Piezoelectric Silicon Micropump for Drug Delivery Applications
Abstract
1. Introduction
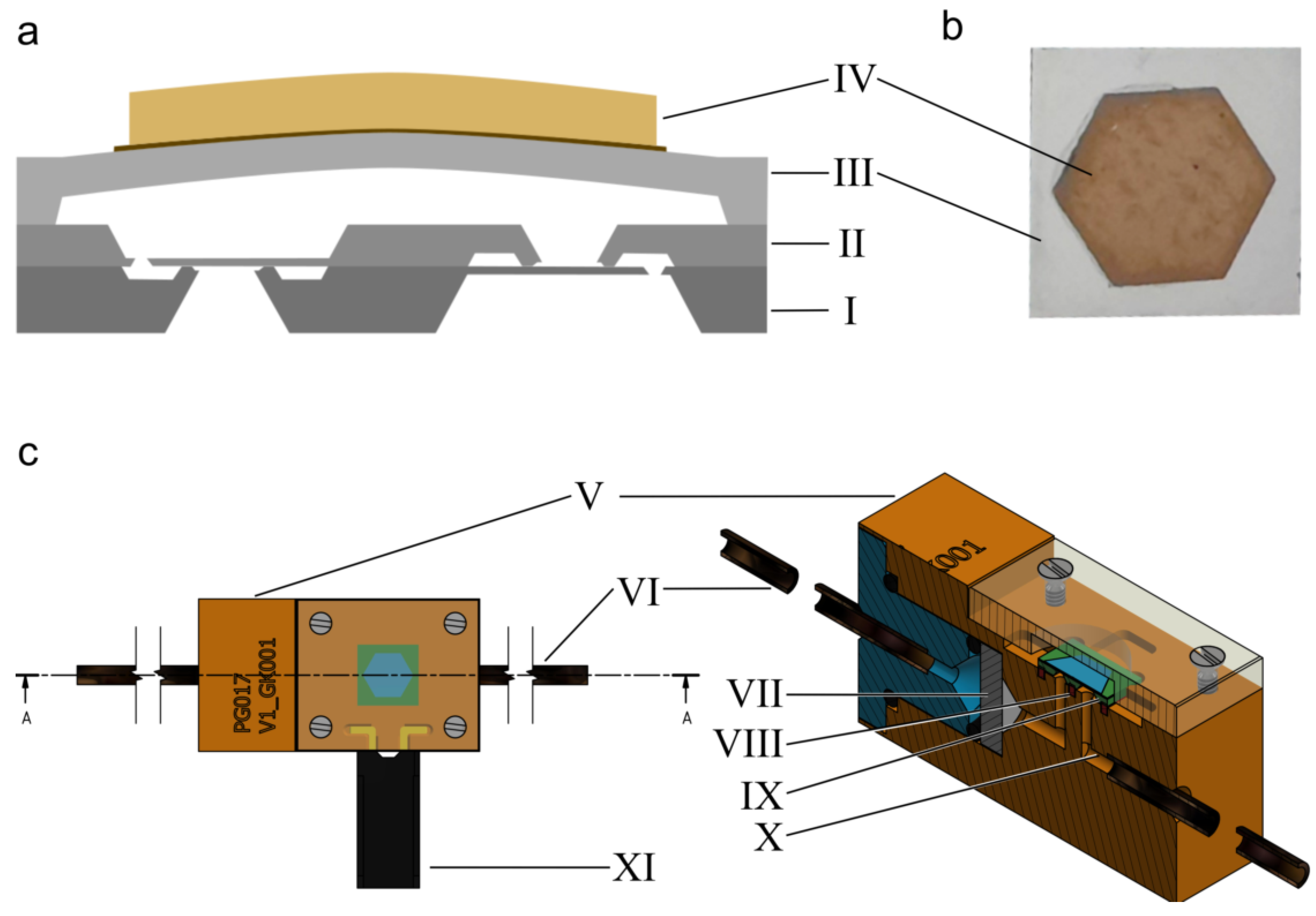
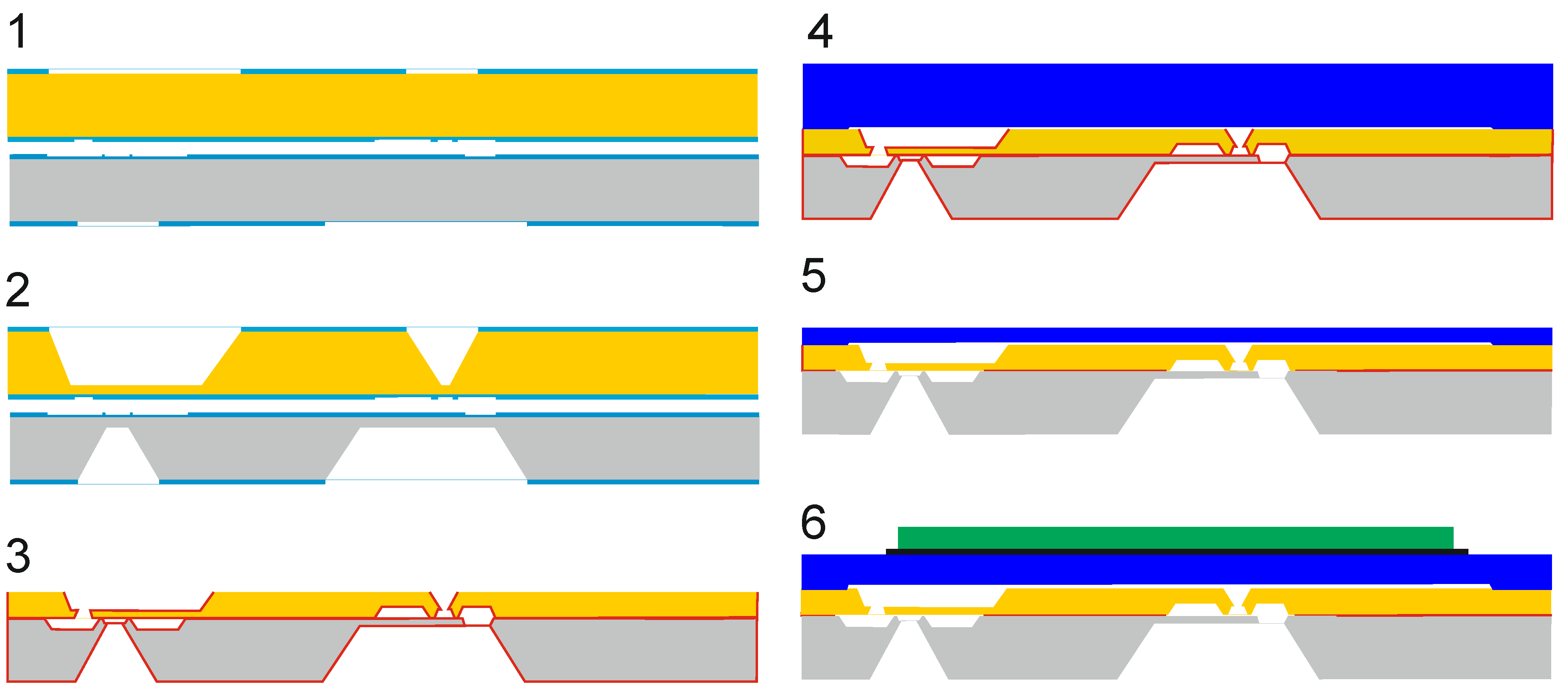
2. Materials and Methods
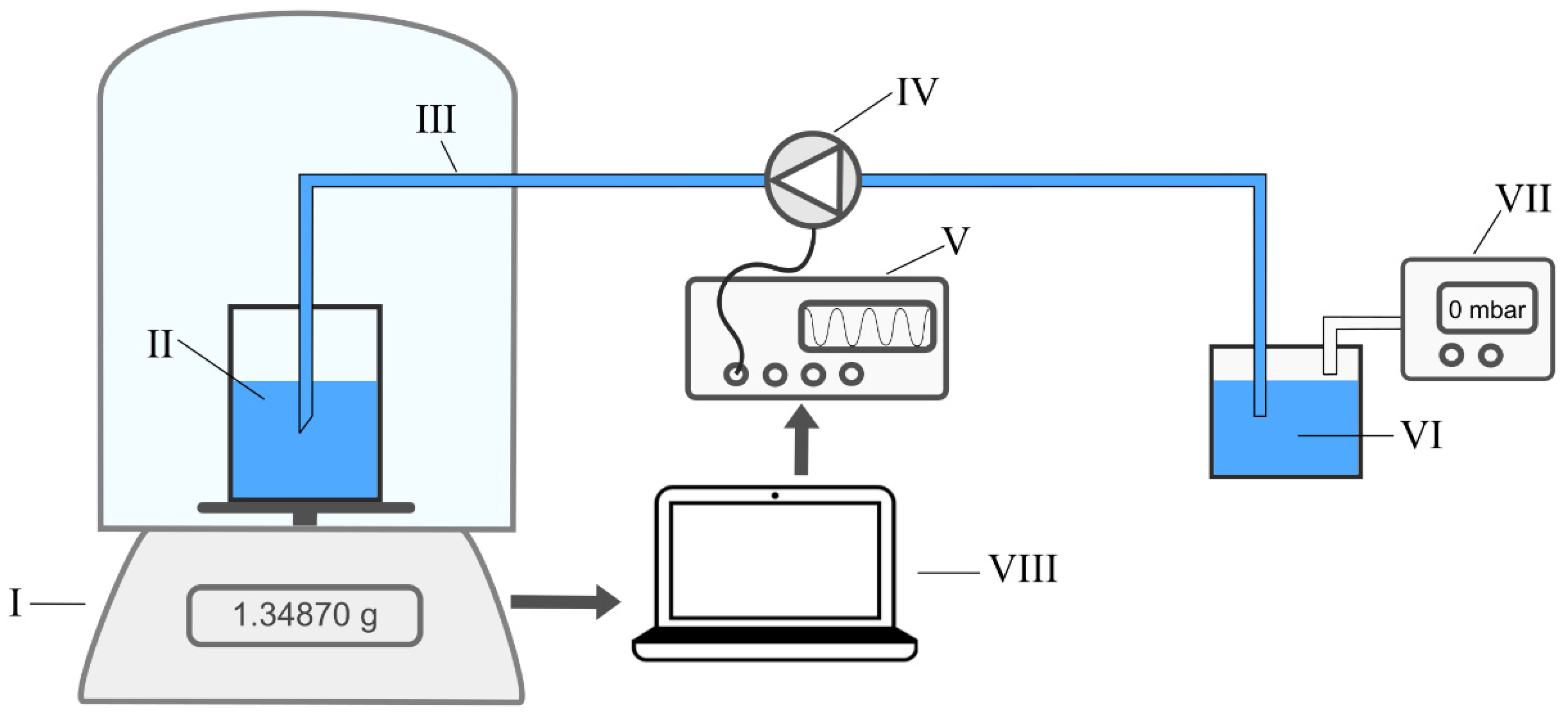
2.1. Basic Characterization
2.2. Bolus Characterization
3. Results and Discussion
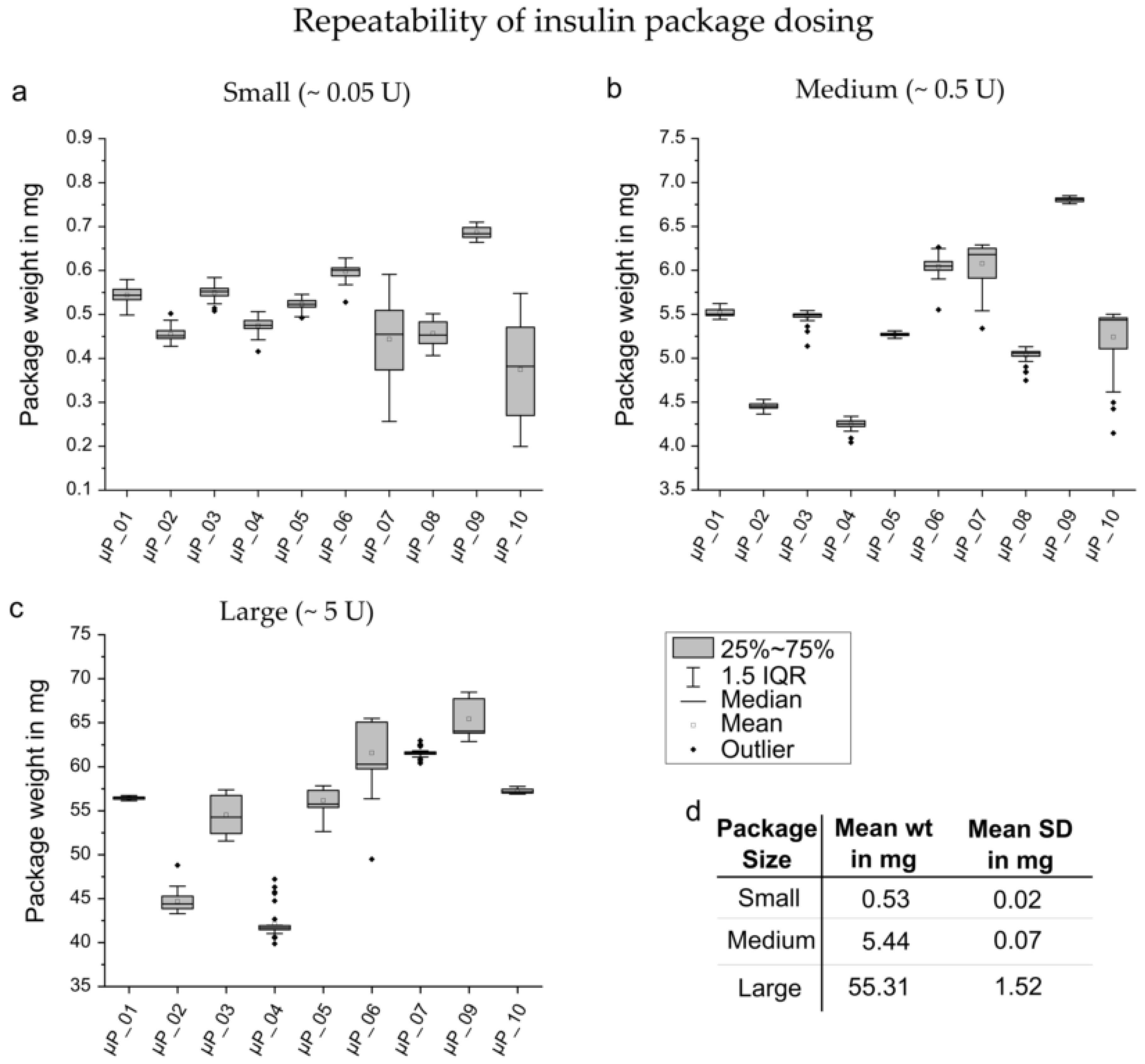
3.1. Standard Characterization
3.2. Bolus Precision Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Item | Description |
|---|---|
| Monocrystalline silicon wafers (n-doped,100) | Base material for manufacturing the silicon micropump consisting (two valve wafers and one actuator wafer) |
| Piezoelectric disc | PIC 252; base material forming the bending actuator together with the actuator wafer |
| 2k-epoxy glue | EPOTEK 353 ND-T, to attach the disc actuator to the pump body |
| O-ring | FKM material that seals the fluidic periphery with the micropump housing |
| Soft sealing | Costume made FKM sealing prevent leakage between the silicon micropump and the housing |
| Micropump housing | Special developed PEEK assembly to house the silicon micropump |
| Filter frit | Stainless steel filter at the inlet of the micropump housing, blocking particles larger than 5 µm |
| Capillaries | PEEK capillaries with 1 mm inner diameter |
| M1 screw | Standard screw to clamp the silicon micropump to the housing with its cover |
References
- The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes 1997, 46, 271–286. [Google Scholar] [CrossRef]
- Zisser, H.; Jovanovic, L. OmniPod Insulin Management System: Patient perceptions, preference, and glycemic control. Diabetes Care 2006, 29, 2175. [Google Scholar] [CrossRef] [PubMed]
- Doyle, E.A.; Weinzimer, S.A.; Steffen, A.T.; Ahern, J.A.H.; Vincent, M.; Tamborlane, W.V. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004, 27, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Jeitler, K.; Horvath, K.; Berghold, A.; Gratzer, T.W.; Neeser, K.; Pieber, T.R.; Siebenhofer, A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: Systematic review and meta-analysis. Diabetologia 2008, 51, 941–951. [Google Scholar] [CrossRef]
- Anhalt, H.; Bohannon, N.J.V. Insulin patch pumps: Their development and future in closed-loop systems. Diabetes Technol. Ther. 2010, 12, S51–S58. [Google Scholar] [CrossRef] [PubMed]
- Zisser, H.C. The OmniPod Insulin Management System: The latest innovation in insulin pump therapy. Diabetes Ther. 2010, 1, 10–24. [Google Scholar] [CrossRef]
- Youan, B.-B.C. Chronopharmaceutics: Gimmick or clinically relevant approach to drug delivery? J. Control. Release 2004, 98, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Focan, C.; Karaboué, A.; De La Valette, V.; Focan-Henrard, D.; Baron, B.; Kreutz, F.; Giacchetti, S. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 1015–1035. [Google Scholar] [CrossRef]
- Bußmann, A.B.; Grünerbel, L.M.; Durasiewicz, C.P.; Thalhofer, T.A.; Wille, A.; Richter, M. Microdosing for drug delivery application—A review. Sens. Actuators A: Phys. 2021, 330, 112820. [Google Scholar] [CrossRef]
- Laubner, K.; Singler, E.; Straetener, J.; Siegmund, T.; Päth, G.; Seufert, J. Comparative dose accuracy of durable and patch insulin pumps under laboratory conditions. Diabetes Technol. Ther. 2019, 21, 371–378. [Google Scholar] [CrossRef]
- Bowen, J.L.; Allender, C.J. A comparative pulse accuracy study of two commercially available patch insulin infusion pumps. Eur. Endocrinol. 2016, 12, 79–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jahn, L.G.; Capurro, J.J.; Levy, B.L. Comparative dose accuracy of durable and patch insulin infusion pumps. J. Diabetes Sci. Technol. 2013, 7, 1011–1020. [Google Scholar] [CrossRef]
- Borot, S.; Franc, S.; Cristante, J.; Penfornis, A.; Benhamou, P.-Y.; Guerci, B.; Hanaire, H.; Renard, E.; Reznik, Y.; Simon, C.; et al. Accuracy of a new patch pump based on a microelectromechanical system (MEMS) compared to other commercially available insulin pumps: Results of the first in vitro and in vivo studies. J. Diabetes Sci. Technol. 2014, 8, 1133–1141. [Google Scholar] [CrossRef]
- Richter, M.; Linnemann, R.; Woias, P. Robust design of gas and liquid micropumps. Sens. Actuators A Phys. 1998, 68, 480–486. [Google Scholar] [CrossRef]
- Kim, L.; Toh, Y.-C.; Voldman, J.; Yu, H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab. Chip 2007, 7, 681–694. [Google Scholar] [CrossRef]
- Chappell, M.A.; Payne, S.J. A physiological model of the release of gas bubbles from crevices under decompression. Respir. Physiol. Neurobiol. 2006, 153, 166–180. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, D.; Zhang, L.; Jeon, H.; Mendoza-Elias, J.E.; Harvat, T.A.; Hassan, S.Z.; Zhou, A.; Eddington, D.T.; Oberholzer, J. Systematic prevention of bubble formation and accumulation for long-term culture of pancreatic islet cells in microfluidic device. Biomed. Microdevices 2012, 14, 419–426. [Google Scholar] [CrossRef]
- Maillefer, D.; Gamper, S.; Frehner, B.; Balmer, P.; van Lintel, H.; Renaud, P. A high-performance silicon micropump for disposable drug delivery systems. Technical Digest; MEMS 2001. In Proceedings of the 14th IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 01CH37090), Interlaken, Switzerland, 25 January 2001; IEEE: Piscataway, NJ, USA, 2002; pp. 413–417. [Google Scholar]
- Maillefer, D.; van Lintel, H.; Rey-Mermet, G.; Hirschi, R. A high-performance silicon micropump for an implantable drug delivery system. In Proceedings of the Technical Digest, IEEE International MEMS 99 Conference, Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 99CH36291), Orlando, FL, USA, 21 January 1999; IEEE: Piscataway, NJ, USA, 2002; pp. 541–546. [Google Scholar] [CrossRef]
- Schneeberger, N.; Blondel, A.; Boutaud, B.; Chappel, E.; Maillefer, D.; Schneider, V. Disposable insulin pump-a medical case study. In Proceedings of the DTIP of MEMS & MOEMS, Montreux, Switzerland, 12–14 May 2004; pp. 25–27. [Google Scholar]
- International Electrotechnical Commission. Medical Electrical Equipment—Part 2–24: Particular Requirements for Basic Safety and Essential Performance of Infusion Pumps and Controllers; IEC 60601-2-24:2012; IEC: Geneva, Switzerland, 2016. [Google Scholar]
- Pleus, S.; Kamecke, U.; Waldenmaier, D.; Freckmann, G. Reporting insulin pump accuracy: Trumpet curves according to IEC 60601-2-24 and beyond. J. Diabetes Sci. Technol. 2019, 13, 592–596. [Google Scholar] [CrossRef]
- Kamecke, U.; Waldenmaier, D.; Haug, C.; Ziegler, R.; Freckmann, G. Establishing methods to determine clinically relevant Bolus and Basal rate delivery accuracy of insulin pumps. J. Diabetes Sci. Technol. 2019, 13, 60–67. [Google Scholar] [CrossRef]
- Freckmann, G.; Kamecke, U.; Waldenmaier, D.; Haug, C.; Ziegler, R. Accuracy of Bolus and Basal rate delivery of different insulin pump systems. Diabetes Technol. Ther. 2019, 21, 201–208. [Google Scholar] [CrossRef]
- Zisser, H.; Breton, M.; Dassau, E.; Markova, K.; Bevier, W.; Seborg, D.; Kovatchev, B. Novel methodology to determine the accuracy of the OmniPod insulin pump: A key component of the artificial pancreas system. J. Diabetes Sci. Technol. 2011, 5, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Wackerle, M.; Richter, M.; Woias, P.; Goldschmidtböing, F. Sub-nl flow measurement method for microfluidic actuators. In Actuator 2000, Proceedings of the 7th International Conference on New Actuators and International Exhibition on Smart Actuators and Drive Systems, Bremen, Germany, 19–21 June 2000; Borgmann, H., Ed.; Messe Bremen GmbH: Bremen, Germany, 2000; pp. 507–510. [Google Scholar]
- Leistner, H.; Wackerle, M.; Congar, Y.; Anheuer, D.; Roehl, S.; Richter, M. Robust Silicon Micropump of Chip Size 5 × 5 × 0.6 mm3 with 4 mL/min Air and 0.5 mL/min Water Flow Rate for Medical and Consumer Applications. In Actuator 2021, Proceedings of the International Conference and Exhibition on New Actuator Systems and Applications (GMM Conference), Online, 17–19 February 2021; Schlaak, H., Ed.; VDE Verlag GmbH: Berlin, Offenbach, 2021; pp. 113–116. ISBN 978-3-8007-5454-0. [Google Scholar]
- Herz, M.; Richter, M.; Wackerle, M. Method for Manufacturing a Bending Transducer, a Micro Pump and a Micro Valve, Micro Pump and Micro Valve. Patent No. US9410641B2, 9 August 2016. [Google Scholar]
- Richter, M.; Wackerle, M.; Congar, Y.; Drost, A.; Häfner, J.; Röhl, S.; Kibler, S.; Kruckow, J.; Kutter, C. Miniaturisierung von Mikromembranpumpen für die Integration in Mobilfunkgeräte. In MicroSystemTechnik Kongress 2017, Proceedings of MST 2017, München, Germany, 23–25 October 2017; VDE Verlag GmbH: Berlin, Germany, 2017. [Google Scholar]
- Woias, P. Micropumps—Past, progress and future prospects. Sens. Actuators B Chem. 2005, 105, 28–38. [Google Scholar] [CrossRef]
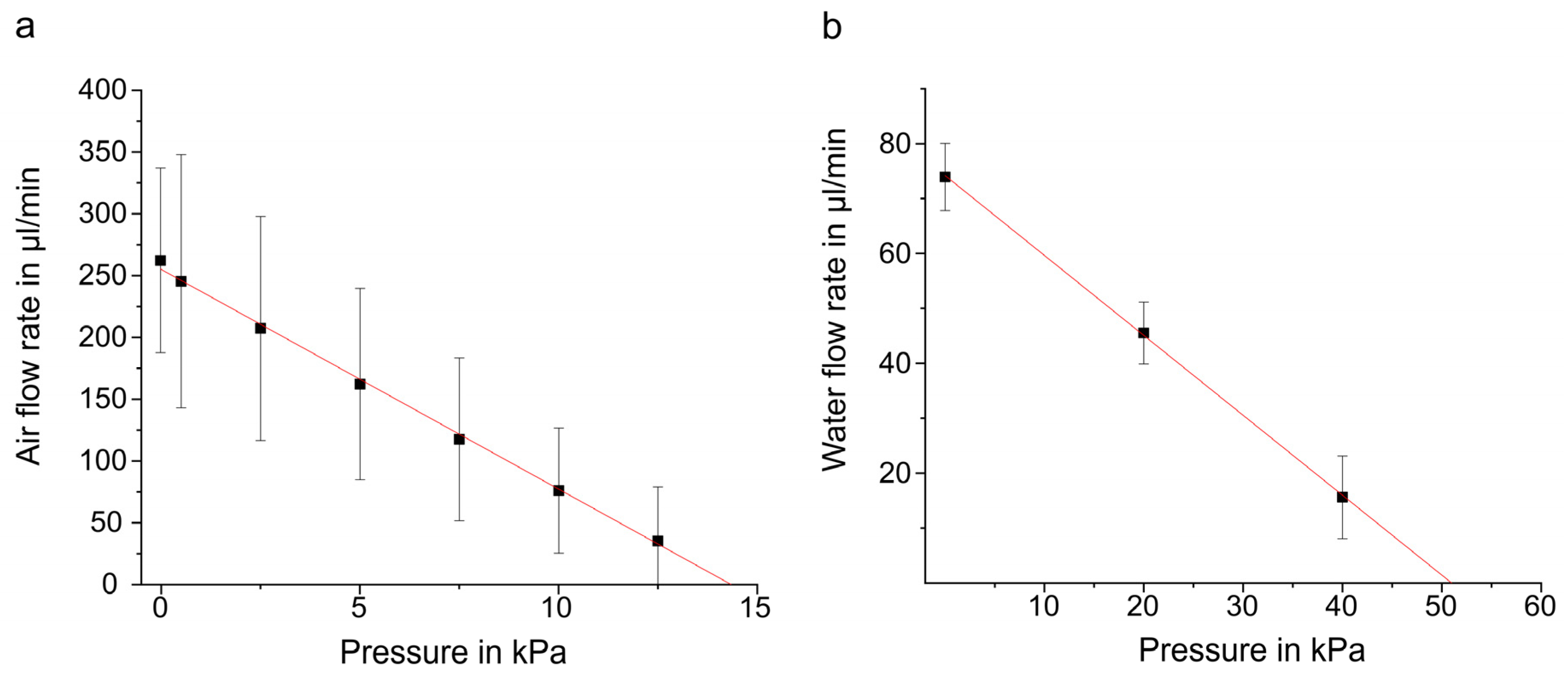

| Fluid | Characteristics | Actuation | Mean Value |
|---|---|---|---|
| - | Actuator stroke | −20/+100 V; quasi-static | µm |
| Air | Flow rate | −50/100 V; 1.6 kHz −30/90 V; 65 Hz | min min |
| Air | Backpressure | −30/90 V; 65 Hz | kPa 1 |
| DI water | Flow rate at 0 kPa | −30/90 V; 15 Hz | min |
| DI water | Blocking pressure | −30/90 V; 15 Hz | kPa 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bußmann, A.; Leistner, H.; Zhou, D.; Wackerle, M.; Congar, Y.; Richter, M.; Hubbuch, J. Piezoelectric Silicon Micropump for Drug Delivery Applications. Appl. Sci. 2021, 11, 8008. https://doi.org/10.3390/app11178008
Bußmann A, Leistner H, Zhou D, Wackerle M, Congar Y, Richter M, Hubbuch J. Piezoelectric Silicon Micropump for Drug Delivery Applications. Applied Sciences. 2021; 11(17):8008. https://doi.org/10.3390/app11178008
Chicago/Turabian StyleBußmann, Agnes, Henry Leistner, Doris Zhou, Martin Wackerle, Yücel Congar, Martin Richter, and Jürgen Hubbuch. 2021. "Piezoelectric Silicon Micropump for Drug Delivery Applications" Applied Sciences 11, no. 17: 8008. https://doi.org/10.3390/app11178008
APA StyleBußmann, A., Leistner, H., Zhou, D., Wackerle, M., Congar, Y., Richter, M., & Hubbuch, J. (2021). Piezoelectric Silicon Micropump for Drug Delivery Applications. Applied Sciences, 11(17), 8008. https://doi.org/10.3390/app11178008






