Reflected Light Spectrometry and AI-Based Data Analysis for Detection of Rapid Chicken Eggshell Change Caused by Mycoplasma Synoviae
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
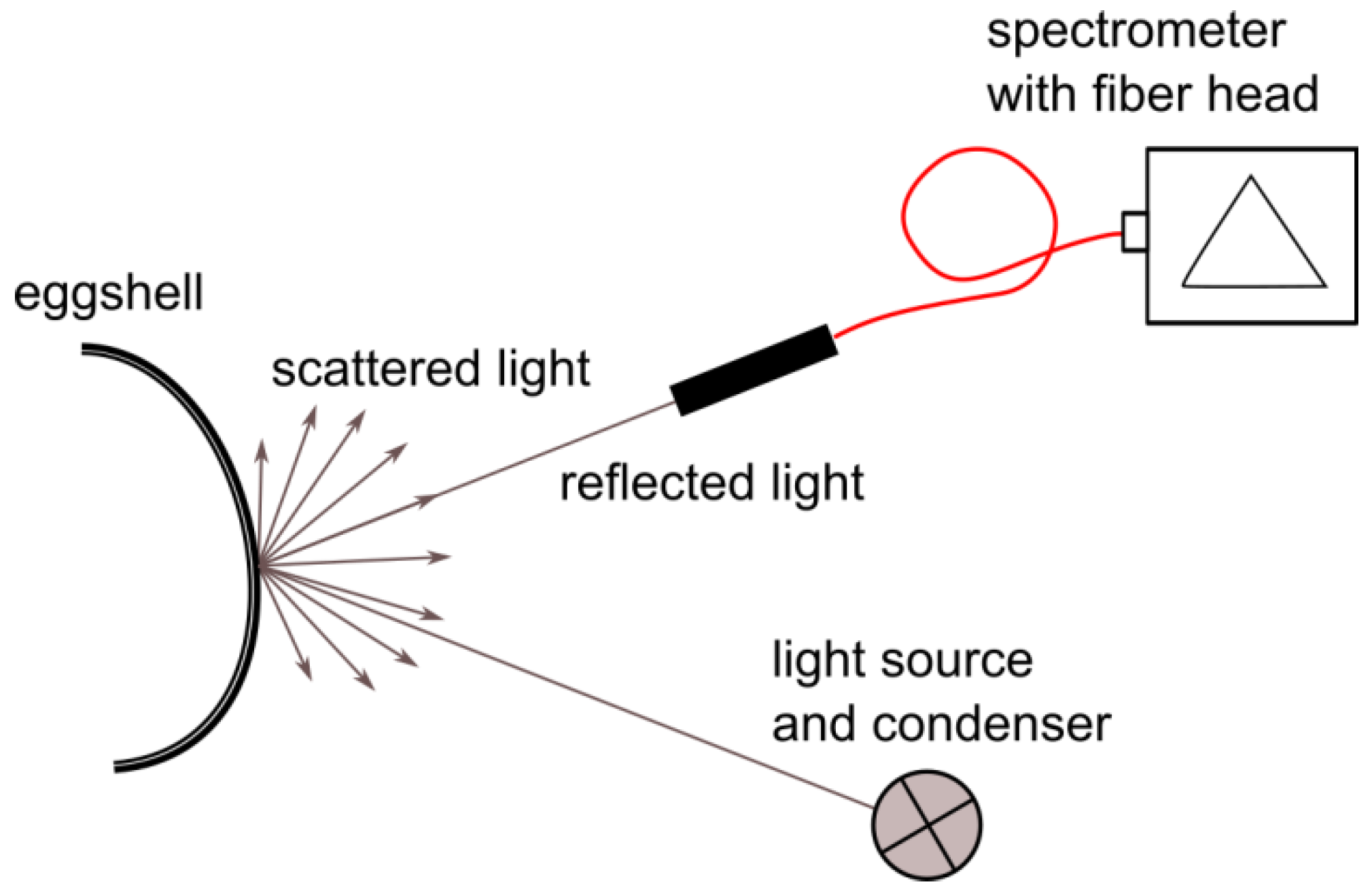
2.1. Spectral Data Acquisition
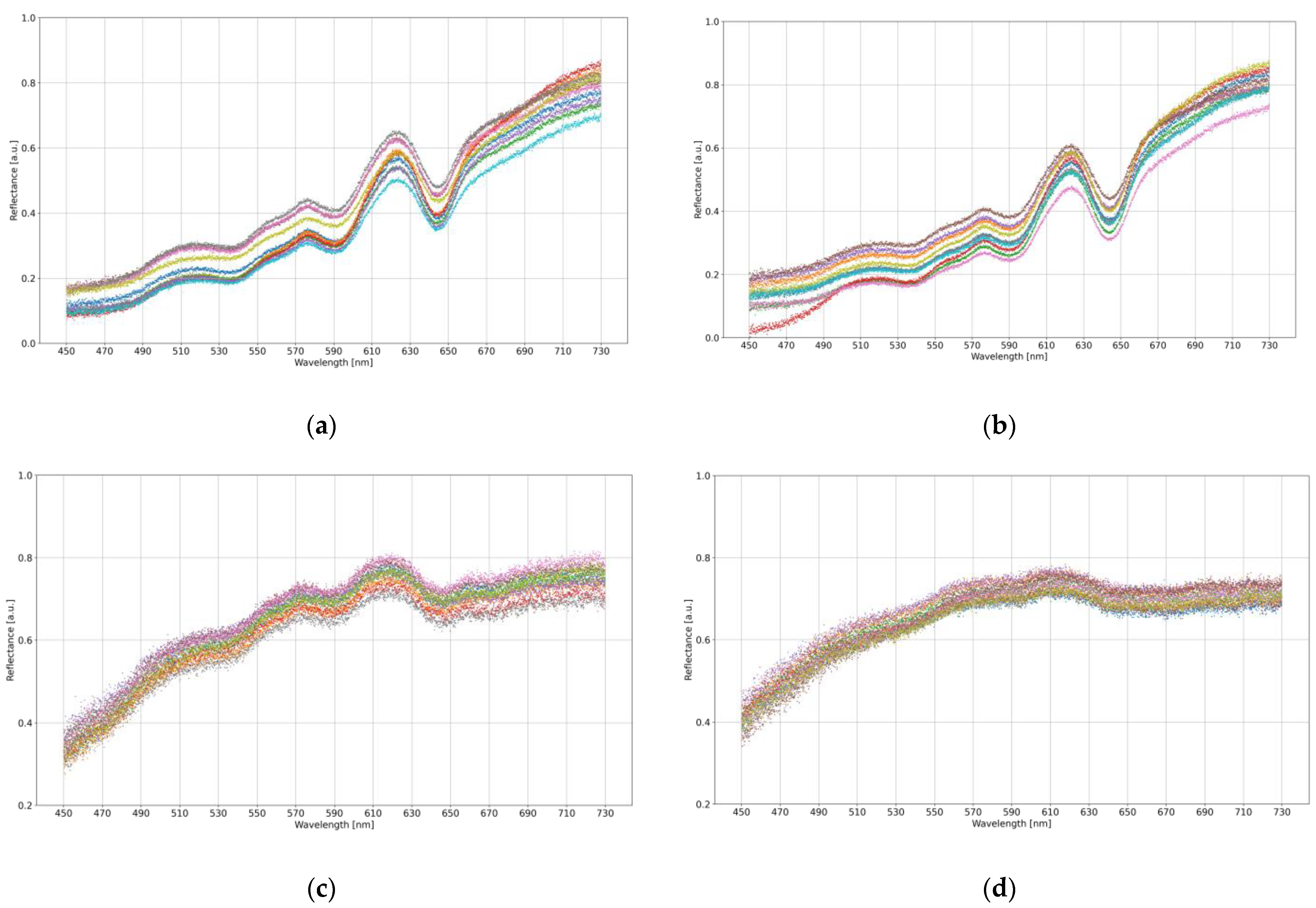
2.2. Obtained Spectral Data—Initial Analysis
2.3. Support Vector Machine
2.4. Metrics
2.5. Hyperparameters Optimization
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleven, S. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult. Sci. 1998, 77, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Landman, W.J.M.; Feberwee, A. Aerosol-induced Mycoplasma synoviae arthritis: The synergistic effect of infectious bronchitis virus infection. Avian Pathol. 2004, 33, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Feberwee, A.; De Wit, J.J.; Landman, W.J.M. Induction of eggshell apex abnormalities by Mycoplasma synoviae: Field and experimental studies. Avian Pathol. 2009, 38, 77–85. [Google Scholar] [CrossRef]
- Kursa, O.; Pakuła, A.; Tomczyk, G.; Paśko, S.; Sawicka, A. Eggshell apex abnormalities caused by two different Mycoplasma synoviae genotypes and evaluation of eggshell anomalies by full-field optical coherence tomography. BMC Vet. Res. 2019, 15, 1. [Google Scholar] [CrossRef]
- Bondoc, I. The veterinary sanitary control of egg and egg products. In Veterinary Health Control of Food Quality and Safety; Bondoc, I., Șindilar, E.V., Eds.; Iași Publishing: Iași, Romania, 2002; Volume I, pp. 377–396. ISBN 973-8014-64-6. [Google Scholar]
- Bondoc, I. The official veterinary sanitary control of table eggs. In Control of Products and Food of Animal Origin; Iași Publishing: Iași, Romania, 2014; Volume I, pp. 222–263. ISBN 978-973-147-139-6. [Google Scholar]
- Solomon, S. The eggshell: Strength, structure and function. Br. Poult. Sci. 2010, 51, 52–59. [Google Scholar] [CrossRef]
- Hunton, P. Research on eggshell structure and quality: An historical overview. Braz. J. Poult. Sci. 2005, 7, 67–71. [Google Scholar] [CrossRef]
- Lierz, A.M.; Obon, E.; Schink, B.; Carbonell, F.; Hafez, H.M. The role of mycoplasmas in a conservation project of the lesser kestrel (Falco naumanni). Avian Dis. 2008, 52, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Kursa, O.; Tomczyk, G.; Sawicka, A. Effect of Mycoplasma synoviae strains isolated from the respiratory and reproductive tracts of chicken on SPF chicken embryos. Med. Weter. Red. 2019, 75, 54–58. [Google Scholar] [CrossRef]
- Ferguson-Noel, N.; Noormohammadi, A.H. Mycoplasma synoviae infection. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V.L., Eds.; Wiley: Ames, IA, USA, 2013; pp. 900–906. [Google Scholar]
- Bencina, D. Haemagglutinins of pathogenic avian mycoplasmas. Avian Pathol. 2002, 31, 535–547. [Google Scholar] [CrossRef]
- OIE. Avian Mycoplasmosis (Mycoplasma gallisepticum, M. synoviae). In OIE Terrestrial Manual; World Organisation for Animal Health: Paris, France, 2018; Chapter 3.3.5; pp. 68–70. [Google Scholar]
- Kleven, S.H.; Eidson, C.S.; Anderson, D.P.; Fletcher, O.J. Decrease of antibody response to Mycoplasma synoviae in chickens infected with Marek’s disease herpesvirus. Am. J. Veter. Res. 1972, 33, 2037–2042. [Google Scholar]
- Feberwee, A.; de Vries, T.S.; Landman, W.J.M. Seroprevalence of Mycoplasma synoviae in Dutch commercial poultry farms. Avian Pathol. 2008, 37, 629–633. [Google Scholar] [CrossRef]
- Hong, Y.; García, M.; Leiting, V.; Bencina, D.; Dufour-Zavala, L.; Zavala, G.; Kleven, S.H. Specific detection and typing of Mycoplasma synoviae strains in poultry with PCR and DNA sequence analysis targeting the hemagglutinin encoding gene vlhA. Avian Dis. 2004, 48, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.P.; de Vargas, T.; Ikuta, N.; Fonseca, A.S.K.; Celmer, Á.J.; Marques, E.K.; Lunge, V.R. A multiplex real-time PCR for detection of Mycoplasma gallisepticum and Mycoplasma synoviae in clinical samples from Brazilian commercial poultry flocks. Braz. J. Microbiol. 2013, 44, 505–510. [Google Scholar] [CrossRef][Green Version]
- Kursa, O.; Woźniakowski, G.; Tomczyk, G.; Sawicka, A.; Minta, Z. Rapid detection of Mycoplasma synoviae by loop-mediated isothermal amplification. Arch. Microbiol. 2014, 197, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.P.; Ramírez, A.S.; Morrow, C.J.; Bradbury, J.M. Development and evaluation of an improved diagnostic PCR for Mycoplasma synoviae using primers located in the haemagglutinin encoding gene vlhA and its value for strain typing. Vet. Microbiol. 2009, 136, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kursa, O.; Tomczyk, G.; Sawicka, A. Prevalence and phylogenetic analysis of Mycoplasma synoviae strains isolated from Polish chicken layer flocks. J. Veter. Res. 2019, 63, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, X.; Chen, Q.; Zuo, K.; Zhou, Y.; Zhang, Z.; Kan, Y.; Yao, L.; Ji, J.; Bi, Y.; et al. Rapid and visible detection of Mycoplasma synoviae using a novel polymerase spiral reaction assay. Poult. Sci. 2019, 98, 5355–5360. [Google Scholar] [CrossRef]
- Lorenc, Z.; Paśko, S.; Kursa, O.; Pakuła, A.; Sałbut, L. Spectral technique for detection of changes in eggshells caused by Mycoplasma synoviae. Poult. Sci. 2019, 98, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Raviv, Z.; Kleven, S.H. The development of diagnostic real-time TaqMan PCRs for the four pathogenic avian mycoplasmas. Avian Dis. 2009, 53, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Patle, A.; Chouhan, D.S. SVM kernel functions for classification. In Proceedings of the 2013 International Conference on Advanced ICT (Information and Communication Technology) for Education (ICAICTE2013), Hainan, China, 20–22 September 2013. [Google Scholar]
- Wang, J.; Chen, Q.; Chen, Y. RBF kernel based support vector machine with universal approximation and its application. Comput. Vis. 2004, 3173, 512–517. [Google Scholar] [CrossRef]
- Bharadwaj, K.; Prakash, B.; Kanagachidambaresan, G.R. Sparse kernel machines. In Pattern Recognition and Machine Learning; Springer: Berlin, Germany, 2021; Chapter 7. [Google Scholar]
- Géron, A. Classification. In Hands-On Machine Learning with Scikit-Learn, Keras, and TensorFlow; O’Reilly Media Inc.: Sebastopol, CA, USA, 2019; Chapter 3. [Google Scholar]
- Lameski, P.; Zdravevski, E.; Mingov, R.; Kulakov, A. SVM parameter tuning with grid search and its impact on reduction of model over-fitting. In Rough Sets, Fuzzy Sets, Data Mining, and Granular Computing; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Luque, A.; Carrasco, A.; Martín, A.; Heras, A.D.L. The impact of class imbalance in classification performance metrics based on the binary confusion matrix. Pattern Recognit. 2019, 91, 216–231. [Google Scholar] [CrossRef]
- Arora, M.; Bhambhu, L.; Scholar, M.T. Role of scaling in data classification using SVM. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2014, 4, 2277. [Google Scholar]
| Origin | Brown | White |
|---|---|---|
| Healthy | 315 | 516 |
| MS-infected | 392 | 252 |
| Total | 707 | 768 |
| 1475 | ||
| Input Variable | Wavelength (nm) | FWHM (nm) |
|---|---|---|
| A0 | 457 | 3 |
| A1 | 473 | 3 |
| A2 | 501 | 3 |
| A3 | 515 | 3 |
| A4 | 523 | 3 |
| A5 | 526 | 3 |
| A6 | 532 | 3 |
| A7 | 543 | 3 |
| A8 | 556 | 3 |
| A9 | 561 | 3 |
| A10 | 589 | 3 |
| A11 | 593 | 3 |
| A12 | 632 | 3 |
| A13 | 660 | 3 |
| A14 | 671 | 3 |
| A15 | 690 | 3 |
| A16 | 729 | 3 |
| Ranking Position | Number of Wavelengths | Combination of Parameters | 5 CV Mean (%) | 5 CV SD |
|---|---|---|---|---|
| 1 | 7 | A10, A11, A12, A13, A14, A15, A0 | 82.473 | 7.683 |
| 2 | 6 | A11, A12, A13, A14, A14, A0 | 82.438 | 8.068 |
| 3 | 5 | A12, A13, A14, A15, A0 | 82.431 | 7.965 |
| 4 | 7 | A11, A12, A13, A14, A15, A16, A0 | 82.421 | 8.247 |
| 5 | 8 | A10, A11, A12, A13, A14, A15, A16, A0 | 82.271 | 8.249 |
| 6 | 9 | A4, A7, A8, A11, A12, A13, A14, A15, A0 | 82.118 | 8.561 |
| 7 | 4 | A11, A12, A13, A0 | 82.061 | 10.84 |
| 8 | 7 | A5, A11, A12, A13, A14, A15, A0 | 81.958 | 8.375 |
| 9 | 7 | A6, A11, A12, A13, A14, A15, A0 | 81.931 | 9.018 |
| 10 | 3 | A12, A13, A0 | 81.928 | 10.986 |
| 11 | 8 | A9, A10, A11, A12, A13, A14, A15, A0 | 81.921 | 8.058 |
| 12 | 7 | A8, A11, A12, A13, A14, A15, A0 | 81.871 | 8.463 |
| 13 | 8 | A8, A11, A12, A13, A14, A15, A16, A0 | 81.825 | 8.654 |
| 14 | 8 | A4, A8, A11, A12, A13, A14, A15, A0 | 81.807 | 8.756 |
| 15 | 5 | A7, A11, A12, A13, A0 | 81.76 | 10.479 |
| 16 | 4 | A12, A13, A16, A0 | 81.744 | 10.881 |
| 17 | 8 | A6, A8, A11, A12, A13, A14, A15, A0 | 81.723 | 8.451 |
| 18 | 8 | A8, A10, A11, A12, A13, A14, A15, A0 | 81.723 | 8.451 |
| 19 | 8 | A5, A8, A11, A12, A13, A14, A15, A0 | 81.68 | 8.671 |
| 20 | 6 | A8, A12, A13, A14, A15, A0 | 81.671 | 8.088 |
| Ranking Position | Number of Wavelengths | Combination of Parameters | 5 CV Mean (%) | 5 CV SD |
|---|---|---|---|---|
| 1 | 2 | A15, A0 | 73.514 | 8.112 |
| 2 | 7 | A3, A4, A5, A7, A8, A14, A15 | 73.291 | 13.072 |
| 3 | 8 | A3, A4, A5, A6, A7, A8, A14, A15 | 73.163 | 13.077 |
| 4 | 1 | A15 | 73.116 | 6.241 |
| 5 | 6 | A4, A5, A6, A8, A14, A15 | 73.079 | 11.901 |
| 6 | 7 | A3, A4, A6, A7, A8, A14, A15 | 73.051 | 12.939 |
| 7 | 5 | A4, A6, A8, A14, A15 | 72.889 | 11.683 |
| 8 | 6 | A4, A6, A7, A8, A14, A15 | 72.88 | 11.742 |
| 9 | 7 | A3, A4, A5, A6, A8, A14, A15 | 72.854 | 12.534 |
| 10 | 4 | A4, A8, A14, A15 | 72.82 | 11.509 |
| 11 | 6 | A3, A4, A7, A8, A14, A15 | 72.813 | 12.653 |
| 12 | 6 | A3, A4, A5, A8, A14, A15 | 72.802 | 12.431 |
| 13 | 7 | A4, A5, A6, A7, A8 A14, A15 | 72.692 | 11.868 |
| 14 | 5 | A3, A4, A8, A14, A15 | 72.597 | 12.627 |
| 15 | 2 | A15, A16 | 72.581 | 6.521 |
| 16 | 5 | A4, A5, A8, A14, A15 | 72.472 | 11.697 |
| 17 | 6 | A3, A4, A6, A8, A14, A15 | 72.383 | 12.773 |
| 18 | 5 | A4, A7, A8, A14, A15 | 72.329 | 11.573 |
| 19 | 6 | A4, A5, A7, A8, A14, A15 | 72.307 | 11.778 |
| 20 | 8 | A3, A4, A5, A6, A8, A9, A14, A15 | 72.238 | 13.728 |
| Ranking Position | Ranking Position w/o Optimization | Combination of Parameters | C | Gamma |
|---|---|---|---|---|
| 1 | 12 | A8, A11, A12, A13, A14, A15, A0 | 110,217.97 | 0.0046 |
| 2 | 13 | A8, A11, A12, A13, A14, A15, A16, A0 | 92,682.9 | 0.0039 |
| 3 | 17 | A6, A8, A11, A12, A13, A14, A15, A0 | 55,108.98 | 0.0055 |
| 4 | 11 | A9, A10, A11, A12, A13, A14, A15, A0 | 46,340.95 | 0.0110 |
| 5 | 18 | A8, A10, A11, A12, A13, A14, A15, A0 | 8192 | 0.0110 |
| 6 | 1 | A10, A11, A12, A13, A14, A15, A0 | 4096 | 0.0312 |
| 7 | 2 | A11, A12, A13, A14, A14, A0 | 27,554.49 | 0.0220 |
| 8 | 6 | A4, A7, A8, A11, A12, A13, A14, A15, A0 | 55,108.98 | 0.0055 |
| 9 | 14 | A4, A8, A11, A12, A13, A14, A15, A0 | 19,483.96 | 0.0065 |
| 10 | 4 | A11, A12, A13, A14, A15, A16, A0 | 608.87 | 0.0625 |
| 11 | 5 | A10, A11, A12, A13, A14, A15, A16, A0 | 9741.98 | 0.0065 |
| 12 | 8 | A5, A11, A12, A13, A14, A15, A0 | 110,217.97 | 0.0046 |
| 13 | 19 | A5, A8, A11, A12, A13, A14, A15, A0 | 27,554.49 | 0.0055 |
| 14 | 9 | A6, A11, A12, A13, A14, A15, A0 | 110,217.97 | 0.0078 |
| 15 | 20 | A8, A12, A13, A14, A15, A0 | 19,483.96 | 0.0110 |
| 16 | 7 | A11, A12, A13, A0 | 128 | 0.1486 |
| 17 | 15 | A7, A11, A12, A13, A0 | 107.63 | 0.0883 |
| 18 | 16 | A12, A13, A16, A0 | 19,483.96 | 0.0032 |
| 19 | 3 | A12, A13, A14, A15, A0 | 27,554.49 | 0.0092 |
| 20 | 10 | A12, A13, A0 | 5792.61 | 0.0131 |
| Ranking Position | Ranking Position w/o Optimization | Combination of Parameters | C | Gamma |
|---|---|---|---|---|
| 1 | 5 | A4, A5, A6, A8, A14, A15 | 4870.99 | 0.0743 |
| 2 | 9 | A3, A4, A5, A6, A8, A14, A15 | 65,536 | 0.0185 |
| 3 | 20 | A3, A4, A5, A6, A8, A9, A14, A15 | 55,108.98 | 0.0065 |
| 4 | 13 | A4, A5, A6, A7, A8 A14, A15 | 4096 | 0.022 |
| 5 | 2 | A3, A4, A5, A7, A8, A14, A15 | 608.87 | 0.0743 |
| 6 | 19 | A4, A5, A7, A8, A14, A15 | 4096 | 0.0312 |
| 7 | 3 | A3, A4, A5, A6, A7, A8, A14, A15 | 13,777.24 | 0.022 |
| 8 | 12 | A3, A4, A5, A8, A14, A15 | 23,170.47 | 0.0312 |
| 9 | 16 | A4, A5, A8, A14, A15 | 4096 | 0.022 |
| 10 | 8 | A4, A6, A7, A8, A14, A15 | 38,967.93 | 0.0312 |
| 11 | 14 | A3, A4, A8, A14, A15 | 23,170.47 | 0.0185 |
| 12 | 6 | A3, A4, A6, A7, A8, A14, A15 | 77,395.87 | 0.0065 |
| 13 | 7 | A4, A6, A8, A14, A15 | 55,108.98 | 0.011 |
| 14 | 10 | A4, A8, A14, A15 | 9741.98 | 0.0312 |
| 15 | 18 | A4, A7, A8, A14, A15 | 16,384 | 0.0185 |
| 16 | 17 | A3, A4, A6, A8, A14, A15 | 38,967.93 | 0.0131 |
| 17 | 11 | A3, A4, A7, A8, A14, A15 | 4096 | 0.0262 |
| 18 | 1 | A15, A0 | 0.062 | 0.0371 |
| 19 | 15 | A15, A16 | 27,554.49 | 0.1051 |
| 20 | 4 | A15 | 8192 | 0.011 |
| Ranking Position | Ranking from Table 3 | C | Gamma | 5 CV Mean (%) | 5 CV SD |
|---|---|---|---|---|---|
| 1 | 5 | 90.5 | 0.125 | 95.755 | 1.248 |
| 2 | 11 | 107.63 | 0.088 | 95.323 | 2.865 |
| 3 | 6 | 107.63 | 0.105 | 95.174 | 1.951 |
| 4 | 13 | 107.63 | 0.105 | 95.159 | 1.184 |
| 5 | 1 | 90.5 | 0.176 | 95.116 | 1.676 |
| 6 | 4 | 90.5 | 0.125 | 94.912 | 2.364 |
| 7 | 14 | 38.05 | 0.148 | 94.717 | 2.008 |
| 8 | 17 | 107.63 | 0.125 | 94.56 | 1.376 |
| 9 | 9 | 107.63 | 0.125 | 94.511 | 0.868 |
| 10 | 8 | 107.63 | 0.176 | 94.324 | 1.249 |
| 11 | 18 | 76.1 | 0.052 | 94.321 | 1.413 |
| 12 | 19 | 107.63 | 0.088 | 94.13 | 0.829 |
| 13 | 2 | 107.63 | 0.25 | 93.957 | 2.271 |
| 14 | 20 | 45.25 | 0.125 | 93.897 | 0.938 |
| 15 | 12 | 107.63 | 0.148 | 93.751 | 0.815 |
| 16 | 3 | 90.5 | 0.353 | 93.181 | 1.657 |
| 17 | 16 | 9.51 | 0.5 | 90.842 | 1.726 |
| 18 | 15 | 107.63 | 0.074 | 90.569 | 0.880 |
| 19 | 7 | 64 | 0.011 | 89.501 | 1.940 |
| 20 | 10 | 16 | 0.018 | 89.07 | 1.477 |
| Ranking Position | Ranking from Table 4 | C | Gamma | 5 CV Mean (%) | 5 CV SD |
|---|---|---|---|---|---|
| 1 | 12 | 107.63 | 0.176 | 86.210 | 0.719 |
| 2 | 20 | 107.63 | 0.297 | 86.089 | 1.509 |
| 3 | 2 | 107.63 | 0.125 | 86.034 | 0.512 |
| 4 | 3 | 90.5 | 0.21 | 85.991 | 1.897 |
| 5 | 9 | 107.63 | 0.148 | 85.377 | 1.641 |
| 6 | 6 | 107.63 | 0.25 | 84.294 | 1.671 |
| 7 | 11 | 107.63 | 0.125 | 84.07 | 1.418 |
| 8 | 13 | 107.63 | 0.176 | 83.981 | 0.612 |
| 9 | 14 | 107.63 | 0.148 | 83.972 | 1.407 |
| 10 | 17 | 107.63 | 0.062 | 83.366 | 2.723 |
| 11 | 19 | 107.63 | 0.176 | 83.154 | 0.673 |
| 12 | 16 | 107.63 | 0.25 | 82.916 | 0.525 |
| 13 | 5 | 107.63 | 0.125 | 82.532 | 0.970 |
| 14 | 8 | 107.63 | 0.21 | 78.482 | 0.893 |
| 15 | 18 | 107.63 | 0.044 | 76.722 | 0.933 |
| 16 | 7 | 76.1 | 0.062 | 76.384 | 1.56 |
| 17 | 1 | 64 | 0.044 | 75.617 | 2.224 |
| 18 | 10 | 6.72 | 0.088 | 75.17 | 1.836 |
| 19 | 4 | 26.9 | 0.011 | 74.639 | 1.425 |
| 20 | 15 | 6.72 | 0.009 | 74.376 | 2.44 |
| Ranking Position | Number of Wavelengths | Combination of Parameters | 5 CV Mean (%) |
|---|---|---|---|
| 1 | 8 | A10, A11, A12, A13, A14, A15, A16, A0 | 95.755 |
| 2 | 8 | A9, A10, A11, A12, A13, A14, A15, A0 | 95.323 |
| 3 | 9 | A4, A7, A8, A11, A12, A13, A14, A15, A0 | 95.174 |
| 4 | 8 | A8, A11, A12, A13, A14, A15, A16, A0 | 95.159 |
| 5 | 8 | A10, A11, A12, A13, A14, A15, A16, A0 | 95.116 |
| 6 | 7 | A11, A12, A13, A14, A15, A16, A0 | 94.912 |
| 7 | 8 | A4, A8, A11, A12, A13, A14, A15, A0 | 94.717 |
| 8 | 8 | A6, A8, A11, A12, A13, A14, A15, A0 | 94.56 |
| 9 | 7 | A6, A11, A12, A13, A14, A15, A0 | 94.511 |
| 10 | 7 | A5, A11, A12, A13, A14, A15, A0 | 94.324 |
| Ranking Position | Number of Wavelengths | Combination of Parameters | 5 CV Mean (%) |
|---|---|---|---|
| 1 | 6 | A3, A4, A5, A8, A14, A15 | 86.210 |
| 2 | 9 | A3, A4, A5, A6, A8, A9, A14, A15 | 86.089 |
| 3 | 7 | A3, A4, A5, A7, A8, A14, A15 | 86.034 |
| 4 | 8 | A3, A4, A5, A6, A7, A8, A14, A15 | 85.991 |
| 5 | 7 | A3, A4, A5, A6, A8, A14, A15 | 85.377 |
| 6 | 7 | A3, A4, A6, A7, A8, A14, A15 | 84.294 |
| 7 | 6 | A3, A4, A7, A8, A14, A15 | 84.07 |
| 8 | 7 | A4, A5, A6, A7, A8, A14, A15 | 83.981 |
| 9 | 5 | A3, A4, A8, A14, A15 | 83.972 |
| 10 | 6 | A3, A4, A6, A8, A14, A15 | 83.366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakuła, A.; Paśko, S.; Kursa, O.; Komar, R. Reflected Light Spectrometry and AI-Based Data Analysis for Detection of Rapid Chicken Eggshell Change Caused by Mycoplasma Synoviae. Appl. Sci. 2021, 11, 7799. https://doi.org/10.3390/app11177799
Pakuła A, Paśko S, Kursa O, Komar R. Reflected Light Spectrometry and AI-Based Data Analysis for Detection of Rapid Chicken Eggshell Change Caused by Mycoplasma Synoviae. Applied Sciences. 2021; 11(17):7799. https://doi.org/10.3390/app11177799
Chicago/Turabian StylePakuła, Anna, Sławomir Paśko, Olimpia Kursa, and Robert Komar. 2021. "Reflected Light Spectrometry and AI-Based Data Analysis for Detection of Rapid Chicken Eggshell Change Caused by Mycoplasma Synoviae" Applied Sciences 11, no. 17: 7799. https://doi.org/10.3390/app11177799
APA StylePakuła, A., Paśko, S., Kursa, O., & Komar, R. (2021). Reflected Light Spectrometry and AI-Based Data Analysis for Detection of Rapid Chicken Eggshell Change Caused by Mycoplasma Synoviae. Applied Sciences, 11(17), 7799. https://doi.org/10.3390/app11177799






