Alginate-Chitosan Microencapsulated Cells for Improving CD34+ Progenitor Maintenance and Expansion
Abstract
1. Introduction
2. Materials and Methods
2.1. CD34+ Cell Isolation
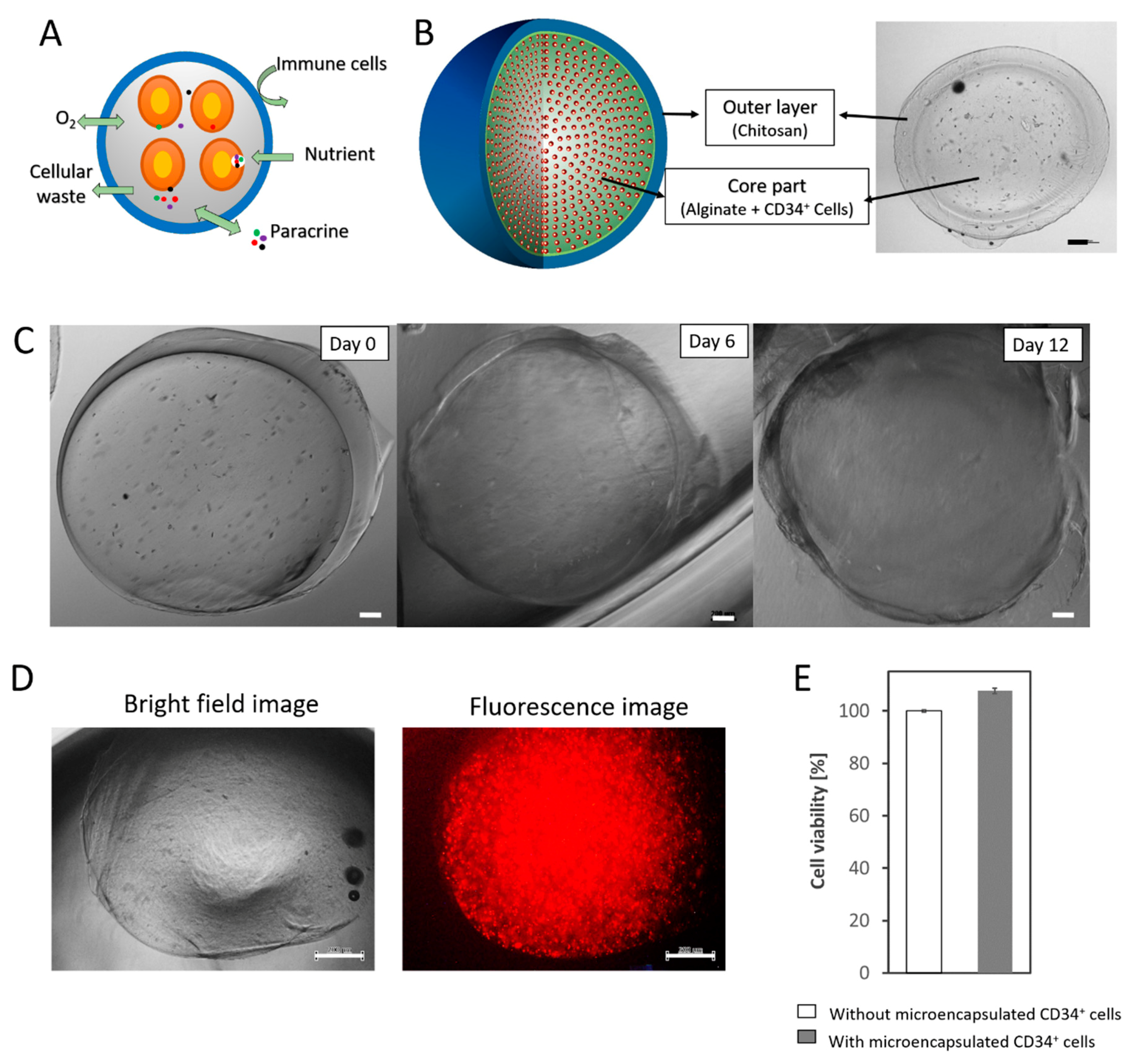
2.2. Alginate-Chitosan Microcapsule Fabrication
2.3. Cytotoxicity Assay
2.4. Cell Leakage Detection Assay
2.5. Co-Cultures of Microencapsulated CD34+ Cells with Bare CD34+ Cells
2.6. Flow Cytometry Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of a Durable Double-Layered Microcapsule
3.2. Paracrine Effects of Microencapsulated CD34+ Cells
3.3. Importance of Microenvironment for CD34+ Progenitor Maintenance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dumphy, S.E.; Dua, H.S. Concise Review: Evidence for CD34 as a Common Marker for Diverse Progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef]
- Nurhayati, R.W.; Ojima, Y.; Taya, M. Recent developments in ex vivo platelet production. Cytotechnology 2016, 68, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, M.; Lübbert, M.; Guo, Y. CD34+ or CD34−: Which is the more primitive? Leukemia 2002, 16, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fackler, M.J.; Leung, W.; Lumkul, R.; Ramirez, M.; Theobald, N.; Malech, H.L.; Civin, C.I. Human CD34+ cell preparations contain over 100-fold greater NOD/SCID mouse engrafting capacity than do CD34- cell preparations. Exp. Hematol. 2001, 29, 910–921. [Google Scholar] [CrossRef]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef]
- Taguchi, A.; Soma, T.; Tanaka, H.; Kanda, T.; Nishimura, H.; Yoshikawa, H.; Tsukamoto, Y.; Iso, H.; Fujimori, Y.; Stern, D.M.; et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Investig. 2004, 114, 330–338. [Google Scholar] [CrossRef]
- Verina, T.; Fatemi, A.; Johnston, M.V.; Comi, A.M. Pluripotent possibilities: Human umbilical cord blood cell treatment after neonatal brain injury. Pediatr. Neurol. 2013, 48, 346–354. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, S.; Li, Y.; Liu, S.; Ma, T.; Wei, W. Umbilical cord blood CD34+ cells administration improved neurobehavioral status and alleviated brain injury in a mouse model of cerebral palsy. Child’s Nerv. Syst. 2021, 37, 1–9. [Google Scholar] [CrossRef]
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef]
- Choi, J.S.; Mahadik, B.; Harley, B.A.C. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol. J. 2015, 10, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Nies, C.; Gottwald, E. Artificial Hematopoietic Stem Cell Niches-Dimensionality Matters. Adv. Tissue Eng. Regen. Med. 2017, 2, 00042. [Google Scholar]
- Yuan, Y.; Tse, K.-T.; Sin, F.W.-Y.; Xue, B.; Fan, H.-H.; Xie, Y. Ex vivo amplification of human hematopoietic stem and progenitor cells in an alginate three-dimensional culture system. Int. J. Lab. Hematol. 2011, 33, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Tarunina, M.; Hernandez, D.; Kronsteiner-Dobramysl, B.; Pratt, P.; Watson, T.; Hua, P.; Gullo, F.; Van Der Garde, M.; Zhang, Y.; Hook, L.; et al. A Novel High-Throughput Screening Platform Reveals an Optimized Cytokine Formulation for Human Hematopoietic Progenitor Cell Expansion. Stem Cells Dev. 2016, 25, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, T.; Kniss, D.A.; Yang, S.-T.; Lasky, L.C. Human Cord Cell Hematopoiesis in Three-Dimensional Nonwoven Fibrous Matrices: In Vitro Simulation of the Marrow Microenvironment. J. Hematother. Stem Cell Res. 2001, 10, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Leisten, I.; Kramann, R.; Ferreira, M.S.V.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knüchel, R.; Schneider, R.K. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef]
- Cook, M.M.; Futrega, K.; Osiecki, M.; Kabiri, M.; Kul, B.; Rice, A.; Atkinson, K.; Brooke, G.; Doran, M. Micromarrows—Three-Dimensional Coculture of Hematopoietic Stem Cells and Mesenchymal Stromal Cells. Tissue Eng. Part C 2012, 18, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, R.W.; Antarianto, R.D.; Pratama, G.; Rahayu, D.; Mubarok, W.; Kobayashi, M.; Hutabarat, M. Encapsulation of human hematopoietic stem cells with a biocompatible polymer. AIP Conf. Proc. 2019, 2092, 020011. [Google Scholar]
- Nurhayati, R.W.; Cahyo, R.D.; Alawiyah, K.; Pratama, G.; Agustina, E.; Antarianto, R.D.; Prijanti, A.R.; Mubarok, W.; Rahyussalim, A.J. Development of Double-Layered Alginate-Chitosan Hydrogels for Human Stem Cell Microencapsulation. AIP Conf. Proc. 2019, 2193, 020004. [Google Scholar]
- Kong, X.; Xu, W. Biodegradation and biocompatibility of a degradable chitosan vascular prosthesis. Int. J. Clin. Exp. Med. 2015, 8, 3498–3505. [Google Scholar]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Batubara, I.; Rahayu, D.; Mohamad, K.; Prasetyaningtyas, W.E. Leydig Cells Encapsulation with Alginate-Chitosan: Optimization of Microcapsule Formation. J. Encapsulation Adsorpt. Sci. 2012, 2, 15–20. [Google Scholar] [CrossRef][Green Version]
- Thu, B.; Smidsrød, O.; Skjåk-Bræk, G. Alginate gels—Some structure-function correlations relevant to their use as immobilization matrix for cells. Immobil. Cells 1996, 11, 19–30. [Google Scholar]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 73, 237–245. [Google Scholar]
- Janowska-Wieczorek, A.; Majka, M.; Ratajczak, J.; Ratajczak, M.Z. Autocrine/Paracrine Mechanisms in Human Hematopoiesis. Stem Cells. 2001, 19, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.; Wang, W.; Qiao, W.; Bornhäuser, M.; Zandstra, P.W.; Werner, C.; Pompe, T. Distinguishing autocrine and paracrine signals in hematopoietic stem cell culture using a biofunctional microcavity platform. Sci. Rep. 2016, 6, 31951. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Duncan, M.T.; Nurhayati, R.W.; Taya, M.; Miller, W. Synergistic effect of hydrogen peroxide on polyploidization during the megakaryocytic differentiation of K562 leukemia cells by PMA. Exp. Cell Res. 2013, 319, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, V.R.; Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 2006, 134, 453–466. [Google Scholar] [CrossRef]
- Sakata, N.; Sumi, S.; Yoshimatsu, G.; Goto, M.; Egawa, S.; Unno, M. Encapsulated islets transplantation: Past, present and future. World J. Gastrointest. Pathophysiol. 2012, 3, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tuch, B.E.; Keogh, G.W.; Williams, L.J.; Wu, W.; Foster, J.L.; Vaithilingam, V.; Philips, R. Safety and Viability of Microencapsulated Human Islets Transplanted Into Diabetic Humans. Diabetes Care 2009, 32, 1887–1889. [Google Scholar] [CrossRef]
- Pan, X.; Sun, Q.; Cai, H.; Gao, Y.; Tan, W.; Zhang, W. Encapsulated feeder cells within alginate beads for ex vivo expansion of cord blood-derived CD34+ cells. Biomater. Sci. 2016, 4, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- del Burgo, L.S.; Ciriza, J.; Noguera, A.E.; Illa, X.; Cabruja, E.; Orive, G.; Hernandez, R.M.; Villa, R.; Pedraz, J.L.; Alvarez, M. 3D Printed porous polyamide macrocapsule combined with alginate microcapsules for safer cell-based therapies. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Lee-Thedieck, C.; Spatz, J.P. Artificial Niches: Biomimetic Materials for Hematopoietic Stem Cell Culture. Macromol. Rapid Commun. 2012, 33, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Rochet, N.; Leroy, P.; Far, D.F.; Ollier, L.; Loubat, A.; Rossi, B. CAL72: A human osteosarcoma cell line with unique effects on hematopoietic cells. Eur. J. Haematol. 2003, 70, 43–52. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurhayati, R.W.; Cahyo, R.D.; Pratama, G.; Anggraini, D.; Mubarok, W.; Kobayashi, M.; Antarianto, R.D. Alginate-Chitosan Microencapsulated Cells for Improving CD34+ Progenitor Maintenance and Expansion. Appl. Sci. 2021, 11, 7887. https://doi.org/10.3390/app11177887
Nurhayati RW, Cahyo RD, Pratama G, Anggraini D, Mubarok W, Kobayashi M, Antarianto RD. Alginate-Chitosan Microencapsulated Cells for Improving CD34+ Progenitor Maintenance and Expansion. Applied Sciences. 2021; 11(17):7887. https://doi.org/10.3390/app11177887
Chicago/Turabian StyleNurhayati, Retno Wahyu, Rafianto Dwi Cahyo, Gita Pratama, Dian Anggraini, Wildan Mubarok, Mime Kobayashi, and Radiana Dhewayani Antarianto. 2021. "Alginate-Chitosan Microencapsulated Cells for Improving CD34+ Progenitor Maintenance and Expansion" Applied Sciences 11, no. 17: 7887. https://doi.org/10.3390/app11177887
APA StyleNurhayati, R. W., Cahyo, R. D., Pratama, G., Anggraini, D., Mubarok, W., Kobayashi, M., & Antarianto, R. D. (2021). Alginate-Chitosan Microencapsulated Cells for Improving CD34+ Progenitor Maintenance and Expansion. Applied Sciences, 11(17), 7887. https://doi.org/10.3390/app11177887





