Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Microorganisms
2.2. Technological Treatment
2.2.1. Fermentation
Preparation of Inocula
Fermentation Process
2.2.2. Juice Pressing
2.2.3. Freeze-Drying
2.2.4. Convective Drying
2.3. Analytical Method
2.3.1. Dry Matter and Water Activity
2.3.2. Color Analysis
2.3.3. Betalain Analysis
2.3.4. Carotenoids Analysis
2.3.5. Microbiological Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Pomace
3.1.1. Pomace Water Activity (aw) and Dry Matter (d.m.)
3.1.2. Pomace Color
3.2. Pigment Content
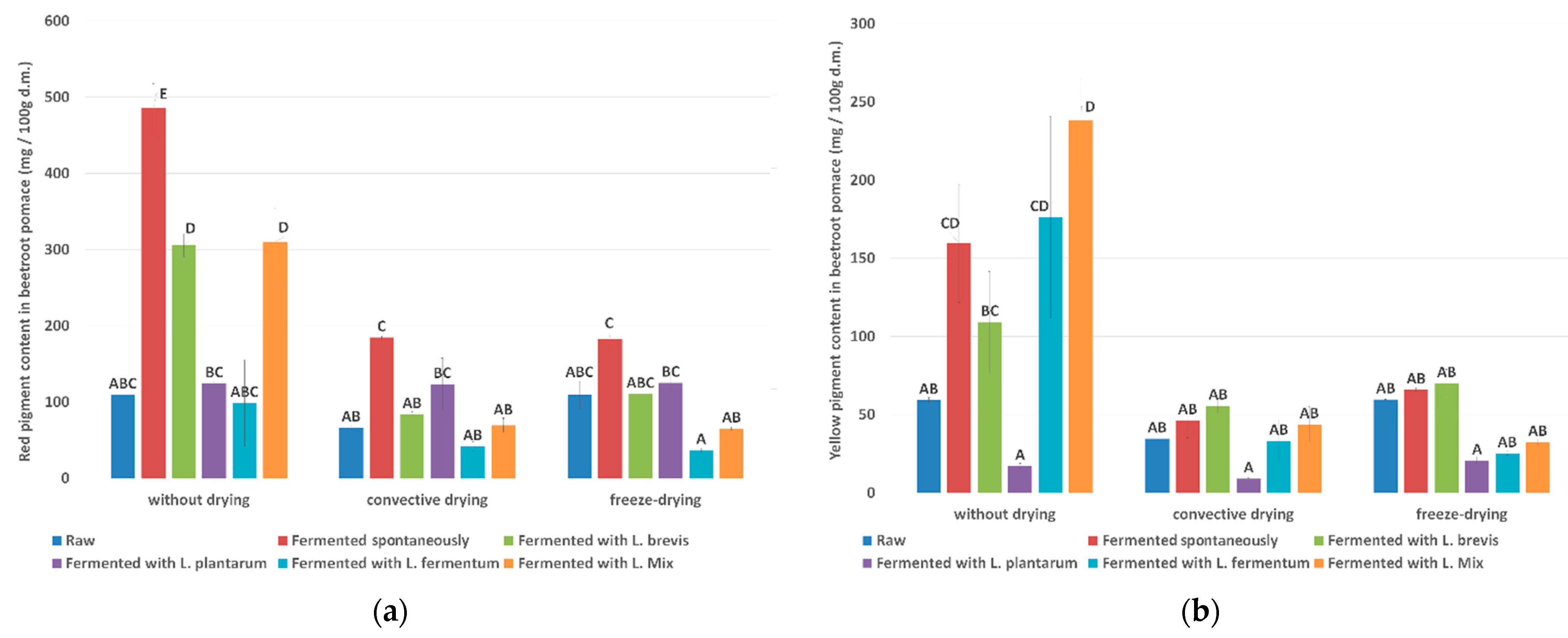
3.2.1. Pigment Content in Beetroot Pomace
3.2.2. Pigment Content in Carrot and Red Pepper Pomace
3.3. Effect of Convection Drying and Freeze-Drying on the Concentration of Lactic Acid Bacteria in Pomace
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaręba, D.; Ziarno, M. Alternatywne probiotyczne napoje warzywne i owocowe. Bromatol. Chem. Toksykol. 2011, 2, 160–168. [Google Scholar]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Nair, M.R.; Chouhan, D.; Sen Gupta, S.; Chattopadhyay, S. Fermented foods: Are they tasty medicines for Helicobacter pylori associated peptic ulcer and gastric cancer? Front. Microbiol. 2016, 7, 1148. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Sant′Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- de Souza, J.V.; Dias, F.S. Protective, technological, and functional properties of select autochthonous lactic acid bacteria from goat dairy products. Curr. Opin. Food Sci. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, X.; Laaksonen, O.; Liu, X.; Shao, Y.; Zhao, H.; Zhang, B.; Zhu, B. Effect of Lactobacillus acidophilus, Oenococcus oeni, and Lactobacillus brevis on composition of bog bilberry juice. Foods 2019, 8, 430. [Google Scholar] [CrossRef] [Green Version]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Malik, M.; Bora, J.; Sharma, V. Growth studies of potentially probiotic lactic acid bacteria (Lactobacillus plantarum, Lactobacillus acidophilus, and Lactobacillus casei) in carrot and beetroot juice substrates. J. Food Process. Preserv. 2019, 43, e14214. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and chemical properties of chokeberry juice fermented by novel lactic acid bacteria with potential probiotic properties during fermentation at 4 degrees C for 4 weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Okonko, I.O.; Adeola, O.; Aloysius, F.; Damilola, A.; Adewale, O. Utilization of food wastes for sustainable development. Electron. J. Environ. Agric. Food Chem. 2009, 8, 263–286. [Google Scholar]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Application of fermentation to recover high-added value compounds from food by-products: Antifungals and Antioxidants. Ferment. Process. Emerg. Conv. Technol. 2021, 195–219. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Grzybowska, E.; Konopka, E.; Witrowa-Rajchert, D. The influence of different pretreatment methods on color and pigment change in beetroot products. Molecules 2021, 26, 3683. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S. Extruded snacks from industrial by-products: A review. Trends Food Sci. Technol. 2020, 99, 284–294. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of different drying techniques on physical properties, total polyphenols and antioxidant capacity of blackcurrant pomace powders. LWT 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Šeregelj, V.; Ćetković, G.; Čanadanović-Brunet, J.; Šaponjac, V.T.; Vulić, J.; Lević, S.; Nedović, V.; Brandolini, A.; Hidalgo, A. Encapsulation of carrot waste extract by freeze and spray drying techniques: An optimization study. LWT-Food Sci. Technol. 2021, 138, 110696. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The quality of red bell pepper subjected to freeze-drying preceded by traditional and novel pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Brzozowska, A.; Gramza-Michałowska, A. The effect of plant additives on the stability of polyphenols in dried black chokeberry (Aronia melanocarpa) fruit. Foods 2021, 10, 44. [Google Scholar] [CrossRef]
- Janowicz, M.; Lenart, A. Selected physical properties of convection dried apples after HHP treatment. LWT-Food Sci. Technol. 2015, 63, 828–836. [Google Scholar] [CrossRef]
- Piotrowski, D.; Kostyra, E.; Grzegory, P.; Janiszewska-Turak, E. Influence of drying methods on the structure, mechanical and sensory properties of strawberries. Eur. Food Res. Technol. 2021, 247, 1859–1867. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef]
- Różyło, R. Recent trends in methods used to obtain natural food colorants by freeze-drying. Trends Food Sci. Technol. 2020, 102, 39–50. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Polak, R.; Biernacka, B. Effect of pre-treatment conditions and freeze-drying temperature on the process kinetics and physicochemical properties of pepper. LWT 2018, 98, 25–30. [Google Scholar] [CrossRef]
- LaTorre-Snyder, M. Lyophilization: The basics. Pharm. Process. 2017, 32, 24–25. [Google Scholar]
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant potential of phytochemicals in pumpkin varieties belonging to Cucurbita moschata and Cucurbita pepo species. CyTA-J. Food 2020, 18, 472–484. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and quality aspects of freeze-drying preceded by traditional and novel pre-treatment methods as exemplified by red bell pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Drobot, V.; Kovbasa, V.; Bondarenko, Y.; Bilyk, O.; Hryshchenko, A. Use of dried carrot pomace in the technology of wheat bread for elderly people. Food Sci. Technol. 2019, 13, 98–105. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Sarkar, B.C.; Sharma, H.K. Mathematical modelling of thin layer hot air drying of carrot pomace. J. Food Sci. Technol. 2012, 49, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Brandolini, A.; Canadanovic-Brunet, J.; Cetkovic, G.; Tumbas Saponjac, V. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef]
- Tumbas Saponjac, V.; Canadanovic-Brunet, J.; Cetkovic, G.; Jakisic, M.; Djilas, S.; Vulic, J.; Stajcic, S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Hornowska, Ł.; Pobiega, K.; Gniewosz, M.; Witrowa-Rajchert, D. The influence of Lactobacillus bacteria type and kind of carrier on the properties of spray-dried microencapsules of fermented beetroot powders. Int. J. Food Sci. Technol. 2021, 56, 2166–2174. [Google Scholar] [CrossRef]
- Janiszewska, E.; Włodarczyk, J. Influence of spray drying conditions on the beetroot pigments retention after microencapsulation process. Acta Agrophysica 2013, 20, 343–356. [Google Scholar]
- Janiszewska-Turak, E.; Witrowa-Rajchert, D. The influence of carrot pretreatment, type of carrier and disc speed on the physical and chemical properties of spray-dried carrot juice microcapsules. Dry. Technol. 2021, 39, 439–449. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Changes of mechanical and thermal properties of cranberries subjected to ultrasound treatment. Int. J. Food Eng. 2017, 13, 20160306. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Nowacka, M.; Witrowa-Rajchert, D. Drying kinetics, microstructure and antioxidant properties of basil treated by ultrasound. J. Food Process. Eng. 2017, 40, e12271. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Janiszewska, E.; Witrowa-Rajchert, D.; Kidon, M.; Czapski, J. Effect of the applied drying method on the physical properties of purple carrot pomace. Int. Agrophys. 2013, 27, 143–149. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Di Scala, K.; Crapiste, G. Drying kinetics and quality changes during drying of red pepper. LWT-Food Sci. Technol. 2008, 41, 789–795. [Google Scholar] [CrossRef]
- Labuza, T.P.; Altunakar, B.; Barbosa-Canovas, G.V.; Fontana, A.J.; Schmidt, S.J. Water Activity in Foods: Fundamentals and Applications; Wiley-Blackwell: Isengard, HD, USA, 2007; p. 109. [Google Scholar]
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water sorption and glass transition temperature of spray dried açai (Euterpe oleracea Mart.) juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- Barbosa, J.; Brandão, T.R.S.; Teixeira, P. Spray drying conditions for orange juice incorporated with lactic acid bacteria. Int. J. Food Sci. Technol. 2017, 52, 1951–1958. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Takhar, P.S. Physical and viscoelastic properties of carrots during drying. J. Texture Stud. 2020, 51, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, İ. Drying kinetics, rehydration and colour characteristics of convective hot-air drying of carrot slices. Heat Mass Transf. 2016, 53, 25–35. [Google Scholar] [CrossRef]
- Pinar, H.; Çetin, N.; Ciftci, B.; Karaman, K.; Kaplan, M. Biochemical composition, drying kinetics and chromatic parameters of red pepper as affected by cultivars and drying methods. J. Food Compos. Anal. 2021, 102, 103976. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red beetroot betalains: Perspectives on extraction, processing, and potential health benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.Y.; Cho, Y.J.; Kim, M.S.; Kim, Y.S. Determination of volatiles and carotenoid degradation compounds in red pepper fermented by Lactobacillus parabuchneri. J. Food Sci. 2018, 83, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Vidmantiene, D.; Juodeikiene, G.; Viskelis, P.; Urbonaviciene, D. Lactic acid fermentation of tomato: Effects on cis/trans lycopene isomer ratio, β-carotene mass fraction and formation of L (+)-and D (−)-lactic acid. Food Technol. Biotechnol. 2013, 51, 471–478. [Google Scholar]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001. [Google Scholar]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar]
- Tang, S.; Cheng, Y.; Wu, T.; Hu, F.; Pan, S.; Xu, X. Effect of Lactobacillus plantarum-fermented mulberry pomace on antioxidant properties and fecal microbial community. LWT 2021, 147, 111651. [Google Scholar] [CrossRef]

| Sample Name | Dry Matter d.m. (%) | Water Activity aw (-) | Color | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| B | 12.1 ± 0.2 b | - | 13.1 ± 1.2 a | 17.7 ± 2.9 efg | 1.9 ± 0.8 d |
| B_CD | 88.5 ± 1.4 c | 0.42 ± 0.01 f | 21.0 ± 0.7 c | 11.9 ± 1.1 abc | 1.9 ± 0.4 d |
| B_FD | 97.5 ± 0.3 f | 0.22 ± 0.00 b | 34.0 ± 0.7 f | 16.1 ± 0.3 def | 5.0 ± 0.4 fgh |
| B_SF | 5.2 ± 0.6 a | - | 27.7 ± 1.7 d | 23.9 ± 0.7 h | −2.8 ± 0.1 b |
| B_SF_CD | 92.6 ± 0.8 de | 0.37 ± 0.00 d | 16.1 ± 2.0 ab | 16.5 ± 2.1 def | 0.1 ± 0.3 c |
| B_SF_FD | 96. ± 0.13 ef | 0.23 ± 0.01 b | 27.7 ± 1.7 d | 23.9 ± 0.7 h | −2.8 ± 0.1 b |
| B_LB | 2.9 ± 0.6 a | - | 18.3 ± 0.4 bc | 14.3 ± 1.2 cde | 3.9 ± 0.5 ef |
| B_LB_CD | 90.5 ± 0.0 cd | 0.41 ± 0.00 e | 13. ± 1.15 a | 11.6 ± 1.3 abc | 2.3 ± 0.4 d |
| B_LB_FD | 95.2 ± 1.3 ef | 0.24 ± 0.01 b | 31.3 ± 0.4 def | 17.6 ± 0.3 def | 3.3 ± 0.3 de |
| B_LP | 9.3 ± 0.1 b | - | 14.3 ± 1.8 ab | 21.1 ± 2.2 gh | −1.7 ± 0.9 b |
| B_LP_CD | 90.2 ± 2.4 cd | 0.46 ± 0.01 g | 16.3 ± 1.2 ab | 9.2 ± 1.2 a | −2.5 ± 0.2 b |
| B_LP_FD | 94.9 ± 0.1 ef | 0.14 ± 0.00 a | 29.4 ± 0.6 de | 18.4 ± 0.3 fg | −5.6 ± 0.2 a |
| B_LF | 4.0 ± 0.0 a | - | 21.1 ± 0.5 c | 13.1 ± 0.9 bcd | 6.0 ± 0.4 h |
| B_LF_CD | 95.3 ± 0.1 ef | 0.27 ± 0.00 c | 15.0 ± 7.3 ab | 9.5 ± 4.7 a | 3.0 ± 1.5 d |
| B_LF_FD | 95.6 ± 0.4 ef | 0.24 ± 0.00 b | 32.8 ± 1.2 ef | 13.3 ± 1.1 bcd | 4.4 ± 0.5 efg |
| B_LMIX | 4.6 ± 0.0 a | - | 16.2 ± 0.3 ab | 18.0 ± 0.7 efg | 5.8 ± 0.4 gh |
| B_LMIX_CD | 90.8 ± 1.6 cd | 0.45 ± 0.01 g | 14.8 ± 1.5 ab | 11.5 ± 1.6 abc | 1.6 ± 0.7 d |
| B_LMIX_FD | 96.4 ± 0.2 ef | 0.24 ± 0.00 b | 33.6 ± 1.1 ef | 17.6 ± 1.0 efg | 3.2 ± 0.4 de |
| C | 16.8 ± 0.1 a | - | 45.3 ± 1.3 b | 26.5 ± 0.4 bcd | 34.9 ± 0.4 ghi |
| C_CD | 89.8 ± 0.1 b | 0.49 ± 0.02 c | 38.1 ± 1.3 a | 23.2 ± 2.0 a | 23.9 ± 2.2 a |
| C_FD | 96.7 ± 0.1 cd | 0.21 ± 0.00 a | 49.2 ± 0.9 c | 23.3 ± 0.4 a | 26.1 ± 0.6 a–d |
| C_SF | 11.6 ± 1.1 a | - | 44.2 ± 1.2 b | 29.0 ± 0.2 de | 32.3 ± 0.3 fgh |
| C_SF_CD | 91.6 ± 0.1 bc | 0.32 ± 0.00 b | 49.5 ± 0.3 c | 30.2 ± 1.2 e | 29.3 ± 0.7 def |
| C_SF_FD | 94.4 ± 0.6 bcd | 0.22 ± 0.01 a | 58.0 ± 1.5 d | 24.4 ± 1.0 ab | 25.6 ± 1.1 ab |
| C_LB | 14.5 ± 0.6 a | - | 45.2 ± 2.2 b | 28.4 ± 1.8 de | 34.9 ± 2.5 ghi |
| C_LB_CD | 93.4 ± 0.4 | 0.35 ± 0.00 b | 42.3 ± 1.9 b | 30.6 ± 1.2 e | 29.5 ± 1.2 def |
| C_LB_FD | 97.9 ± 0.4 d | 0.17 ± 0.01 a | 58.0 ± 1.6 d | 26.7 ± 0.6 b-d | 30.3 ± 0.6 ef |
| C_LP | 14.7 ± 0.0 a | - | 44.3 ± 1.0 b | 30.7 ± 0.3 e | 36.2 ± 0.3 hi |
| C_LP_CD | 95.0 ± 0.1 bcd | 0.45 ± 0.07 c | 38.7 ± 2.7 a | 24.0 ± 3.7 ab | 25.3 ± 5.5 ab |
| C_LP_FD | 97.3 ± 0.7 cd | 0.22 ± 0.01 a | 58.1 ± 2.5 d | 26.8 ± 1.7 b–e | 30.5 ± 1.0 b–f |
| C_LF | 12.9 ± 0.5 a | - | 45.6 ± 1.1 b | 29.2 ± 0.4 de | 35.3 ± 1.0 ghi |
| C_LF_CD | 91.6 ± 0.1 bc | 0.46 ± 0.01 c | 43.0 ± 1.9 b | 27.8 ± 1.4 cde | 27.4 ± 1.0 a–e |
| C_LF_FD | 93.6± 2.7 bdc | 0.14 ± 0.01 a | 58.3 ± 0.5 d | 24.4 ± 1.2 ab | 27.6 ± 0.6 a–e |
| C_LMIX | 11.0 ± 0.4 a | - | 44.9 ± 1.2 b | 29.8 ± 1.7 e | 37.3 ± 1.6 i |
| C_LMIX_CD | 89.5 ± 2.4 b | 0.35 ± 0.00 b | 43.9 ± 2.4 b | 28.3 ± 1.9 de | 29.4 ± 1.8 def |
| C_LMIX_FD | 97.4 ± 0.1 cd | 0.16 ± 0.00 a | 55.6 ± 0.1 d | 25.5 ± 1.0 abc | 28.7 ± 0.0 b–e |
| P | 13.0 ± 0.5 a | - | 30.7 ± 1.8 abc | 27.0 ± 2.7 bcd | 27.9 ± 2.3 e–h |
| P_CD | 81.9 ± 0.1 b | 0.48 ± 0.07 bc | 34.9 ± 1.9 cd | 24.2 ± 0.6 abc | 20.3 ± 1.7 abc |
| P_FD | 91.7 ± 0.3 cd | 0.19 ± 0.01 a | 38.8 ± 4.8 de | 20.5 ± 3.9 a | 19.0 ± 2.2 a |
| P_SF | 9.9 ± 0.5 a | - | 31.0 ± 0.6 abc | 31.3 ± 3.8 def | 22.9 ± 2.6 a–d |
| P_SF_CD | 82.9 ± 0.4 b | 0.50 ± 0.00 d | 38.3 ± 1.7 de | 32.2 ± 1.7 ef | 25.7 ± 1.8 d–g |
| P_SF_FD | 93.2 ± 0.0 d | 0.31 ± 0.00 b | 37.0 ± 3.3 de | 24.5 ± 3.3 abc | 26.8 ± 1.2 d–g |
| P_LB | 8.6 ± 0.4 a | - | 26.9 ± 0.8 a | 30.7 ± 1.9 def | 30.0 ± 1.5 ghi |
| P_LB_CD | 83.9 ± 3.6 b | 0.48 ± 0.00 c | 38.3 ± 1.4 de | 31.5 ± 1.5 def | 23.9 ± 1.9 b–e |
| P_LB_FD | 95.0 ± 0.1 d | 0.12 ± 0.00 a | 40.7 ± 4.5 ef | 21.9 ± 3.5 ab | 22.9 ± 3.4 a–d |
| P_LP | 8.6 ± 0.2 a | - | 28.9 ± 0.6 a | 35.8 ± 1.0 f | 33.1 ± 2.3 i |
| P_LP_CD | 86.6 ± 1.7 bc | 0.42 ± 0.00 c | 44.6 ± 2.1 f | 27.5 ± 3.1 cde | 29.1 ± 2.5 e–h |
| P_LP_FD | 96.3 ± 0.3 d | 0.31 ± 0.00 b | 30.4 ± 3.0 abc | 26.7 ± 4.9 bcd | 19.2 ± 4.8 ab |
| P_LF | 8.4 ± 0.0 a | - | 26.9 ± 1.6 a | 31.5 ± 1.0 def | 29.9 ± 2.3 f–i |
| P_LF_CD | 82.5 ± 0.2 b | 0.42 ± 0.00 c | 37.5 ± 1.2 de | 31.8 ± 0.6 def | 27.8 ± 1.1 e–h |
| P_LF_FD | 96.7 ± 0.0 d | 0.31 ± 0.00 a | 40.6 ± 1.8 ef | 25.3 ± 1.3 abc | 31.4 ± 1.8 hi |
| P_LMIX | 9.0 ± 0.2 a | - | 29.9 ± 0.8 ab | 34.3 ± 1.4 f | 33.4 ± 2.0 i |
| P_LMIX_CD | 94.1 ± 0.2 d | 0.44 ± 0.00 c | 31.5 ± 1.1 abc | 24.7 ± 0.6 abc | 20.6 ± 1.2 abc |
| P_LMIX_FD | 94.3 ± 0.0 d | 0.33 ± 0.00 b | 34.5 ± 5.6 bcd | 23.4 ± 2.2 abc | 24.6 ± 4.5 c–f |
| Raw | FD | CD | |
|---|---|---|---|
| log CFU/gs.d. ± SD | |||
| B_LB | 6.70 ± 0.02 | 2.93 ± 0.06 | <1 |
| B_LP | 6.61 ± 0.02 | 2.46 ± 0.12 | <1 |
| B_LF | 6.63 ± 0.03 | 2.82 ± 0.06 | <1 |
| B_LMIX | 5.06 ± 0.08 | 2.04 ± 0.03 | <1 |
| C_LB | 6.29 ± 0.20 | 2.56 ± 0.06 | <1 |
| C_LP | 6.05 ± 0.06 | 3.06 ± 0.05 | <1 |
| C_LF | 6.72 ± 0.06 | 3.34 ± 0.04 | <1 |
| C_LMIX | 5.65 ± 0.01 | 2.86 ± 0.03 | <1 |
| P_LB | 6.65 ± 0.04 | 2.95 ± 0.04 | <1 |
| P_LP | 7.40 ± 0.02 | 3.48 ± 0.04 | <1 |
| P_LF | 7.16 ± 0.06 | 3.62 ± 0.03 | <1 |
| P_LMIX | 6.07 ± 0.09 | 2.94 ± 0.02 | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Appl. Sci. 2021, 11, 7864. https://doi.org/10.3390/app11177864
Janiszewska-Turak E, Kołakowska W, Pobiega K, Gramza-Michałowska A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Applied Sciences. 2021; 11(17):7864. https://doi.org/10.3390/app11177864
Chicago/Turabian StyleJaniszewska-Turak, Emilia, Weronika Kołakowska, Katarzyna Pobiega, and Anna Gramza-Michałowska. 2021. "Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria" Applied Sciences 11, no. 17: 7864. https://doi.org/10.3390/app11177864
APA StyleJaniszewska-Turak, E., Kołakowska, W., Pobiega, K., & Gramza-Michałowska, A. (2021). Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Applied Sciences, 11(17), 7864. https://doi.org/10.3390/app11177864









