Glassy Carbon Electrochemical Sensor for Gallic and Vanillic Acid Detection in Aqueous Solutions
Abstract
1. Introduction
2. Experiments
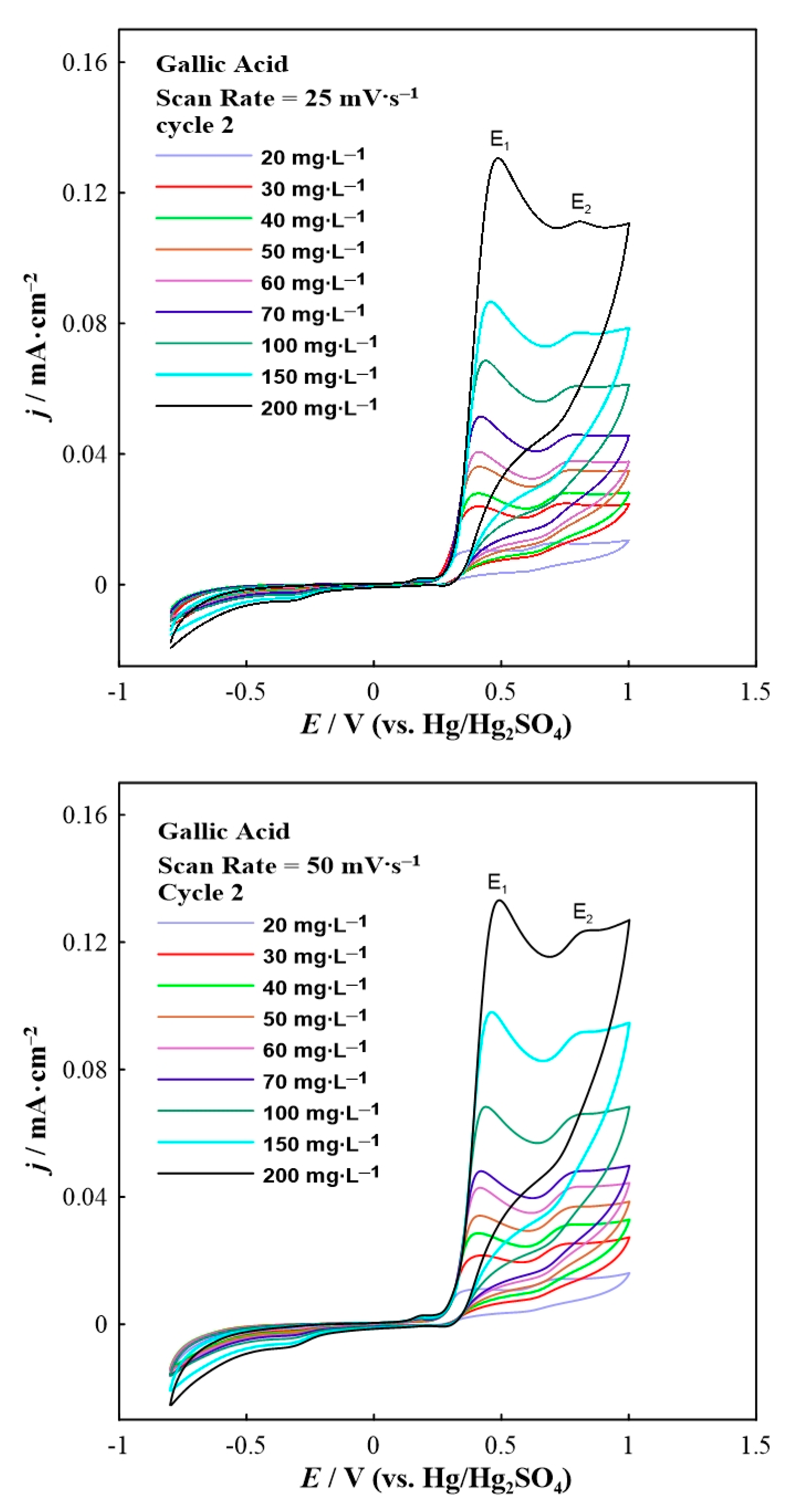
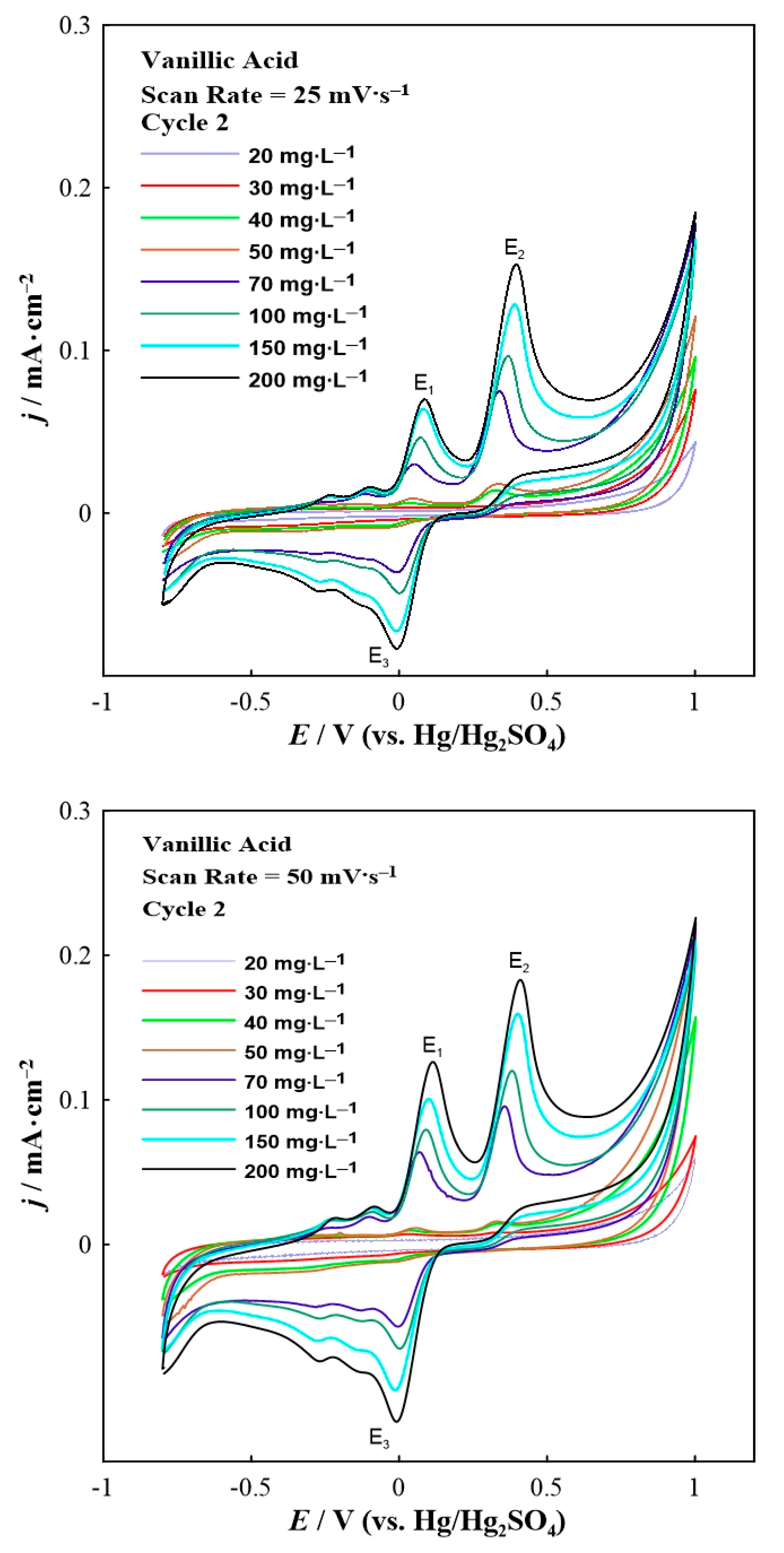
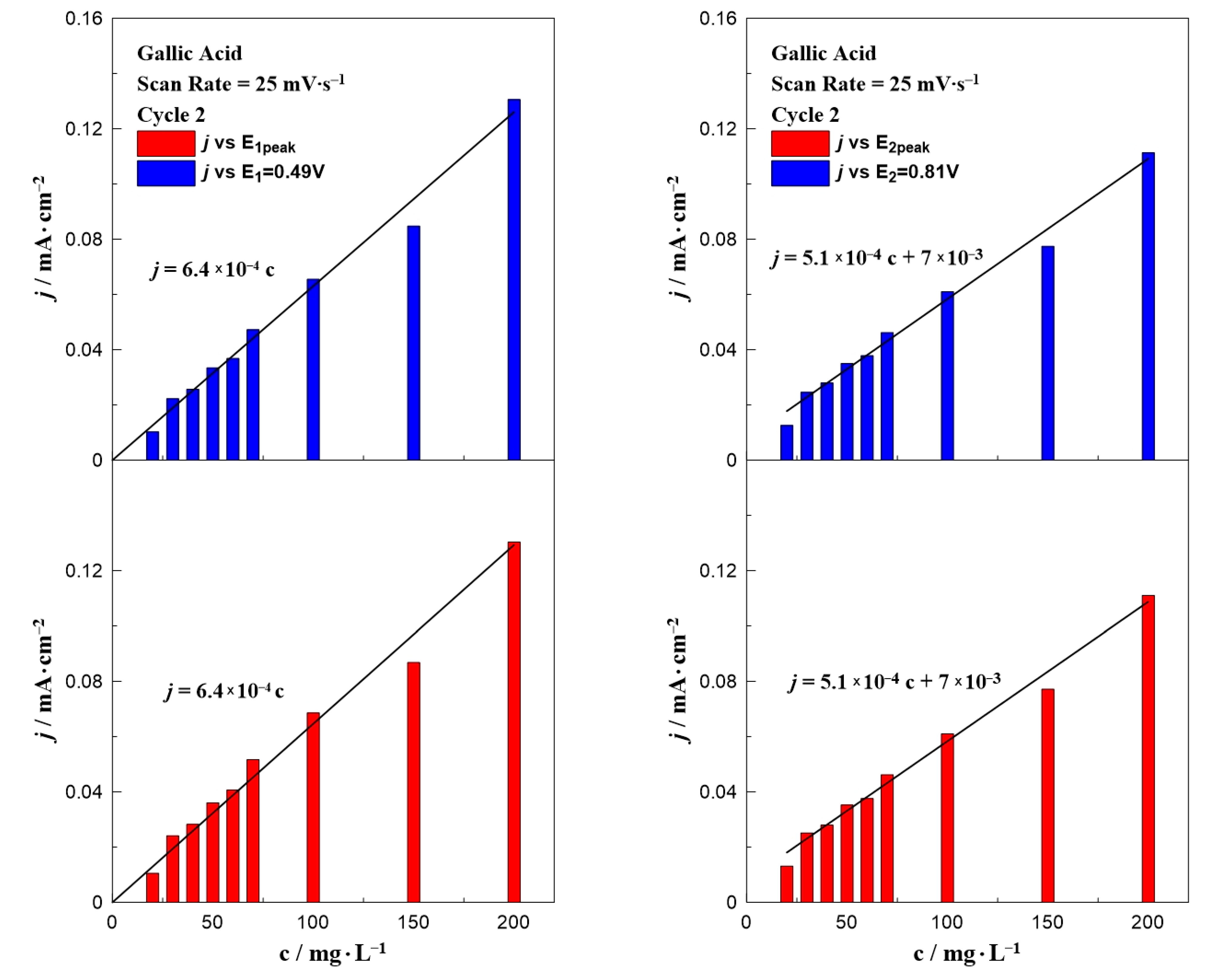
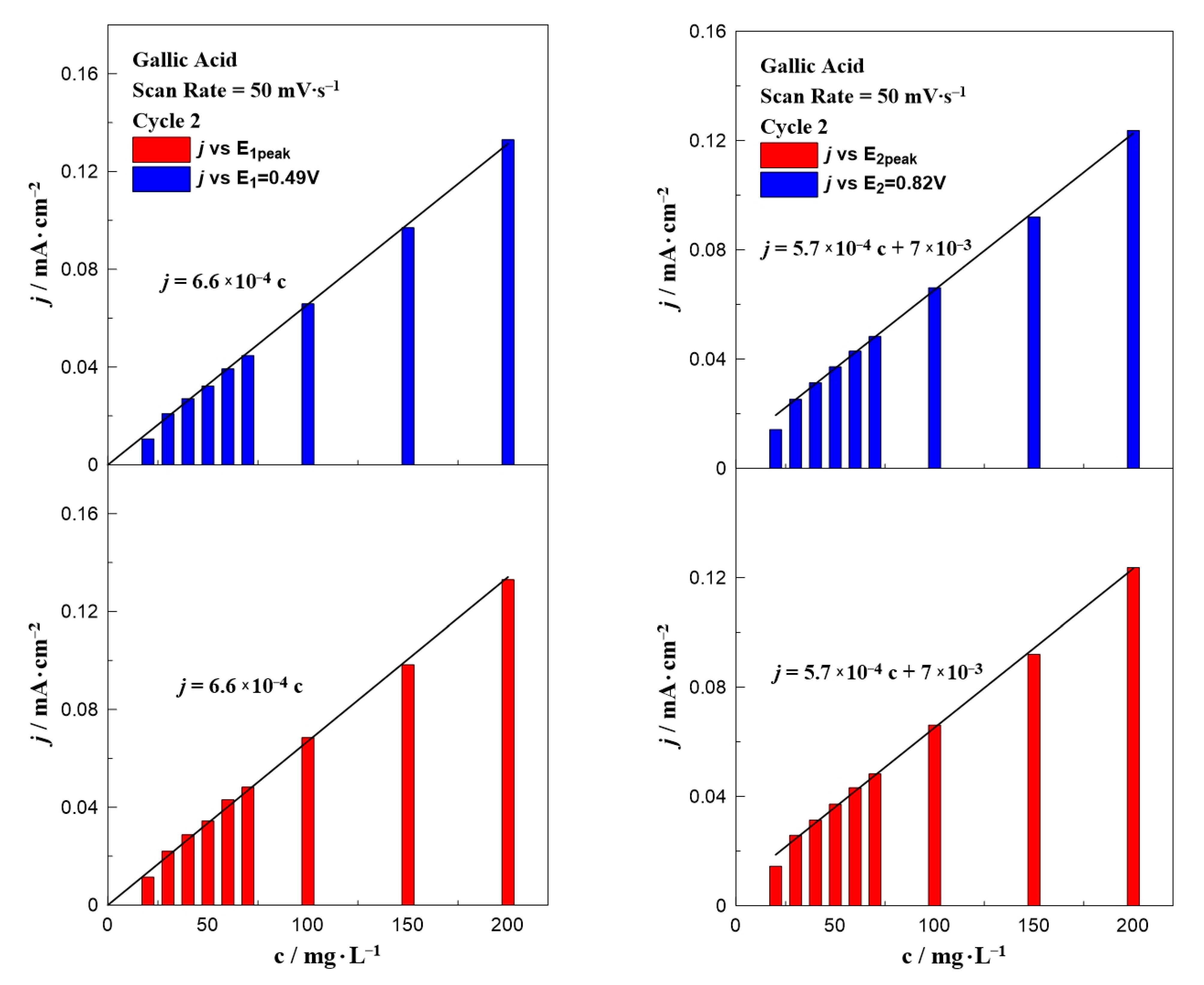
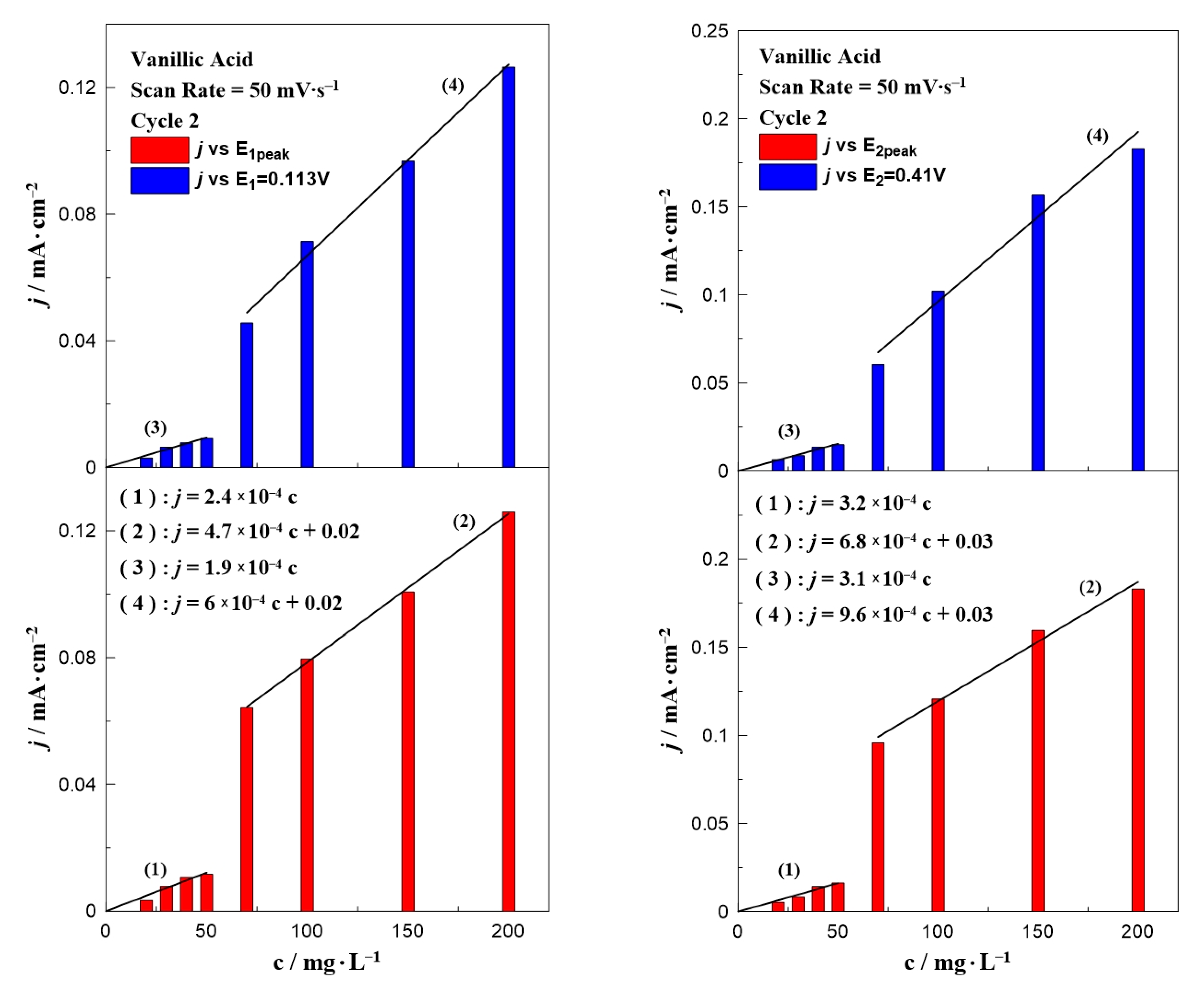
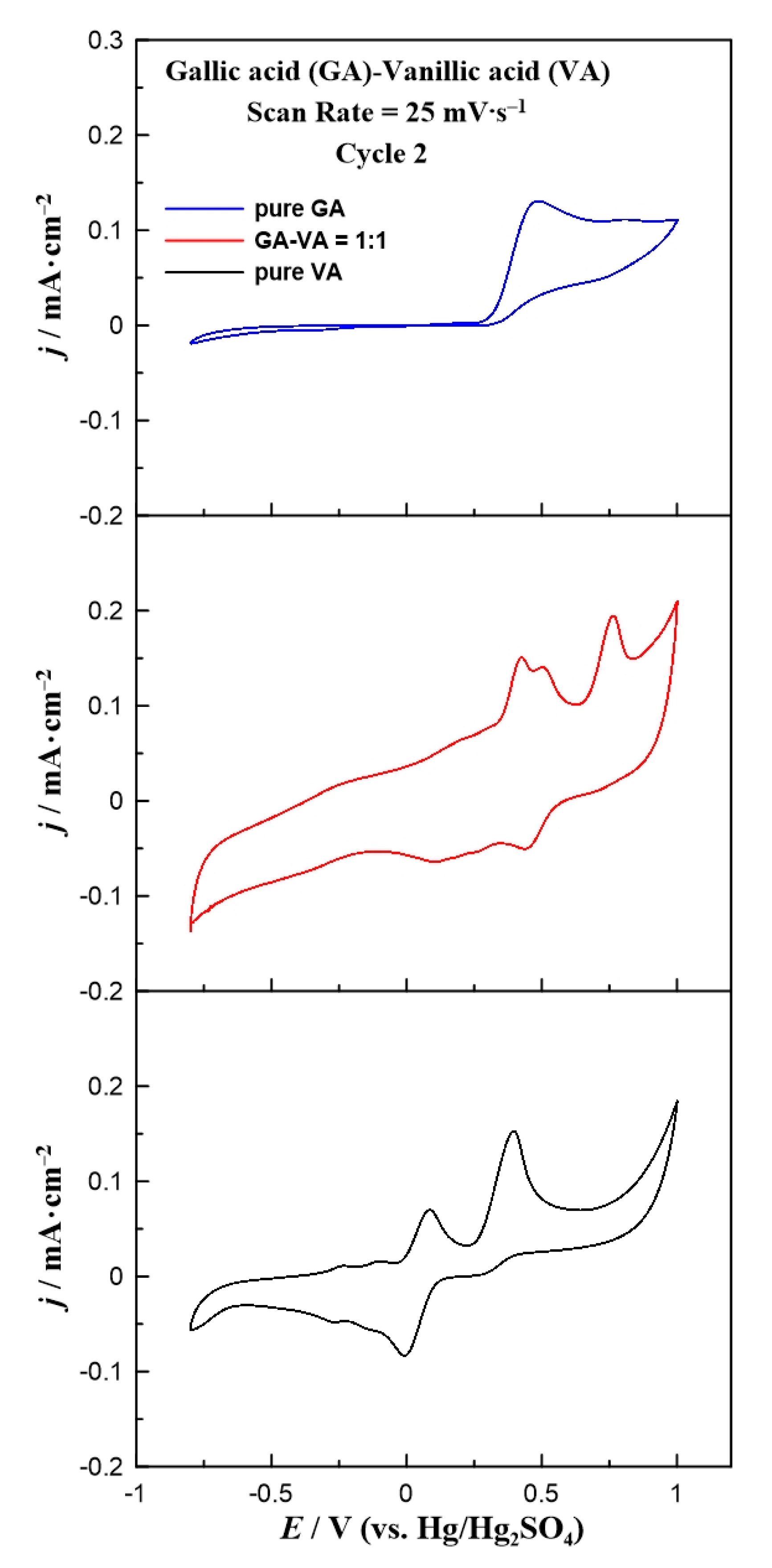
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Clarke, C. Nanostructured chemiresistive gas sensors for medical applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef]
- Janata, J. Introduction: Modern topics in chemical sensing. Chem. Rev. 2008, 108, 327–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catterall, R. Chemical Sensors; Oxford University Press: Oxford, UK, 1997; pp. 4–30. [Google Scholar] [CrossRef]
- Monea, B.F.; Ionete, E.I.; Spiridon, S.I.; Ion-Ebrasu, D.; Petre, E. Carbon nanotubes and carbon nanotube structures used for temperature measurement. Sensors 2019, 19, 2464. [Google Scholar] [CrossRef]
- Tundis, A.; Faizan, A.; Mühlhäuser, M. A feature-based model for the identification of electrical devices in smart environments. Sensors 2019, 19, 2611. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.C.; Silva, A.F. Smart Devices: Micro- and Nanosensors; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007464. [Google Scholar]
- Simões, F.R.; Xavier, M.G. Electrochemical Sensors; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323497800. [Google Scholar]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Napolitano-Tabares, P.I.; Negrín-Santamaría, I.; Gutiérrez-Serpa, A.; Pino, V. Recent efforts to increase greenness in chromatography. Curr. Opin. Green Sustain. Chem. 2021, 32, 100536–100547. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef]
- Pelle, F.D.; Compagnone, D. Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Anodic oxidation of phenol for waste water treatment. J. Appl. Electrochem. 1991, 21, 703–708. [Google Scholar] [CrossRef]
- Iotov, P.I.; Kalcheva, S.V. Mechanistic approach to the oxidation of phenol at a platinum/gold electrode in an acid medium. J. Electroanal. Chem. 1998, 442, 19–26. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T.; Piotrowska, G. Electrooxidation of phenol on PtRh and PtRu alloys in 0.1 M NaOH solution. Int. J. Electrochem. Sci. 2015, 10, 2432–2438. [Google Scholar]
- Hart, J.P.; Crew, A.; Crouch, E.; Honeychurch, K.C.; Pemberton, R.M. Some Recent Designs and Developments of Screen-Printed Carbon Electrochemical Sensors/Biosensors for Biomedical, Environmental, and Industrial Analyses. Anal. Lett. 2004, 37, 789–830. [Google Scholar] [CrossRef]
- Gattrell, M.; Kirk, D.W. The Electrochemical Oxidation of Aqueous Phenol Carbon Electrode. Can. J. Chem. Eng. 1990, 68, 997–1003. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Mathiyarasu, J.; Joseph, J.; Phani, K.L.N.; Yegnaraman, V. Electrochemical detection of phenol in aqueous solutions. Indian J. Chem. Technol. 2004, 11, 797–803. [Google Scholar]
- Govindhan, M.; Lafleur, T.; Adhikari, B.R.; Chen, A. Electrochemical Sensor Based on Carbon Nanotubes for the Simultaneous Detection of Phenolic Pollutants. Electroanalysis 2015, 27, 902–909. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.L.; Liang, Y.M.; Liu, W. A graphene-based electrochemical sensor for rapid determination of phenols in water. Sensors 2013, 13, 6204. [Google Scholar] [CrossRef]
- Chand, R.; Molina, R.; Johnson, I.; Hans, A.; Bremner, D.H. Activated carbon cloth: A potential adsorbing/oxidizing catalyst for phenolic wastewater. Water Sci. Technol. 2010, 61, 2817–2823. [Google Scholar] [CrossRef]
- Dedelaite, L.; Kizilkaya, S.; Incebay, H.; Ciftci, H.; Ersoz, M.; Yazicigil, Z.; Oztekin, Y.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical determination of Cu(II) ions using glassy carbon electrode modified by some nanomaterials and 3-nitroaniline. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 279–284. [Google Scholar] [CrossRef]
- Oztekin, Y.; Yazicigil, Z.; Ramanaviciene, A.; Ramanavicius, A. Polyphenol-modified glassy carbon electrodes for copper detection. Sens. Actuators B Chem. 2011, 152, 37–48. [Google Scholar] [CrossRef]
- Oztekin, Y.; Yazicigil, Z.; Solak, A.O.; Ustundag, Z.; Okumus, A.; Kilic, Z.; Ramanaviciene, A.; Ramanavicius, A. Phenanthroline derivatives electrochemically grafted to glassy carbon for Cu(II) ion detection. Sens. Actuators B Chem. 2012, 166–167, 117–127. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Romeo, R.; Cassano, A. Concentration of bioactive phenolic compounds in olive mill wastewater by direct contact membrane distillation. Molecules 2021, 26, 1808. [Google Scholar] [CrossRef]
- Hussain, A.; Dubey, S.K.; Kumar, V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour. Ind. 2015, 11, 81–90. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Electrochemical behavior of antioxidants: I. Mechanistic study on electrochemical oxidation of gallic acid in aqueous solutions at glassy-carbon electrode. J. Electroanal. Chem. 2011, 657, 107–112. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Apetrei, C.; Apetrei, I.M.; de Saja, J.A.; Rodriguez-Mendez, M.L. Carbon paste electrodes made from different carbonaceous materials: Application in the study of antioxidants. Sensors 2011, 11, 1328–1344. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, S.; Zhu, H. Investigation on the electrochemical behaviors of phenol oxidation on GCE (Glassy Carbon Electrode). Adv. Mater. Res. 2013, 652–654, 1684–1687. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript.. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagoraios, D.; Ioakeimidis, C.; Kyriakou, G.; Katsaounis, A. Glassy Carbon Electrochemical Sensor for Gallic and Vanillic Acid Detection in Aqueous Solutions. Appl. Sci. 2021, 11, 8045. https://doi.org/10.3390/app11178045
Zagoraios D, Ioakeimidis C, Kyriakou G, Katsaounis A. Glassy Carbon Electrochemical Sensor for Gallic and Vanillic Acid Detection in Aqueous Solutions. Applied Sciences. 2021; 11(17):8045. https://doi.org/10.3390/app11178045
Chicago/Turabian StyleZagoraios, Dimitrios, Charis Ioakeimidis, Georgios Kyriakou, and Alexandros Katsaounis. 2021. "Glassy Carbon Electrochemical Sensor for Gallic and Vanillic Acid Detection in Aqueous Solutions" Applied Sciences 11, no. 17: 8045. https://doi.org/10.3390/app11178045
APA StyleZagoraios, D., Ioakeimidis, C., Kyriakou, G., & Katsaounis, A. (2021). Glassy Carbon Electrochemical Sensor for Gallic and Vanillic Acid Detection in Aqueous Solutions. Applied Sciences, 11(17), 8045. https://doi.org/10.3390/app11178045







