Protective Effects of Tocotrienols in Cerebral and Myocardial Ischemia-Reperfusion Injury: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
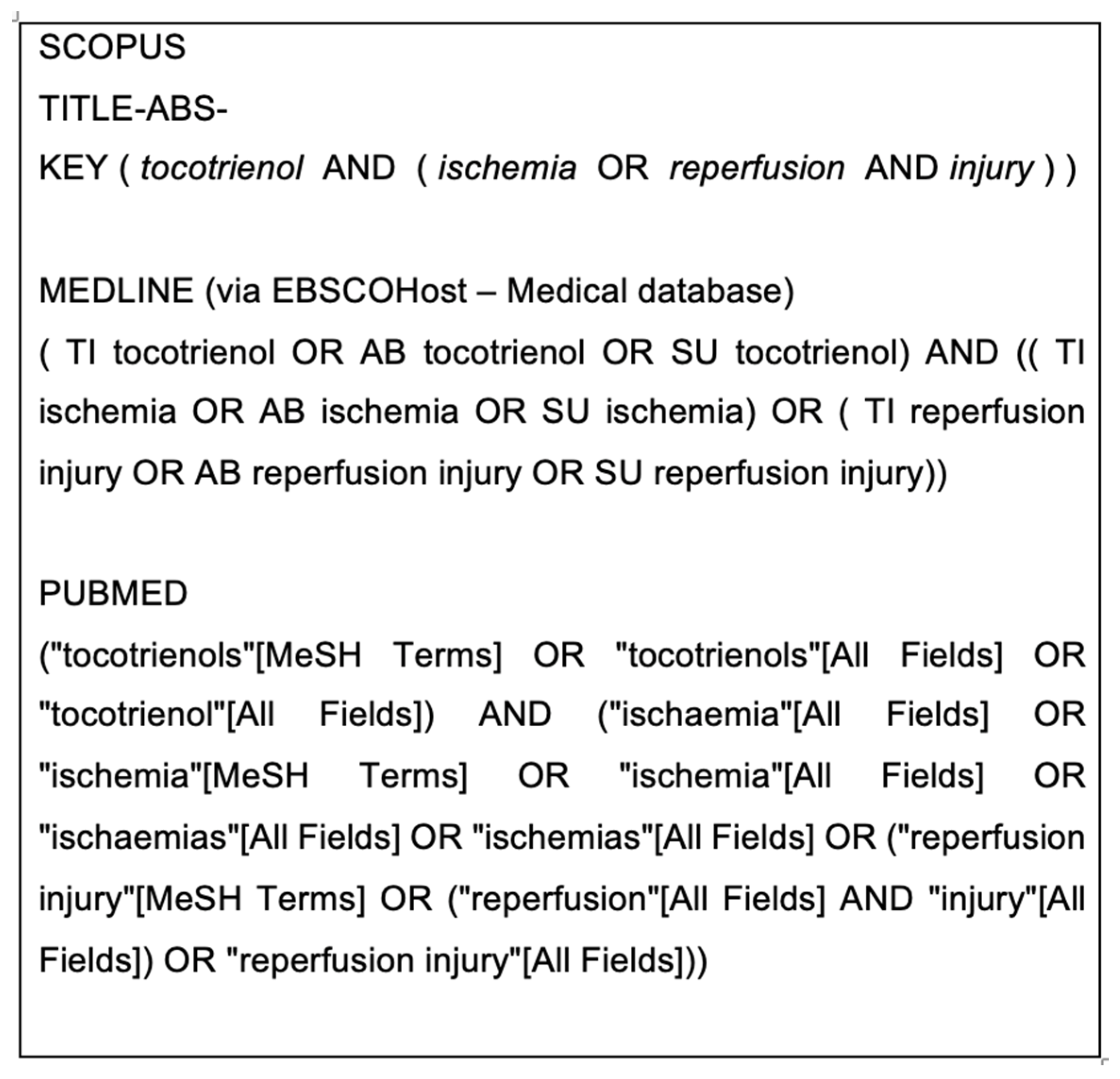
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Analysis
3. Results
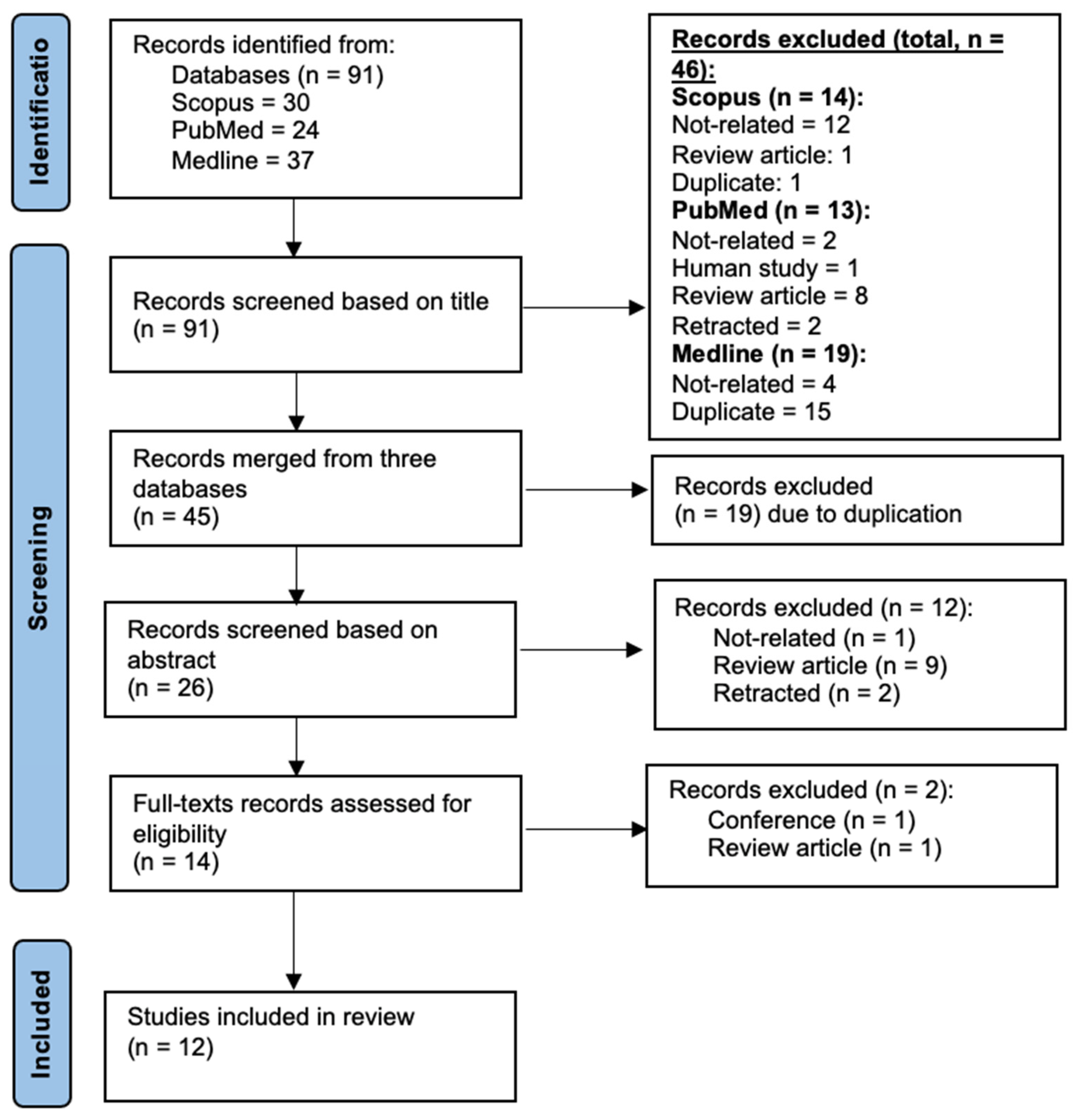
3.1. Description of Article Selection Process
3.2. Characteristics of Included Studies
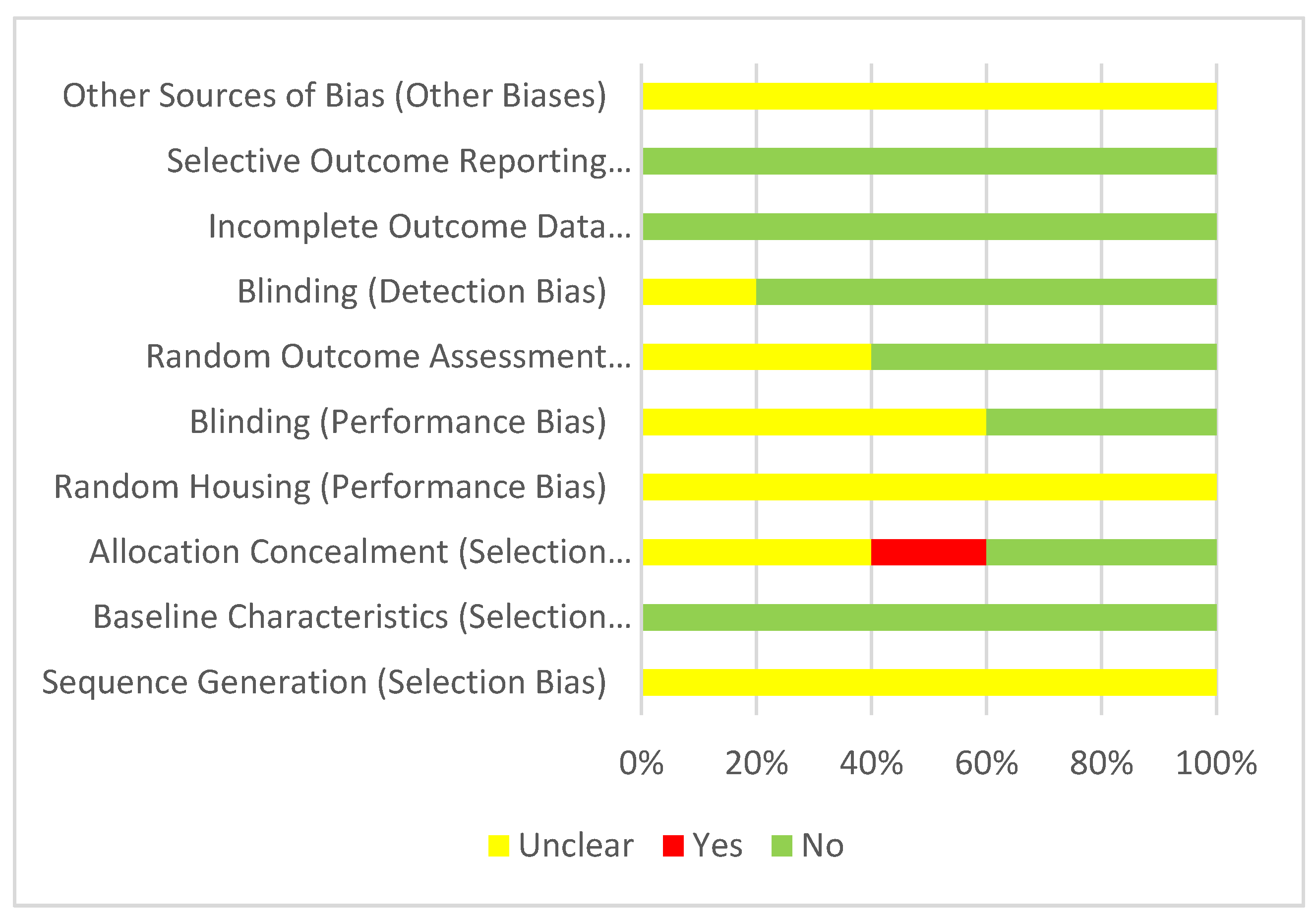
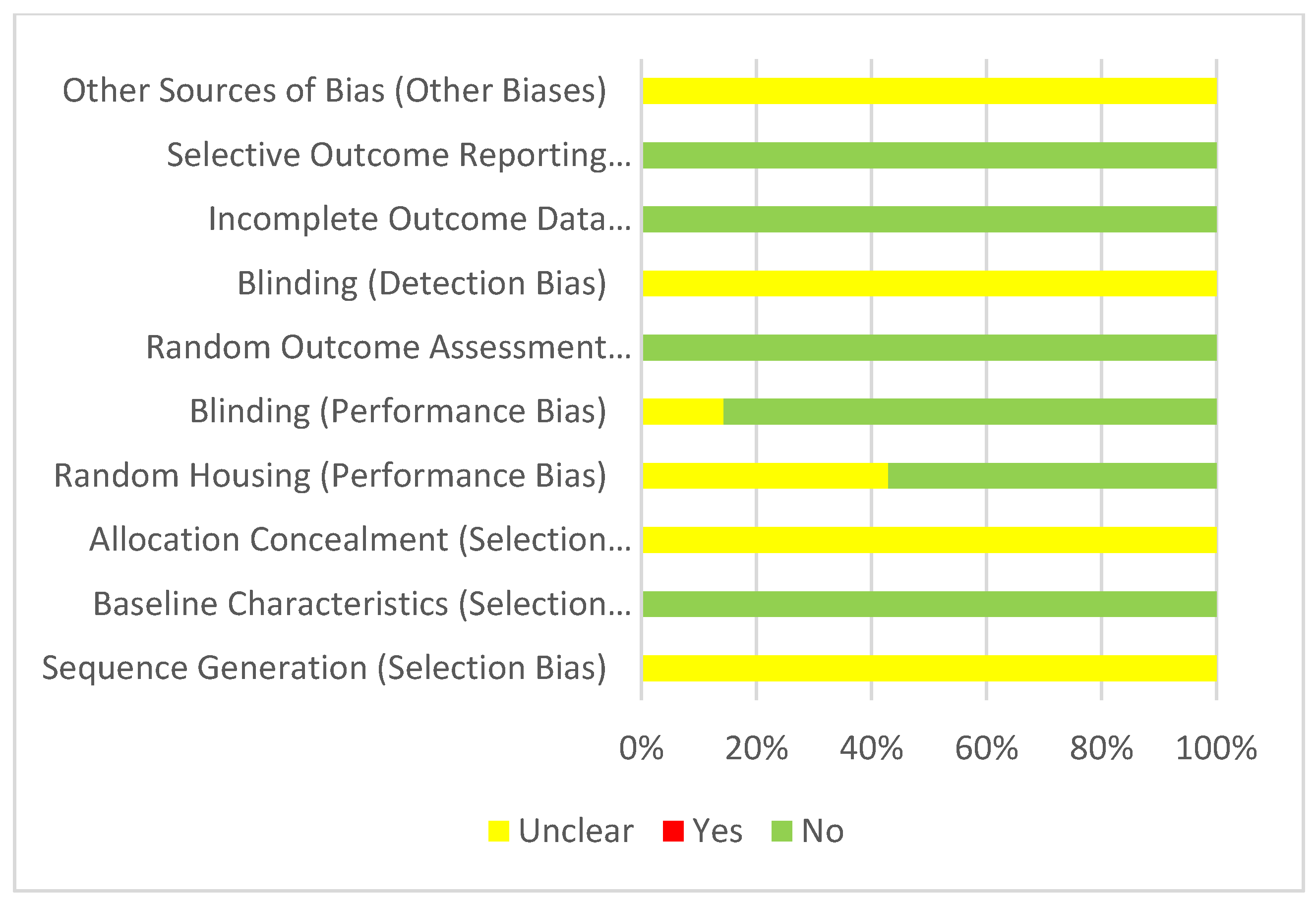
3.3. Risk of Bias Assessment
3.3.1. Cerebral Ischemia-Reperfusion Injury
3.3.2. Myocardial Ischemia-Reperfusion Injury
3.4. Tocotrienol’s Effects on Cerebral Ischaemia Reperfusion Injury
3.5. Tocotrienol’s Effects in Myocardial Ischemia-Reperfusion Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Chelluboina, B.; Vemuganti, R. Therapeutic potential of nutraceuticals to protect brain after stroke. Neurochem. Int. 2021, 142, 104908. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Penson, P.; Banach, M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 7, S21–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; De Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Loh, H.C.; Lim, R.; Lee, K.W.; Ooi, C.Y.; Chuan, D.R.; Looi, I.; Hay, Y.K.; Khan, N.A.K. Effects of vitamin E on stroke: A systematic review with meta-analysis and trial sequential analysis. Stroke Vasc. Neurol. 2021, 6, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Di Castelnuovo, A.; Iacoviello, L.; De Gaetano, G.; Violi, F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 354–363. [Google Scholar] [CrossRef]
- Das, M.; Das, S.; Wang, P.; Powell, S.R.; Das, D.K. Caveolin and proteasome in tocotrienol mediated myocardial protection. Cell. Physiol. Biochem. 2008, 22, 287–294. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, S.; Lekli, I.; Gurusamy, N.; Bardhan, J.; Raychoudhury, U.; Chakravarty, R.; Banerji, S.; Knowlton, A.A.; Das, D.K. Tocotrienols confer resistance to ischemia in hypercholesterolemic hearts: Insight with genomics. Mol. Cell Biochem. 2012, 360, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Esterhuyse, A.J.; Du Toit, E.; Van Rooyen, J. Dietary red palm oil supplementation protects against the consequences of global ischemia in the isolated perfused rat heart. Asia Pac. J. Clin. Nutr. 2005, 14, 340–347. [Google Scholar]
- Esterhuyse, J.S.; van Rooyen, J.; Strijdom, H.; Bester, D.; du Toit, E.F. Proposed mechanisms for red palm oil induced cardioprotection in a model of hyperlipidaemia in the rat. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lekli, I.; Ray, D.; Mukherjee, S.; Gurusamy, N.; Ahsan, M.K.; Juhasz, B.; Bak, I.; Tosaki, A.; Gherghiceanu, M.; Popescu, L.M.; et al. Co-ordinated autophagy with resveratrol and gamma-tocotrienol confers synergetic cardioprotection. J. Cell. Mol. Med. 2010, 14, 2506–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Das, S.; Ahsan, M.K.; Otani, H.; Das, D.K. Modulation of microRNA 20b with resveratrol and longevinex is linked with their potent anti-angiogenic action in the ischaemic myocardium and synergestic effects of resveratrol and γ-tocotrienol. J. Cell. Mol. Med. 2012, 16, 2504–2517. [Google Scholar] [CrossRef]
- Serbinova, E.; Khwaja, S.; Catudioc, J.; Ericson, J.; Torres, Z.; Gapor, A.; Kagan, V.; Packer, L. Palm oil vitamin E protects against ischemia/reperfusion injury in the isolated perfused langendorff heart. Nutr. Res. 1992, 12, S203–S215. [Google Scholar] [CrossRef]
- Jiao, Y.; Shang, J.; Ohta, Y.; Yan, H.; Liu, X.; Li, X.; Morihara, R.; Nakano, Y.; Fukui, Y.; Shi, X.; et al. Neuroprotective Effects of Tocovid Pretreatment in a Mouse Stroke Model. J. Stroke Cerebrovasc. Dis. 2018, 27, 2166–2174. [Google Scholar] [CrossRef]
- Mishima, K.; Tanaka, T.; Pu, F.; Egashira, N.; Iwasaki, K.; Hidaka, R.; Matsunaga, K.; Takata, J.; Karube, Y.; Fujiwara, M. Vitamin E isoforms alpha-tocotrienol and gamma-tocopherol prevent cerebral infarction in mice. Neurosci. Lett. 2003, 337, 56–60. [Google Scholar] [CrossRef]
- Park, H.A.; Kubicki, N.; Gnyawali, S.; Chan, Y.C.; Roy, S.; Khanna, S.; Sen, C.K. Natural vitamin E α-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke 2011, 42, 2308–2314. [Google Scholar] [CrossRef] [Green Version]
- Rink, C.; Christoforidis, G.; Khanna, S.; Peterson, L.; Patel, Y.; Khanna, S.; Abduljalil, A.; Irfanoglu, O.; Machiraju, R.; Bergdall, V.K.; et al. Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J. Cereb. Blood Flow Metab. 2011, 31, 2218–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, J.; Yan, H.; Jiao, Y.; Ohta, Y.; Liu, X.; Li, X.; Morihara, R.; Nakano, Y.; Fukui, Y.; Shi, X.; et al. Therapeutic Effects of Pretreatment with Tocovid on Oxidative Stress in Postischemic Mice Brain. J. Stroke Cerebrovasc. Dis. 2018, 27, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Tosaki, A.; Juhasz, B.; Lekli, I.; Varadi, J.; Nesaretam, K.; Maulik, N.; Das, D.K. Tocotrienols in cardioprotection: Role of different isomers. FASEB J. 2007, 21, A1112. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Yu, Z. Ischemia-reperfusion Injury in the Brain: Mechanisms and Potential Therapeutic Strategies. Biochem. Pharmacol. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Kumari Naga, K.; Panigrahi, M.; Prakash Babu, P. Changes in endogenous antioxidant enzymes during cerebral ischemia and reperfusion. Neurol. Res. 2007, 29, 877–883. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Mhadu, N.H.; Al-Dalain, S.M.; Martínez, G.; León, O.S. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci. Res. 2001, 41, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Y.; Akamatsu, Y.; Lee, C.C.; Stetler, R.A.; Lawton, M.T.; Yang, G.Y. Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Prog. Neurobiol. 2014, 115, 138–156. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, G.A.; Cunningham, L.A.; Wallace, J.; Alexander, S.; Estrada, E.Y.; Grossetete, M.; Razhagi, A.; Miller, K.; Gearing, A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: Activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001, 893, 104–112. [Google Scholar] [CrossRef]
- Tejima, E.; Guo, S.; Murata, Y.; Arai, K.; Lok, J.; van Leyen, K.; Rosell, A.; Wang, X.; Lo, E.H. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J. Neurotrauma 2009, 26, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, J.; Hoehn, M. Poststroke angiogenesis, con: Dark side of angiogenesis. Stroke 2015, 46, e103–e104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalothorn, D.; Zhang, H.; Smith, J.E.; Edwards, J.C.; Faber, J.E. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ. Res. 2009, 105, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.H.; Zhang, X.J.; Shang, H.Q.; Wang, X.; Rong, D. Glutamine protects myocardial ischemia-reperfusion injury in rats through the PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, K.; Hu, P. The Role of Autophagy in Acute Myocardial Infarction. Front. Pharmacol. 2019, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Z.; Hu, W.; Wang, D.; Jiang, S.; Fan, C.; Di, S.; Liu, D.; Sun, Y.; Yi, W. Caveolin-1/-3: Therapeutic targets for myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2016, 111, 45. [Google Scholar] [CrossRef] [PubMed]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Food and Drug Administration: Rockville, MD, USA, 2005. [Google Scholar]
- Qureshi, A.A.; Khan, D.A.; Silswal, N.; Saleem, S.; Qureshi, N. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J. Clin. Exp. Cardiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hosomi, A.; Goto, K.; Kondo, H.; Iwatsubo, T.; Yokota, T.; Ogawa, M.; Arita, M.; Aoki, J.; Arai, H.; Inoue, K. Localization of alpha-tocopherol transfer protein in rat brain. Neurosci. Lett. 1998, 256, 159–162. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.; Wang, T.; Dolde, D.; Xin, H. Tocopherol and annatto tocotrienols distribution in laying-hen body. Poult. Sci. 2015, 94, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Animal Characteristics | Treatment | Effects |
|---|---|---|---|
| Brain | |||
| Jiao [17] (2018) | ICR mice weighing 23–25 g aged 6 weeks. | 200 mg/kg/day Tocovid for 1 month, given orally. | Neuroprotection via anti-inflammatory effects of Tocovid. |
| Shang [21] (2018) | ICR mice weighing 23–25 g aged 6 weeks. | 200 mg/kg/day Tocovid for 1 month, given orally. | Neuroprotection via antioxidative effects. |
| Rink [20] (2011) | Mongrel canines weighing 26.6 ± 2.6 kg aged 2.4 ± 0.9 years. | 200 mg mixed tocotrienols two times a day for 10 weeks. | Neuroprotective effects via collateral circulation. |
| Park [19] (2011) | C57BL/6 mice aged 5 weeks. Mouse hippocampal HT4 neural cells—primary cortical neurons (Sprague-Dawley rats). | 50 mg/kg/day α-tocotrienol for 13 weeks, given orally via gavage; 1 μmol/L α-tocotrienol added 6 h before the glutamate treatment. | Regulation of MRP1 by α-tocotrienols. |
| Mishima [18] (2003) | Male ddY mice weighing 25–35 g. | 0.2 mM or 2 mM of α-/γ-/𝛿-tocotrienol, α-/γ-/𝛿-tocopherol, given by bolus intravenously 3 h before and after MCA occlusion. | Reduction of cerebral infarct volume is more effective in α-tocotrienol and γ-tocopherol. |
| Heart | |||
| Mukhopadhyay [15] (2012) | Male Sprague-Dawley rats weighing 250–300 g. | 5 mg/kg/day of γ-tocotrienol for 21 days via gavage. | Modulation of microRNA that regulates angiogenesis. |
| Das [11] (2012) | Adult New Zealand rabbits (male and female) weighing 2.4–3.0 kg—I/R model. Genomic study: Adult Sprague-Dawley rat (male). | 20 µmol/kg/day of α-/γ-/𝛿-tocotrienols on top of 2% cholesterol diet. Control received 2% cholesterol diet only for 4 weeks. | Gamma tocotrienol is superior in cardioprotection, modulating numerous genes’ expression, including antioxidants, energy production, fatty acid metabolism and calcium channels. Interpretation of LPL, MMP-2, MMP-9, TGF-β, p-Akt, ERα, ERβ, p-FoxO4, and Spot-14 were not clear as the text description were not parallel to the figures provided. The data were based on the text description. |
| Lekli [14] (2010) | Male Sprague-Dawley rats weighing 250–300 g. | 0.3 mg/kg/day of γ-tocotrienol for 30 days via gavage. | Autophagy coordination and regulation of PI3K/Akt/mTOR-signaling pathway. |
| Das [10] (2008) | Male Sprague Dawley rats weighing 250–300 g. | α-/γ-/𝛿-tocotrienols with a dose of 0.3 mg/kg for 30 days via gavage. | Caveolin and proteasome regulation of p38MAPK-signaling pathway. |
| Esterhuyse [13] (2006) | Male Wistar rats, 300–400 g (post); 7 weeks old. | Treatment group received rat chow with 7 g of RPO/kg for 6 weeks. | |
| Esterhuyse [12] (2005) | Male Long-Evans rats. | Treatment group received rat chow with 7 g of RPO/kg diet. Control received only rat chow for 6 weeks. | |
| Serbinova [16] (1992) | Male Sprague-Dawley rats (350–400 g). | Diet supplemented with 7 g palm oil vitamin E/kg (55% tocotrienols: 45% tocopherols). Control received 20 g vitamin E acetate/kg for 6 weeks. | |
| Authors | Infarct Size | LDH | MRP1 | Rotarod Time | Bederson Score | Corner Test |
|---|---|---|---|---|---|---|
| Jiao [17] | ↓ TV | ↑ | NS | NS | ||
| Shang [21] | ↓ TV | ↑ | ↑ | NS | NS | |
| Rink [20] | ↓ TE | |||||
| Park [19] | ↓ α-TC | ↓ | ↑ | |||
| Mishima [18] | ↓ α-TC, α-TH, γ-TC | ↓ |
| Author | IS | HR | CF | AF | AOR | LVDP | LV dp/dt |
|---|---|---|---|---|---|---|---|
| Mukhopadhyay [15] | ↓ | NS | ↑ | ↑ | ↑ | ||
| Lekli [14] | ↓ | NS | ↑ | ↑ | ↑ | ||
| Esterhuyse [13] | NS | NS | ↑ | NS | |||
| Esterhuyse [12] | ↑ | NS | |||||
| Serbinova [16] | ↑ | ||||||
| Das [11] | M: ↓γ F: ↓γα | M: NS γα𝛿 F: ↑γ (γ > α), (Fγ > Mγ), NS α𝛿 | M: ↑γ (γ > α), NS α𝛿 F: ↑γ (γ > α), α (F > M), NS α𝛿 | M: ↑γα (γ > α), NS 𝛿 F: ↑γ (γ > α),(F > M), α (F > M), NS 𝛿 | M: ↑γα (γ > α), NS 𝛿 F: ↑γ (γ > α), (F > M), α (F > M), NS 𝛿 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, F.F.; Ali, A.; Ibrahim, N.’I. Protective Effects of Tocotrienols in Cerebral and Myocardial Ischemia-Reperfusion Injury: A Systematic Review. Appl. Sci. 2021, 11, 7994. https://doi.org/10.3390/app11177994
Ramli FF, Ali A, Ibrahim N’I. Protective Effects of Tocotrienols in Cerebral and Myocardial Ischemia-Reperfusion Injury: A Systematic Review. Applied Sciences. 2021; 11(17):7994. https://doi.org/10.3390/app11177994
Chicago/Turabian StyleRamli, Fitri Fareez, Adli Ali, and Nurul ’Izzah Ibrahim. 2021. "Protective Effects of Tocotrienols in Cerebral and Myocardial Ischemia-Reperfusion Injury: A Systematic Review" Applied Sciences 11, no. 17: 7994. https://doi.org/10.3390/app11177994
APA StyleRamli, F. F., Ali, A., & Ibrahim, N. ’I. (2021). Protective Effects of Tocotrienols in Cerebral and Myocardial Ischemia-Reperfusion Injury: A Systematic Review. Applied Sciences, 11(17), 7994. https://doi.org/10.3390/app11177994







