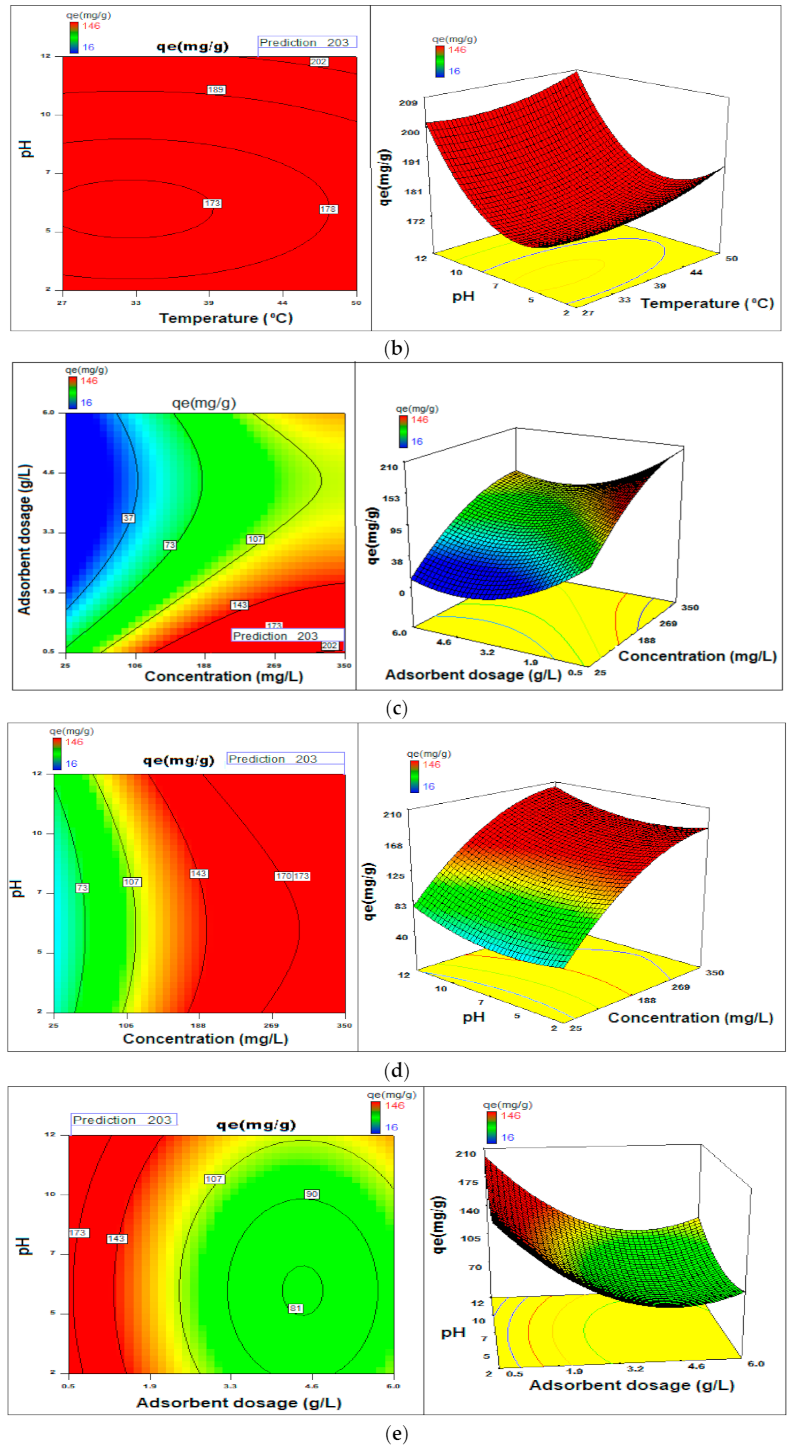
2.4. Statistical Optimization of the Process Parameters
3. Test Data Analysis
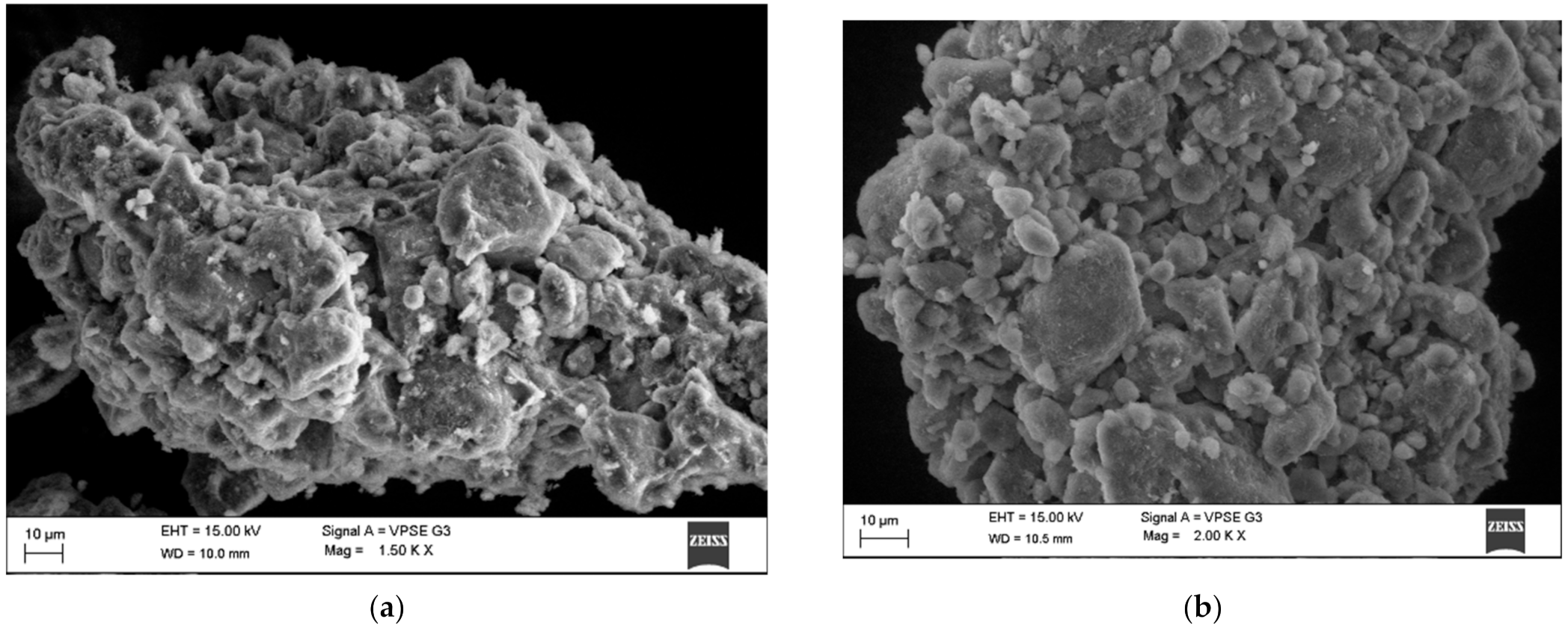
3.1. HNT/BG-HNT Surface Syntesis
Analyses of SEM Images and FTIR Spectrum
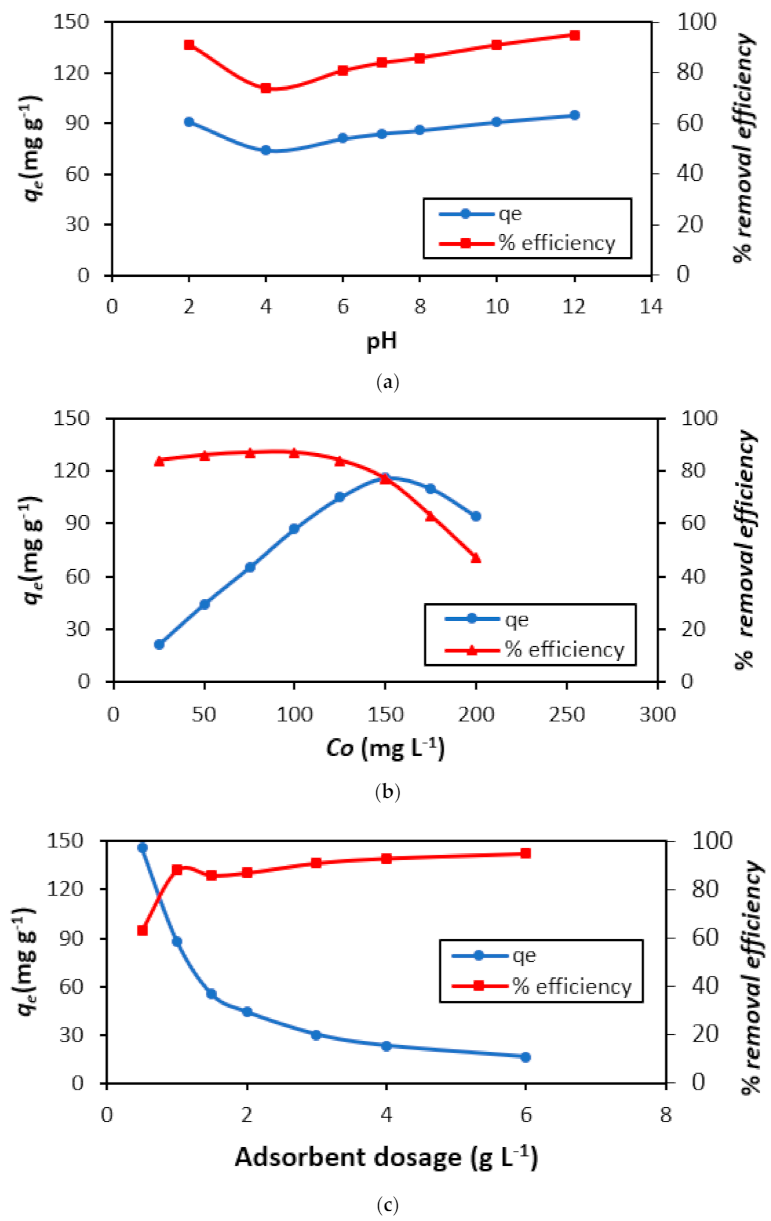
3.2. Parametric Impact
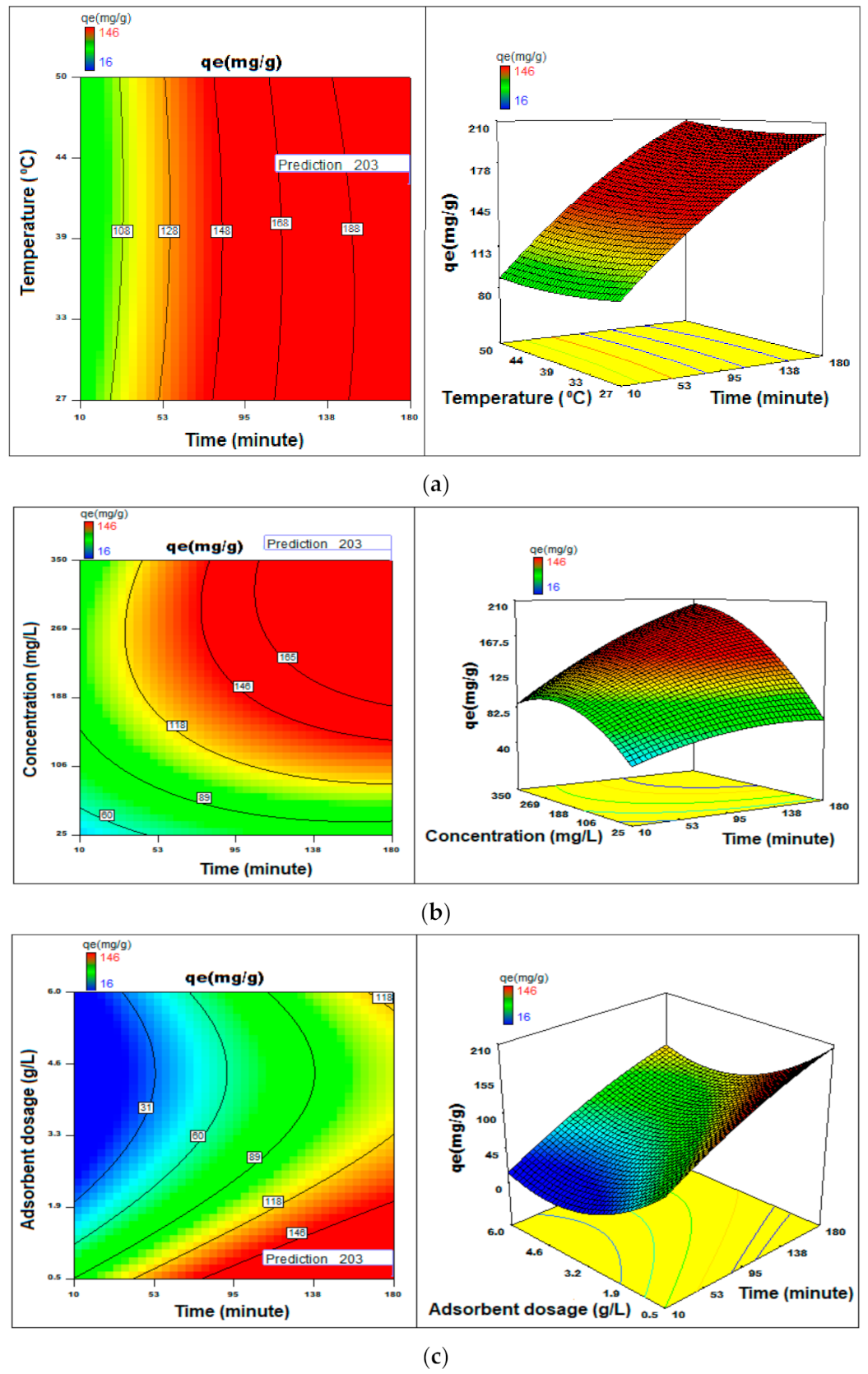
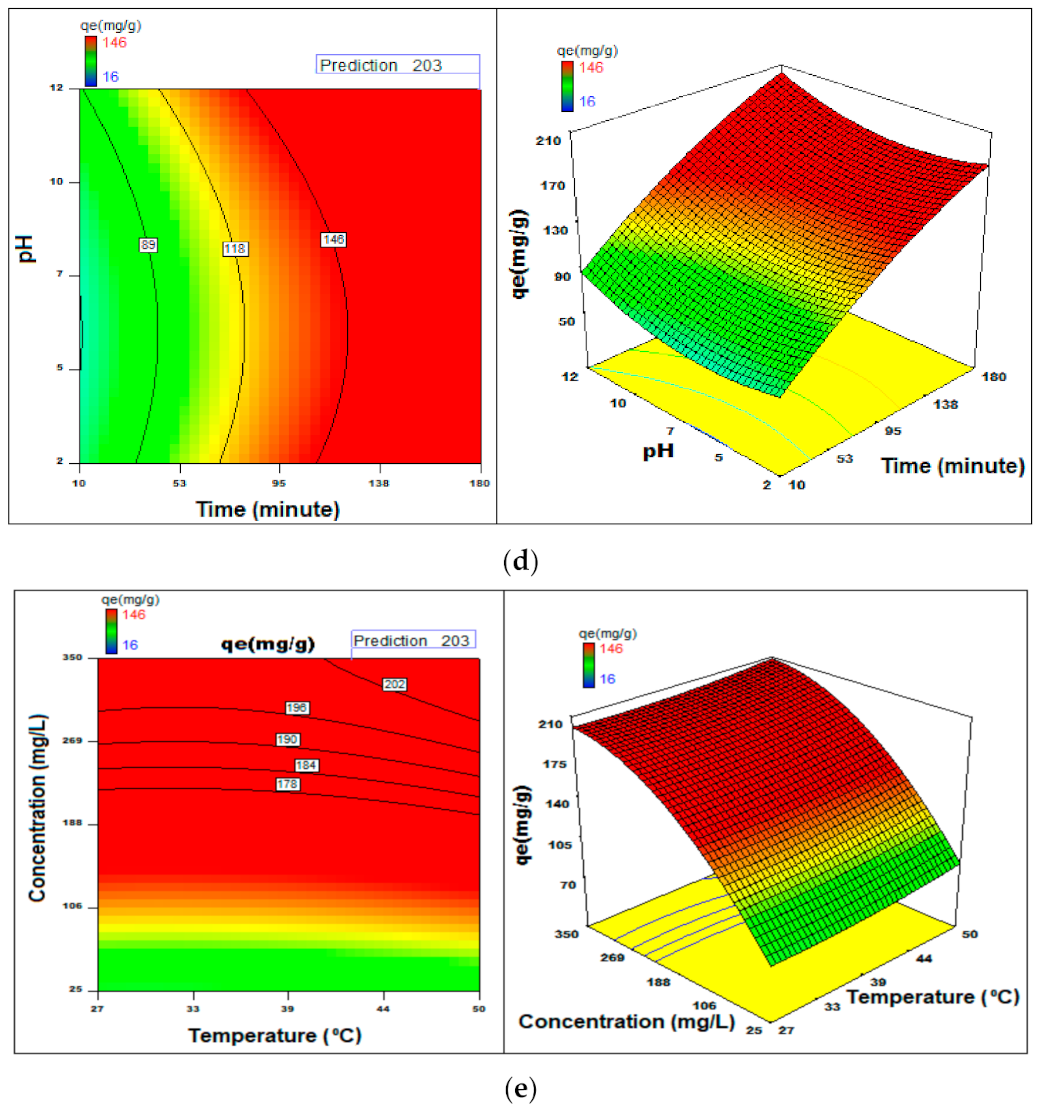
3.2.1. pH Effect
3.2.2. Initial BG Dye Concentration
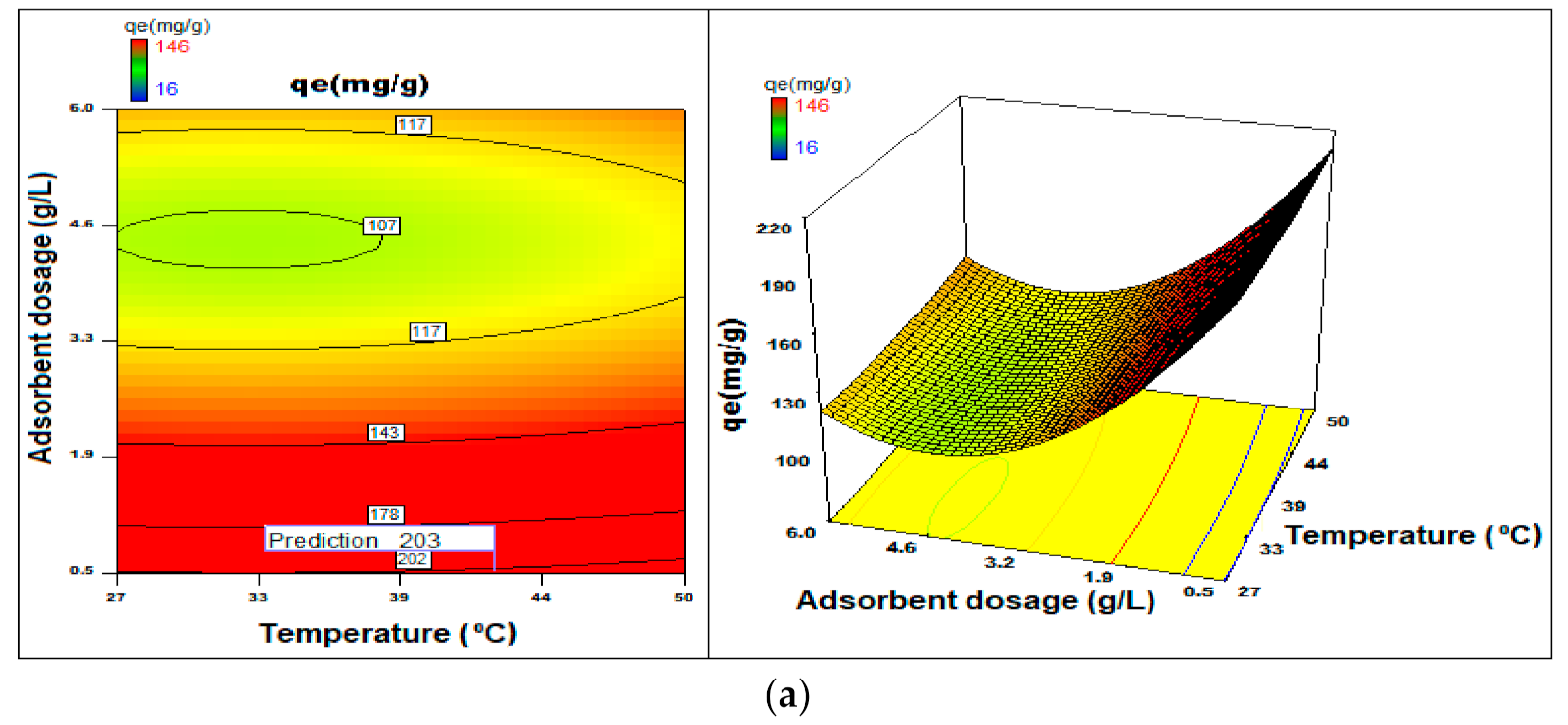
3.2.3. Quantity of Adsorbent
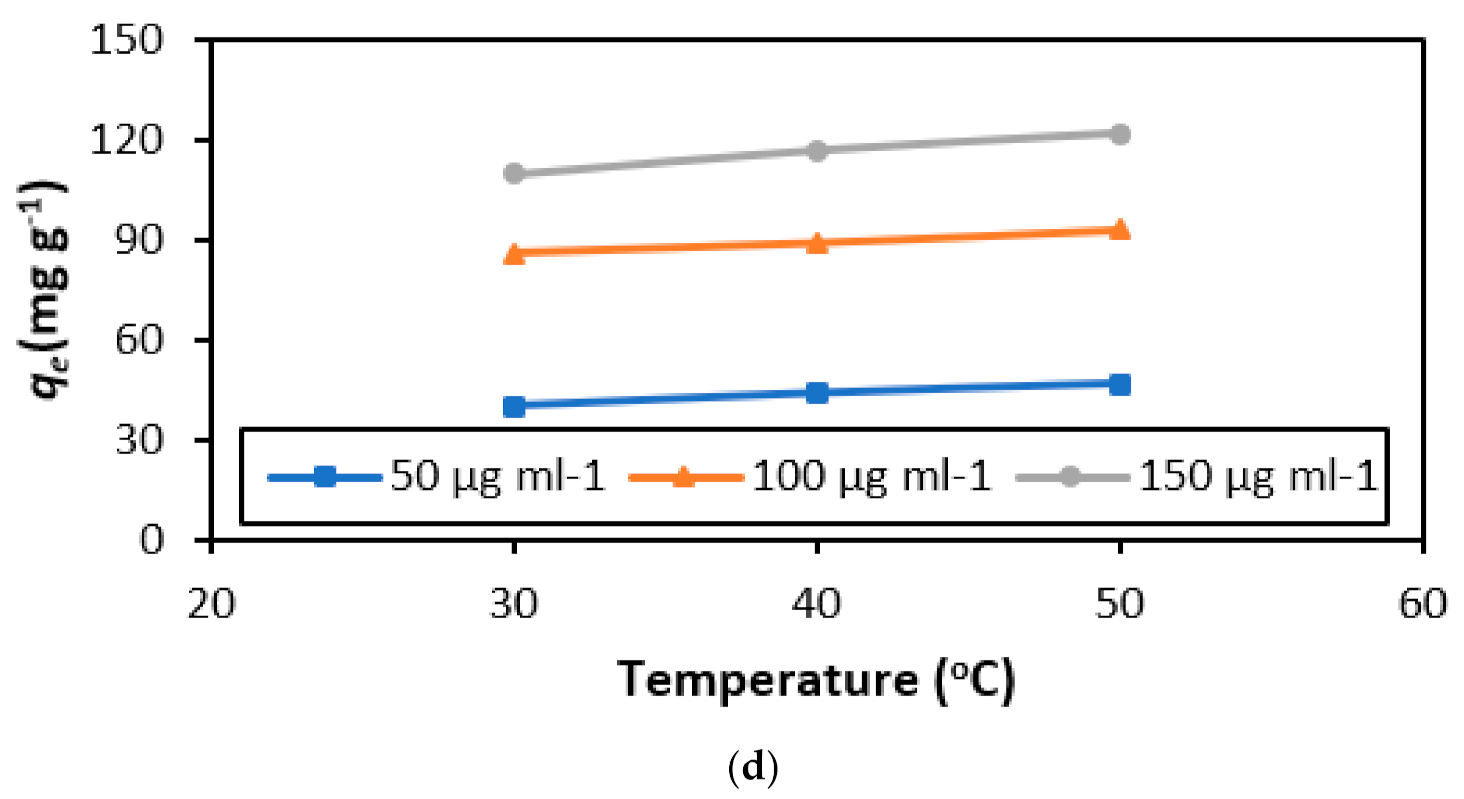
3.2.4. Thermal Influence
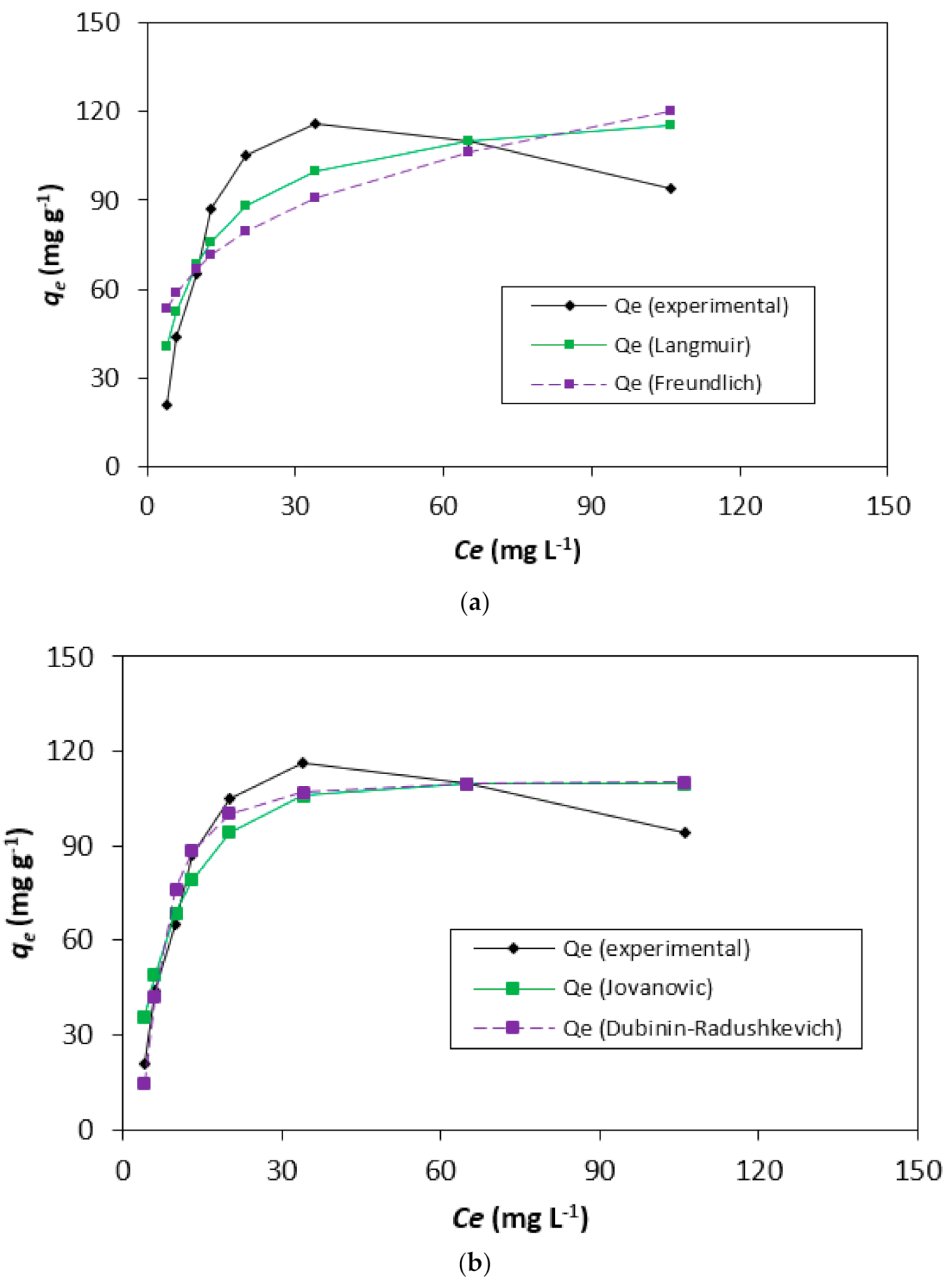
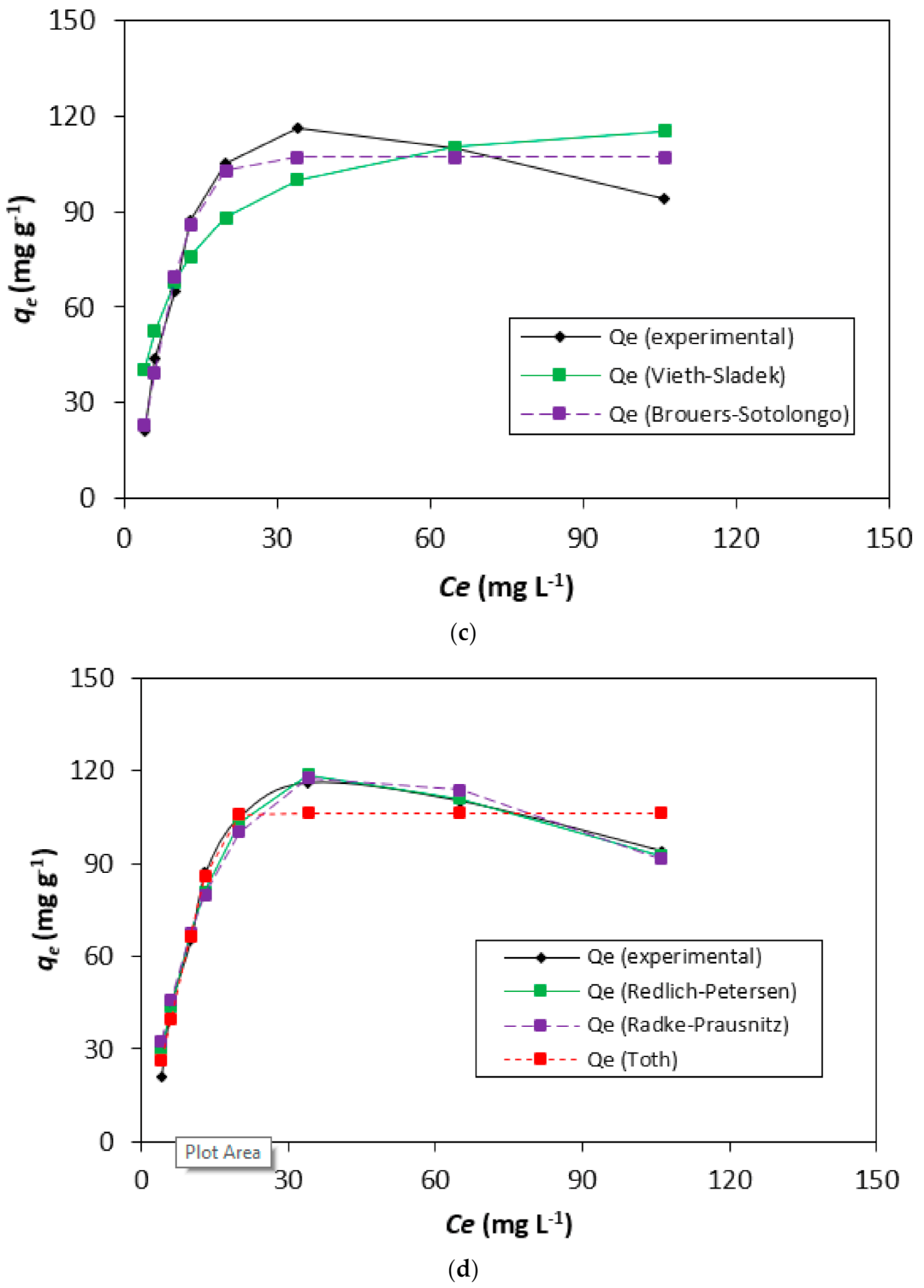
3.3. Adsorption Isotherms-Modeling Analysis
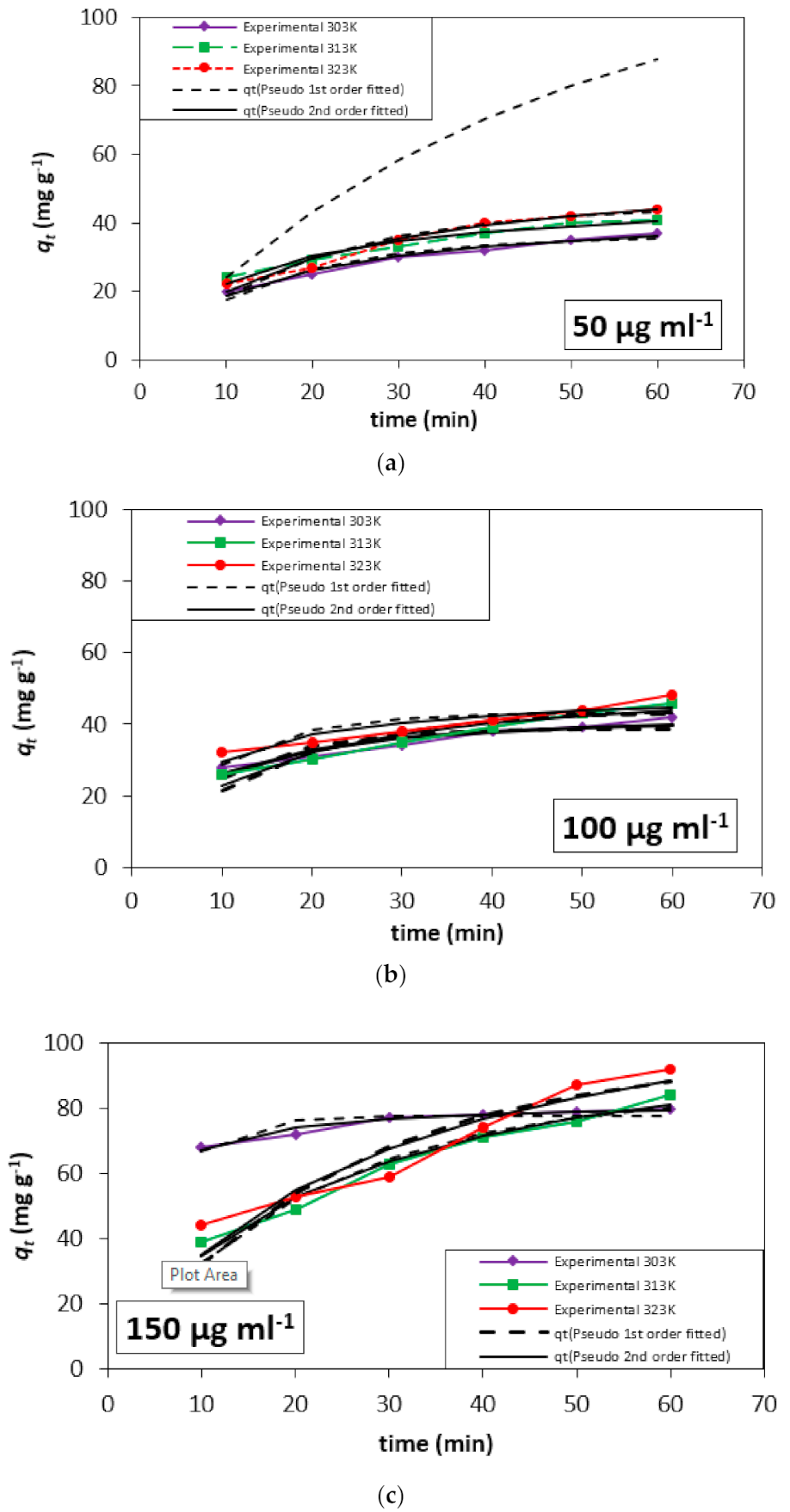
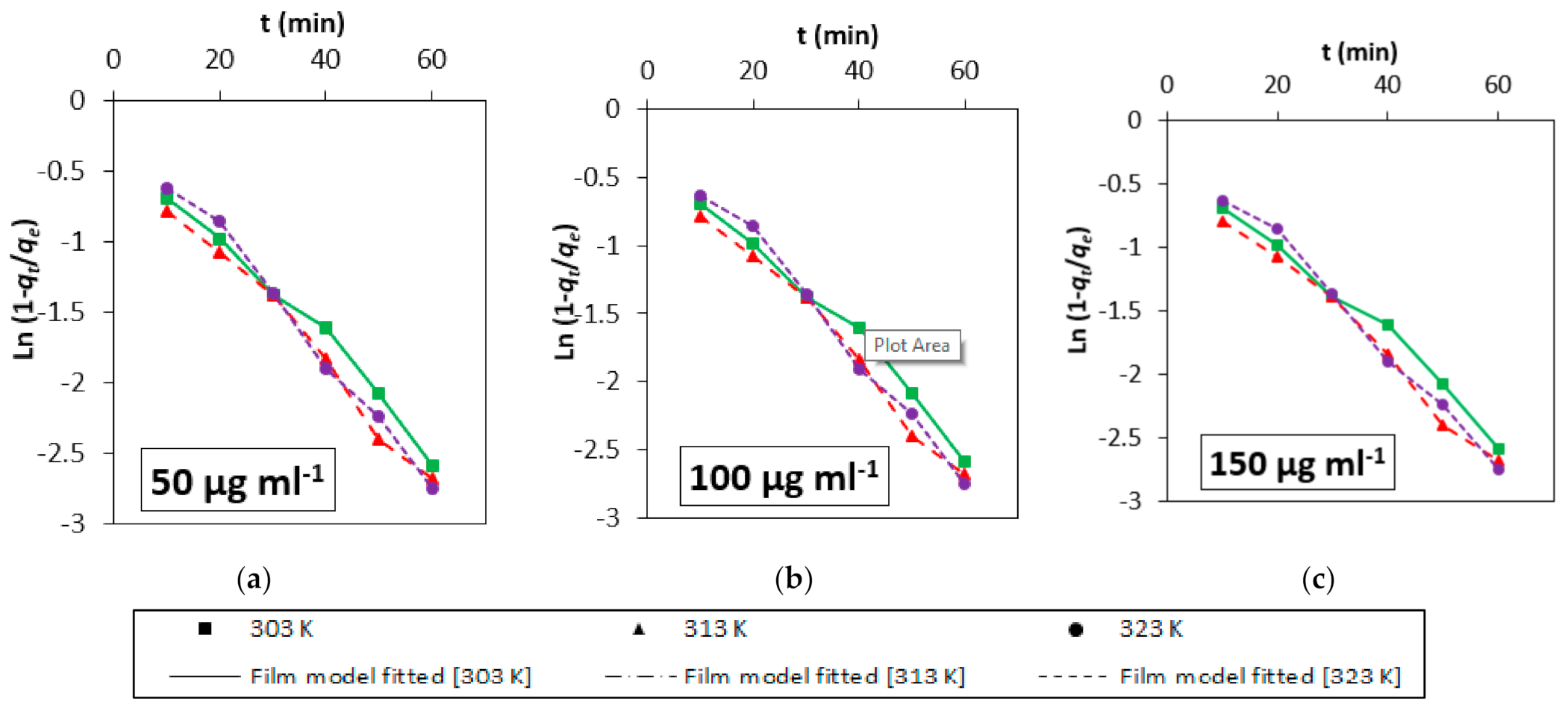
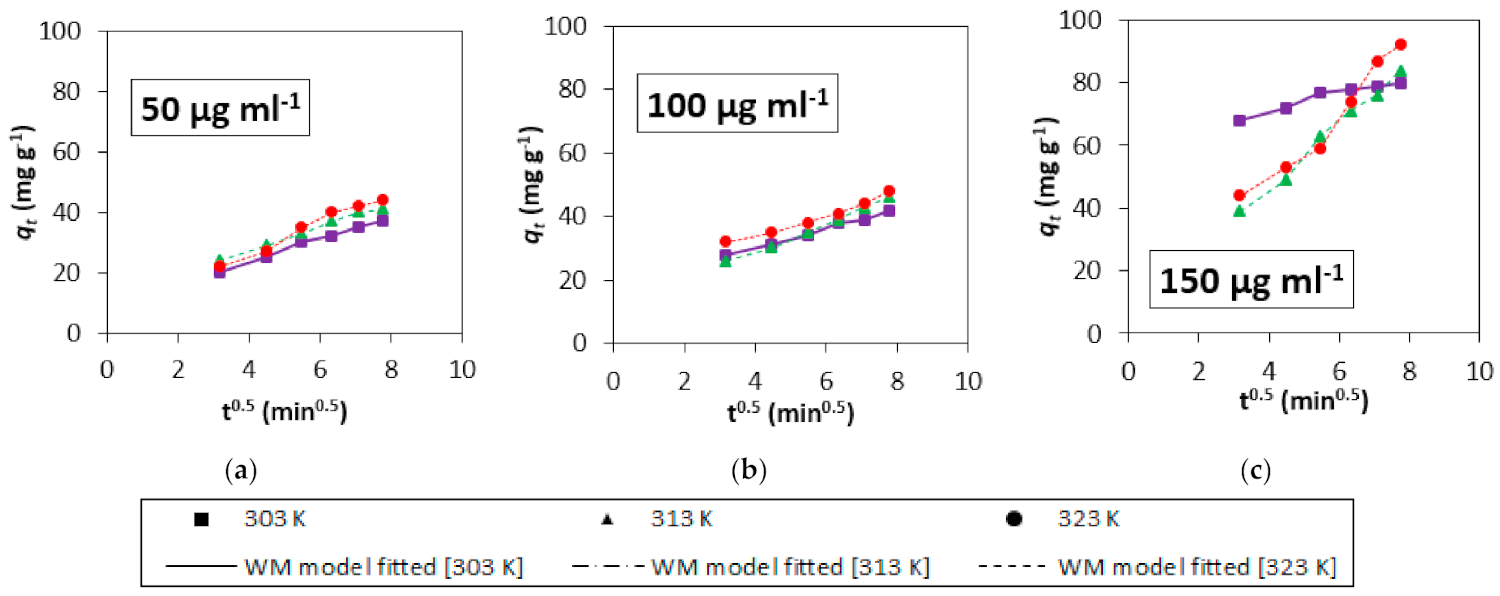
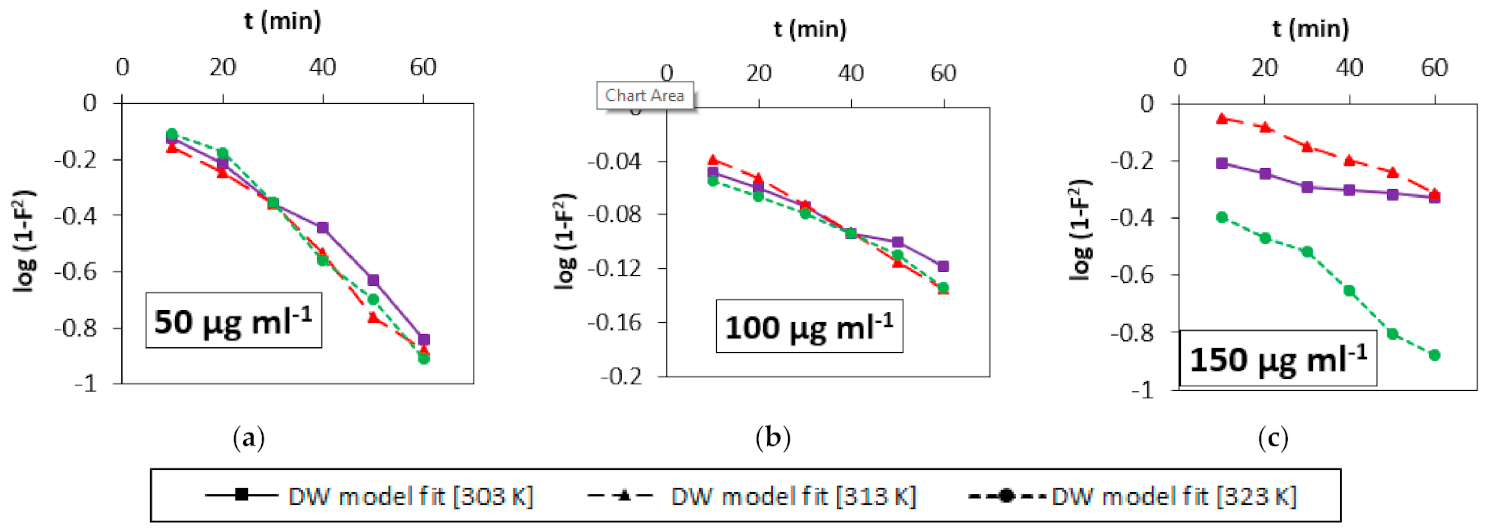
3.4. Adsorption Kinetics
3.5. Mechanistic Study
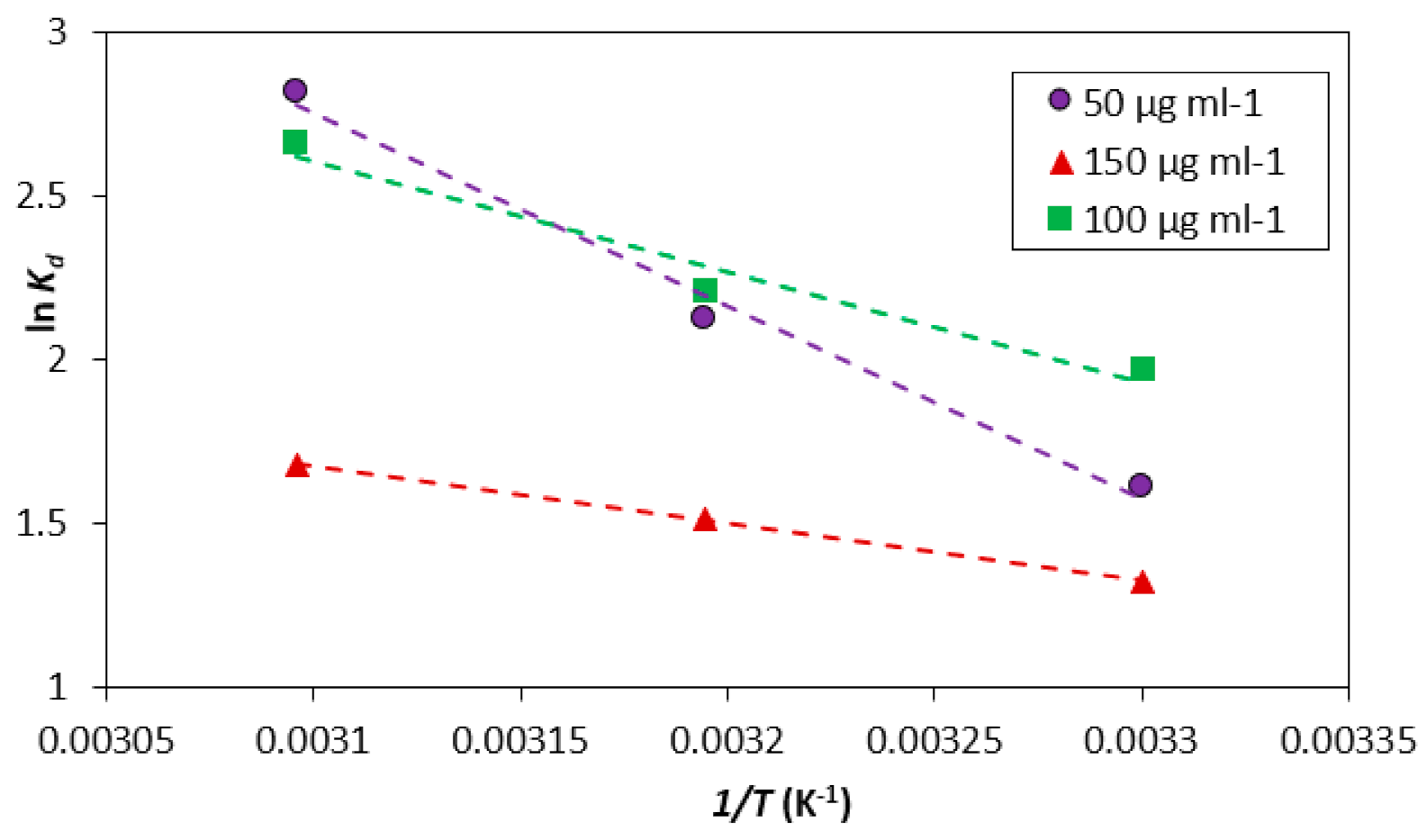
3.6. Thermodynamics of the Adsorption Process
3.7. Process Optimization
3.8. Mechanism of Adsorption of BG Dye onto H.N.T.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sustainable Fabrics Market Information: By Product Type (Organic, Regenerated, Recycled, Natural), Application (Clothing, Furnishing, Medical, Others), and Region—Global Forecast Till 2027. Available online: https://www.marketresearchfuture.com/reports/sustainable-fabrics-market-7435 (accessed on 10 July 2021).
- Anjaneyulu, Y.; Chary, N.S.; Raj, D.S.S. Decolourization of industrial effluents–available methods and emerging technologies–a review. Rev. Environ. Sci. Biotechnol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Tan, Y.; Wang, L.; Shen, M. Decoupling water consumption and environmental impact on textile industry by using water footprint method: A case study in China. Water 2017, 9, 124. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Chequer, F.D.; de Oliveira, G.A.R.; Ferraz, E.R.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Eco-Friendly Textile Dyeing and Finishing; InTech: Rijeka, Croatia, 2013; Volume 6, pp. 151–176. [Google Scholar]
- Püntener, A.; Page, C. European Ban on Certain Azo Dyes, Quality & Environment. Eur. J. 2004, 14, 231–458. Available online: http://www.dyediet.com/wp-content/uploads/2012/01/European-Ban-on-certain-Azo-Dyes.pdf (accessed on 24 August 2021).
- Glavič, P.; Lukman, R. Review of sustainability terms and their definitions. J. Clean. Prod. 2007, 15, 1875–1885. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. BioloSSgical methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Saraf, S.; Vaidya, V.K. Statistical optimization of biosorption of Reactive Orange 13 by dead biomass of Rhizopus arrhizus NCIM 997 using response surface methodology. Int. J. Ind. Chem. 2015, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; Igwegbe, C.A.; Rahdar, S. The application of thermally activated persulfate for degradation of Acid Blue 92 in aqueous solution. Int. J. Ind. Chem. 2019, 10, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kaur, A. Various methods for removal of dyes from industrial effluents-a review. Indian J. Sci. Technol. 2018, 11, 1–21. [Google Scholar] [CrossRef]
- Tabaï, A.; Bechiri, O.; Abbessi, M. Degradation of organic dye using a new homogeneous Fenton-like system based on hydrogen peroxide and a recyclable Dawson-type heteropolyanion. Int. J. Ind. Chem. 2017, 8, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Zauro, S.A.; Vishalakshi, B. Amphoteric gellan gum-based terpolymer–montmorillonite composite: Synthesis, swelling, and dye adsorption studies. Int. J. Ind. Chem. 2017, 8, 345–362. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Li, X.D.; Zhai, Q.Z. Evaluation of eosin Y removal from aqueous solution using nano-mesoporous material MCFs: Adsorption equilibrium, kinetics, and adsorption isotherms. Int. J. Ind. Chem. 2020, 11, 55–67. [Google Scholar] [CrossRef]
- Regti, A.; Laamari, M.R.; Stiriba, S.E.; El Haddad, M. Removal of Basic Blue 41 dyes using Persea americana-activated carbon prepared by phosphoric acid action. Int. J. Ind. Chem. 2017, 8, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Sanghi, R.; Bhattacharya, B. Review on decolorisation of aqueous dye solutions by low cost adsorbents. Color. Technol. 2002, 118, 256–269. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Papegowda, P.K.; Syed, A.A. Isotherm, kinetic and thermodynamic studies on the removal of methylene blue dye from aqueous solution using Saw Palmetto spent. Int. J. Environ. Res. 2017, 11, 91–98. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. A novel sustainable design to develop polypropylene and unsaturated polyester resin polymer composites from waste of major polluting industries and investigation on their physicomechanical and wear properties. Polym. Compos. 2019, 40, 1142–1157. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. Development of sustainable dye adsorption system using nutraceutical industrial fennel seed spent—studies using Congo red dye. Int. J. Phytoremediat. 2017, 19, 686–694. [Google Scholar] [CrossRef]
- Vinokurov, V.; Novikov, A.; Rodnova, V.; Anikushin, B.; Kotelev, M.; Ivanov, E.; Lvov, Y. Cellulose nanofibrils and tubular halloysite as enhanced strength gelation agents. Polymers 2019, 11, 919. [Google Scholar] [CrossRef] [Green Version]
- Oplatowska, M.; Donnelly, R.F.; Majithiya, R.J.; Kennedy, D.G.; Elliott, C.T. The potential for human exposure, direct and indirect, to the suspected carcinogenic triphenylmethane dye Brilliant Green from green paper towels. Food. Chem. Toxicol. 2011, 49, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Kiani, G.; Dostali, M.; Rostami, A.; Khataee, A.R. Adsorption studies on the removal of Malachite Green from aqueous solutions onto halloysite nanotubes. Appl. Clay Sci. 2011, 54, 34–39. [Google Scholar] [CrossRef]
- Dhaif-Allah, M.A.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Kinetic and isotherm modeling for acid blue 113 dye adsorption onto low-cost nutraceutical industrial fenugreek seed spent. Appl. Water Sci. 2020, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. Valorization of nutraceutical industrial coriander seed spent by the process of sustainable adsorption system of Acid Black 52 from aqueous solution. Int. J. Environ. Res. 2019, 13, 639–659. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. Adsorption of Acid Blue 113 from aqueous solution onto nutraceutical industrial coriander seed spent: Isotherm, kinetics, thermodynamics and modeling studies. Desalin. Water Treat. 2019, 153, 321–337. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Baral, S.S.; Das, N.; Chaudhury, G.R.; Das, S.N. A preliminary study on the adsorptive removal of Cr (VI) using seaweed, Hydrilla verticillata. J. Hazard. Mater. 2009, 171, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Sulthana, R.; Taqui, S.N.; Zameer, F.; Syed, U.T.; Syed, A.A. adsorption of ethidium bromide from aqueous solution onto nutraceutical industrial fennel seed spent: Kinetics and thermodynamics modeling studies. Int. J. Phytoremediat. 2018, 20, 1075–1086. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Uber die adsorption in lo sungen. Z. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Jovanović, D.S. Physical adsorption of gases. Colloid Polym. Sci. 1969, 235, 1203–1213. [Google Scholar]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Yakuth, S.A.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Nutraceutical industrial chillies stalk waste as a new adsorbent for the removal of Acid Violet 49 from water and textile industrial effluent: Adsorption isotherms and kinetic models. Desalin. Water Treat. 2019, 155, 94–112. [Google Scholar] [CrossRef] [Green Version]
- Toth, J. State equation of the solid-gas interface layers. Acta Chim. Hung. 1971, 69, 311–328. [Google Scholar]
- Brouers, F.; Sotolongo, O.; Marquez, F.; Pirard, J.P. Microporous and heterogeneous surface adsorption isotherms arising from Levy distributions. Physica A Stat. Mech. Appl. 2005, 349, 271–282. [Google Scholar] [CrossRef]
- Vieth, W.R.; Sladek, K.J. A model for diffusion in a glassy polymer. J. Coll. Sci. 1965, 20, 1014–1033. [Google Scholar] [CrossRef]
- Radke, C.J.; Prausnitz, J.M. Adsorption of organic solutes from dilute aqueous solution of activated carbon. Ind. Eng. Chem. Fundam. 1972, 11, 445–451. [Google Scholar] [CrossRef]
- Redlich, O.J.D.L.; Peterson, D.L. A useful adsorption isotherm. J. Phy. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Chen, J.L.; Zhai, Z.C. Study on thermodynamics and kinetics of adsorption of p-toluidine from aqueous solution by hypercrosslinked polymeric adsorbents. Environ. Chem. 2004, 23, 192–196. [Google Scholar]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Noto, R.; Riela, S. Halloysite nanotubes: A green resource for materials and life sciences. Rend. Fis. Acc. Lincei 2020, 31, 213–221. [Google Scholar] [CrossRef]
- Saki, H.; Alemayehu, E.; Schomburg, J.; Lennartz, B. Halloysite nanotubes as adsorptive material for phosphate removal from aqueous solution. Water 2019, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- Dhaif-Allah, M.A.H.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Development of sustainable acid blue 113 dye adsorption system using nutraceutical industrial Tribulus terrestris spent. SN Appl. Sci. 2019, 1, 330. [Google Scholar] [CrossRef] [Green Version]
- Bagherzadeh, S.A.; D’Orazio, A.; Karimipour, A.; Goodarzi, M.; Bach, Q.V. A novel sensitivity analysis model of EANN for F-MWCNTs-Fe3O4/EG nanofluid thermal conductivity: Outputs predicted analytically instead of numerically to more accuracy and less costs. Phys. A-Stat. Mech. Appl. 2019, 521, 406–415. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Safaei, M.R.; Goodarzi, M.; Alrashed, A.; Nguyen, T.K. New temperature, interfacial shell dependent dimensionless model for thermal conductivity of nanofluids. Int. J. Heat Mass Transf. 2017, 114, 207–210. [Google Scholar] [CrossRef]
- Peng, Y.; Khaled, U.; Al-Rashed, A.A.; Meer, R.; Goodarzi, M.; Sarafraz, M.M. Potential application of Response Surface Methodology (RSM) for the prediction and optimization of thermal conductivity of aqueous CuO (II) nanofluid: A statistical approach and experimental validation. Phys. A: Stat. Mech. Appl. 2020, 554, 124353. [Google Scholar] [CrossRef]
- Wu, H.; Bagherzadeh, S.A.; D’Orazio, A.; Habibollahi, N.; Karimipour, A.; Goodarzi, M.; Bach, Q.V. Present a new multi objective optimization statistical Pareto frontier method composed of artificial neural network and multi objective genetic algorithm to improve the pipe flow hydrodynamic and thermal properties such as pressure drop and heat transfer coefficient for non-Newtonian binary fluids. Phys. A Stat. Mech. Appl. 2019, 535, 122409. [Google Scholar]
- Peng, Y.; Parsian, A.; Khodadadi, H.; Akbari, M.; Ghani, K.; Goodarzi, M.; Bach, Q.V. Develop optimal network topology of artificial neural network (AONN) to predict the hybrid nanofluids thermal conductivity according to the empirical data of Al2O3–Cu nanoparticles dispersed in ethylene glycol. Phys. A Stat. Mech. Appl. 2020, 549, 124015. [Google Scholar] [CrossRef]
- Mehrdad, S.; Dadsetani, R.; Amiriyoon, A.; Leon, A.S.; Reza Safaei, M.; Goodarzi, M. Exergo-economic optimization of organic rankine cycle for saving of thermal energy in a sample power plant by using of strength pareto evolutionary algorithm II. Processes 2020, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Taqui, S.N.; Mohan, C.S.; Khatoon, B.A.; Soudagar, M.E.M.; Khan, T.Y.; Mujtaba, M.A.; Ahmed, W.; Elfasakhany, A.; Kumar, R.; Pruncu, C.I. Sustainable adsorption method for the remediation of malachite green dye using nutraceutical industrial fenugreek seed spent. Biomass. Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Taqui, S.N.; Mohan, C.S.; Goodarzi, M.S.; Elkotb, M.A.; Khatoon, B.A.; Soudagar, M.E.M.; Koki, I.B.; Elfasakhany, A.; Khalifa, A.S.; Ali, M.A.; et al. Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent. Appl. Sci. 2021, 11, 7635. [Google Scholar] [CrossRef]
- Kamble, R.; Ghag, M.; Gaikawad, S.; Panda, B.K. Halloysite nanotubes and applications: A review. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Mitra, G.B.; Bhattacherjee, S. The structure of halloysite. Acta Crystallogr. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 2851–2857. [Google Scholar] [CrossRef]
- Shu, Z.; Chen, Y.; Zhou, J.; Li, T.; Yu, D.; Wang, Y. Nanoporous-walled silica and alumina nanotubes derived from halloysite: Controllable preparation and their dye adsorption applications. Appl. Clay Sci. 2015, 112, 17–24. [Google Scholar] [CrossRef]
| Feature | Variable | Dimensional Value | Valuemin | Valuemax |
|---|---|---|---|---|
| A | Duration | minute | 0 | 180 |
| B | Temperature | °C | 27 | 50 |
| C | Concentration | mgL−1 | 25 | 200 |
| D | Adsorbent quantity | gL−1 | 0.5 | 6.0 |
| E | pH | - | 2 | 12 |
| Two-Parameter Isotherms | Three-Parameter Isotherms | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir | Freundlich | Jovanovic | Dubinin- Radushkevich | Toth | Brouers-Sotolongo | Vieth-Sladek | Radke-Prausnitz | Redlich-Peterson | |||||||||
| Qm | 124.2 | KF | 37.9 | Qm | 109.7 | Qs | 110.6 | Qm | 106.4 | Qm | 107.1 | Qm | 124.2 | Qm | 774.8 | ARP | 7.8 |
| KS | 0.12 | nF | 4.06 | KJ | 0.09 | Kad | 6.43 × 10−6 | nT0 | 12.50 | KBS | 0.02 | KVS | 1 × 10−7 | krp | 0.01 | BRP | 0.01 |
| bT0 | 1.13 × 1015 | α | 1.61 | βVS | 0.12 | mrp | 2.89 | g | 1.64 | ||||||||
| Isotherms | Langmuir | Freundlich | Jovanovic | Dubinin-Radushkevich | Toth | Brouers-Sotolongo | Vieth-Sladek | Radke-Prausnitz | Redlich-Peterson |
|---|---|---|---|---|---|---|---|---|---|
| SSE | 1581.8 | 3506.7 | 777.9 | 544.4 | 311.1 | 311.8 | 1581.8 | 244.5 | 140.7 |
| χ2 | 31.28 | 77.08 | 12.84 | 7.82 | 4.47 | 3.61 | 31.29 | 7.70 | 4.63 |
| R2 | 0.91 | 0.92 | 0.99 | 0.94 | 0.96 | 0.96 | 0.82 | 0.98 | 0.98 |
| Initial Concentration (µg mL−1) | Temp (K) | qe, expt (mg g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe, pred (mg g−1) | K1 | R2 | χ2 | qe, pred (mg g−1) | K2 | R2 | Χ2 | |||
| 50 | 303 | 40 | 35.96 | 6.63 × 10−2 | 0.94 | 0.59 | 44.26 | 1.64 × 10−3 | 0.97 | 3.94 |
| 313 | 44 | 117.53 | 2.28 × 10−2 | 1.00 | 149.57 | 48.08 | 1.79 × 10−3 | 0.96 | 0.32 | |
| 323 | 47 | 45.09 | 5.32 × 10−2 | 0.96 | 0.80 | 57.85 | 9.04 × 10−4 | 0.97 | 0.48 | |
| 100 | 303 | 86 | 38.86 | 1.02 × 10−1 | 0.91 | 8.51 | 44.68 | 3.12 × 10−3 | 0.91 | 0.48 |
| 313 | 89 | 43.90 | 4.39 × 10−1 | 0.93 | 1.54 | 65.88 | 1.71 × 10−3 | 0.93 | 1.65 | |
| 323 | 93 | 43.32 | 1.07 × 10−1 | 0.94 | 1.58 | 49.70 | 2.94 × 10−3 | 0.94 | 0.76 | |
| 150 | 303 | 110 | 77.79 | 1.95 × 10−1 | 0.91 | 0.36 | 82.43 | 5.33 × 10−3 | 0.95 | 0.07 |
| 313 | 117 | 85.27 | 4.76 × 10−2 | 0.94 | 1.65 | 111.58 | 4.01 × 10−4 | 0.97 | 0.92 | |
| 323 | 122 | 96.90 | 4.04 × 10−2 | 0.96 | 4.99 | 127.72 | 2.95 × 10−4 | 0.90 | 3.61 | |
| Initial Concentration (µg mL−1) | Temp (K) | Film Diffusion Model | Weber-Morris Model | Dumwald-Wagner | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R (min−1) | R2 | χ2 | Kist (mg g−1 s−0.5) | R2 | χ2 | K (min−1) | R2 | χ2 | ||
| 50 | 303 | 0.0372 | 0.98 | 0.511 | 3.73 | 0.99 | 0.050 | 0.032 | 0.97 | 0.057 |
| 313 | 0.0397 | 0.99 | 0.682 | 3.88 | 0.99 | 0.045 | 0.035 | 0.98 | 0.045 | |
| 323 | 0.0437 | 0.99 | 0.111 | 5.12 | 0.97 | 0.273 | 0.038 | 0.98 | 0.178 | |
| 100 | 303 | 0.0055 | 0.99 | 5.158 | 3.08 | 0.98 | 0.056 | 0.032 | 0.99 | 0.201 |
| 313 | 0.0078 | 0.99 | 2.191 | 4.49 | 0.99 | 0.078 | 0.005 | 0.99 | 0.030 | |
| 323 | 0.0060 | 0.99 | 5.121 | 3.42 | 0.97 | 0.117 | 0.004 | 0.98 | 0.192 | |
| 150 | 303 | 0.0066 | 0.90 | 4.282 | 2.67 | 0.94 | 0.086 | 0.005 | 0.90 | 2.156 |
| 313 | 0.0171 | 0.99 | 0.762 | 9.95 | 0.99 | 0.204 | 0.012 | 0.99 | 0.387 | |
| 323 | 0.0202 | 0.96 | 0.477 | 11.09 | 0.96 | 1.314 | 0.023 | 0.97 | 0.907 | |
| Initial Concentration | Temp | ΔG° | ΔS° | ΔH° | ln A | Ea |
|---|---|---|---|---|---|---|
| (µg mL−1) | (K) | (kJ mol-1) | (J mol−1 K−10) | (kJ mol−1) | (kJ mol−1) | |
| 50 | 303 | −6.54 | 486.19 | 120.45 | 2.90 | 38.33 |
| 313 | −6.37 | |||||
| 323 | −6.15 | |||||
| 100 | 303 | −5.95 | 645.33 | 233.61 | 4.51 | 59.69 |
| 313 | −5.74 | |||||
| 323 | −5.14 | |||||
| 150 | 303 | −3.93 | 862.91 | 432.70 | 6.46 | 73.76 |
| 313 | −3.62 | |||||
| 323 | −3.31 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 60,415.24 | 13 | 4647.33 | 30.12 | <0.001 |
| A | 18,483.5 | 1 | 18,483.5 | 119.8 | <0.0001 |
| B | 66.8 | 1 | 66.8 | 0.4 | 0.5128 |
| C | 17,876.0 | 1 | 17,876.0 | 115.8 | <0.0001 |
| D | 6660.9 | 1 | 6660.9 | 43.2 | <0.0001 |
| E | 479.8 | 1 | 479.8 | 3.1 | 0.0821 |
| AB | 217.6 | 1 | 217.6 | 1.4 | 0.2389 |
| AC | 1176.8 | 1 | 1176.8 | 7.6 | 0.0073 |
| BC | 1.32 | 1 | 1.3 | 0.0086 | 0.9265 |
| A2 | 736.5 | 1 | 736.5 | 4.8 | 0.0322 |
| B2 | 100.7 | 1 | 100.7 | 0.7 | 0.4218 |
| C2 | 223.0 | 1 | 223.0 | 1.4 | 0.2333 |
| D2 | 2437.6 | 1 | 2437.6 | 15.8 | 0.0002 |
| E2 | 494.3 | 1 | 494.3 | 3.2 | 0.0777 |
| F2 | 552.6 | 1 | 736.5 | 4.8 | 0.0322 |
| Residual | 10,955.8 | 71 | 154.3 | ||
| Total | 71,371.0 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukkund, S.J.; Puthiyillam, P.; Alshehri, H.M.; Goodarzi, M.; Taqui, S.N.; Anqi, A.E.; Safaei, M.R.; Ali, M.A.; Syed, U.T.; Mir, R.A.; et al. Adsorption Method for the Remediation of Brilliant Green Dye Using Halloysite Nanotube: Isotherm, Kinetic and Modeling Studies. Appl. Sci. 2021, 11, 8088. https://doi.org/10.3390/app11178088
Ukkund SJ, Puthiyillam P, Alshehri HM, Goodarzi M, Taqui SN, Anqi AE, Safaei MR, Ali MA, Syed UT, Mir RA, et al. Adsorption Method for the Remediation of Brilliant Green Dye Using Halloysite Nanotube: Isotherm, Kinetic and Modeling Studies. Applied Sciences. 2021; 11(17):8088. https://doi.org/10.3390/app11178088
Chicago/Turabian StyleUkkund, Shareefraza J., Prasad Puthiyillam, Hashim M. Alshehri, Marjan Goodarzi, Syed Noeman Taqui, Ali E. Anqi, Mohammad Reza Safaei, Masood Ashraf Ali, Usman Taqui Syed, Rayees Afzal Mir, and et al. 2021. "Adsorption Method for the Remediation of Brilliant Green Dye Using Halloysite Nanotube: Isotherm, Kinetic and Modeling Studies" Applied Sciences 11, no. 17: 8088. https://doi.org/10.3390/app11178088
APA StyleUkkund, S. J., Puthiyillam, P., Alshehri, H. M., Goodarzi, M., Taqui, S. N., Anqi, A. E., Safaei, M. R., Ali, M. A., Syed, U. T., Mir, R. A., Elfasakhany, A., Eed, E. M., Siddiqui, M. I. H., Mokashi, I., & Soudagar, M. E. M. (2021). Adsorption Method for the Remediation of Brilliant Green Dye Using Halloysite Nanotube: Isotherm, Kinetic and Modeling Studies. Applied Sciences, 11(17), 8088. https://doi.org/10.3390/app11178088











