Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent
Abstract
:1. Introduction
2. Experiment Details
2.1. Materials
2.2. Parametric Effect Study
2.3. Characterization Methods
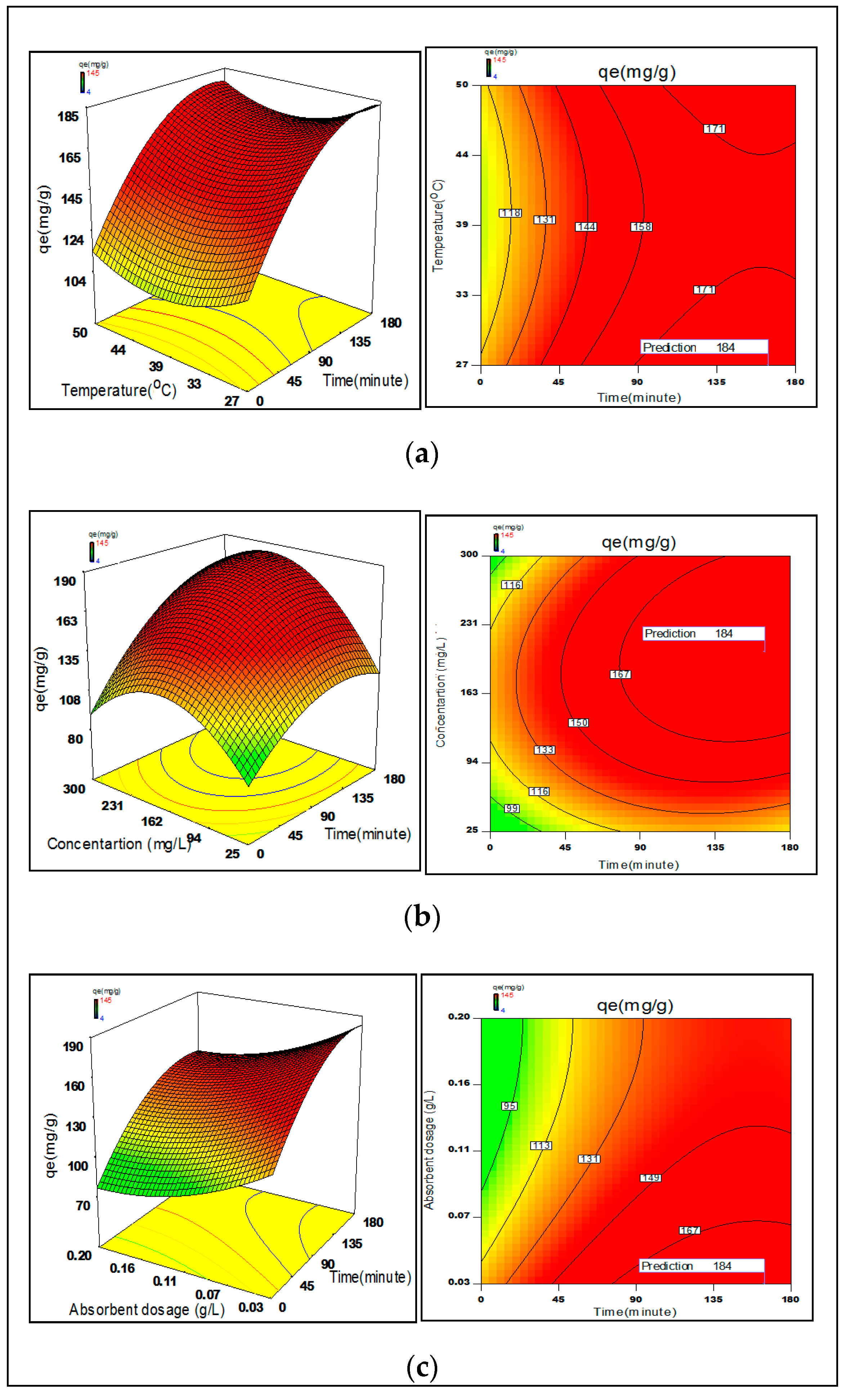
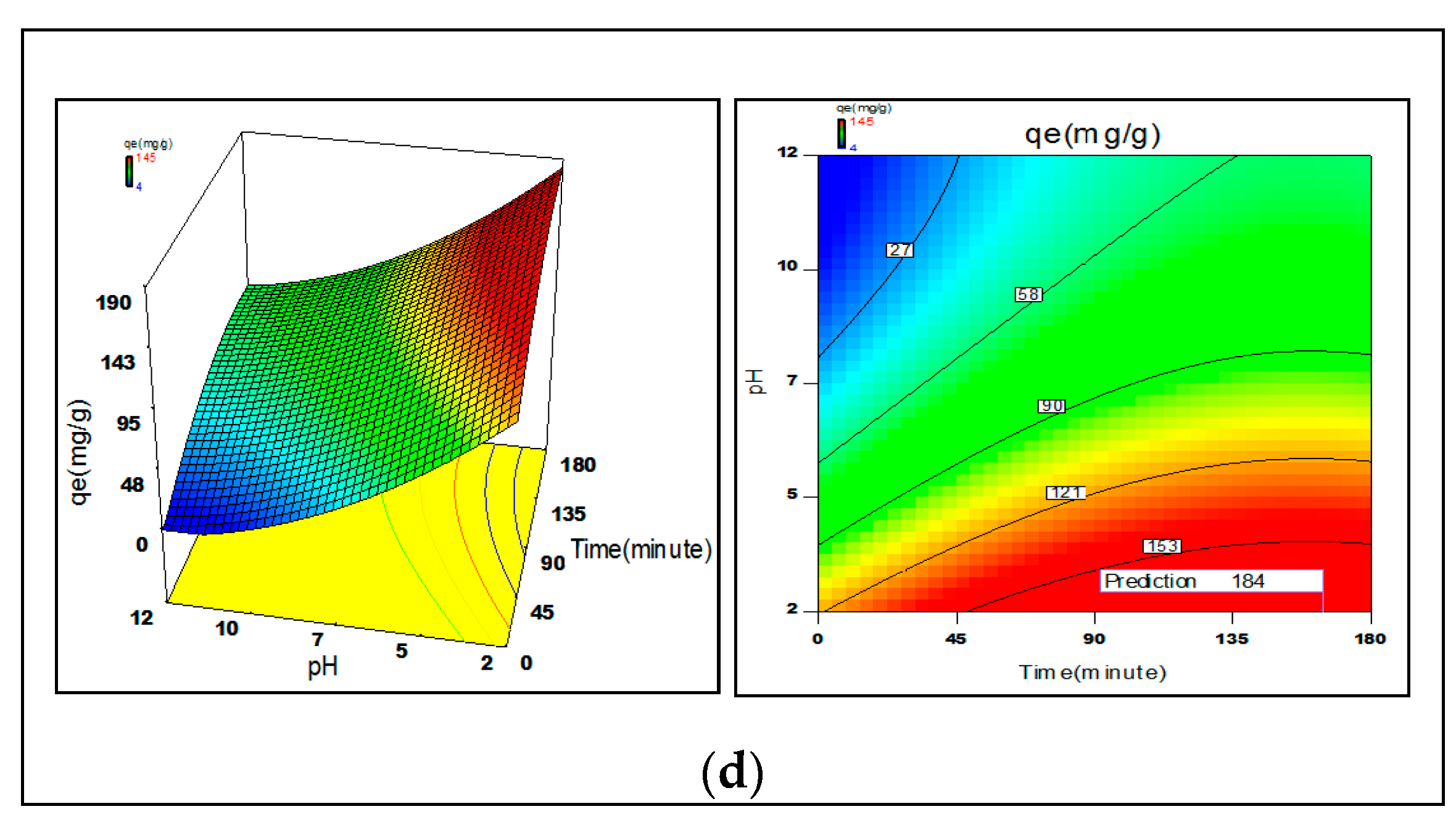
2.4. Statistical Optimization of Process Parameters
3. Experimental Outcomes
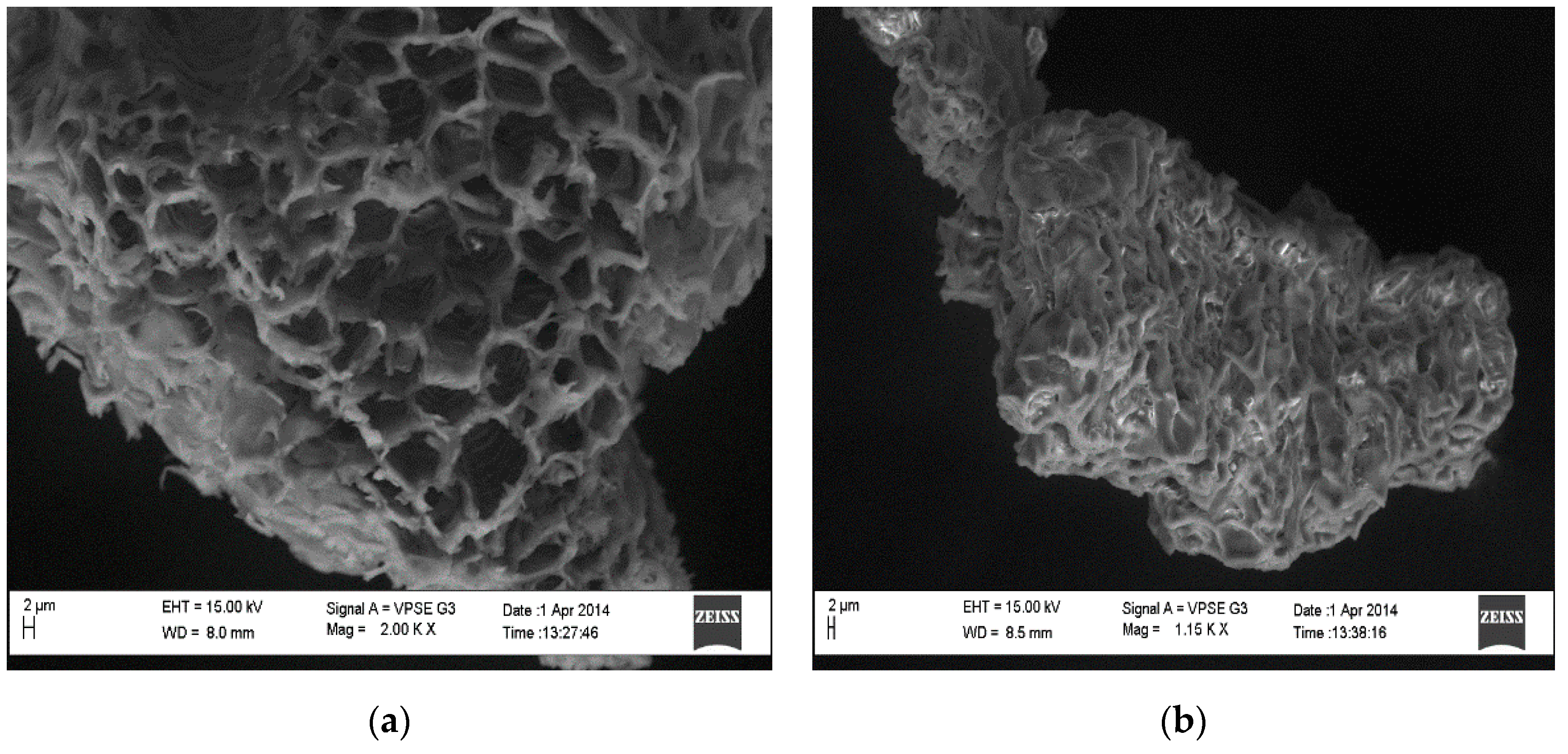
3.1. Analyses of SEM Images
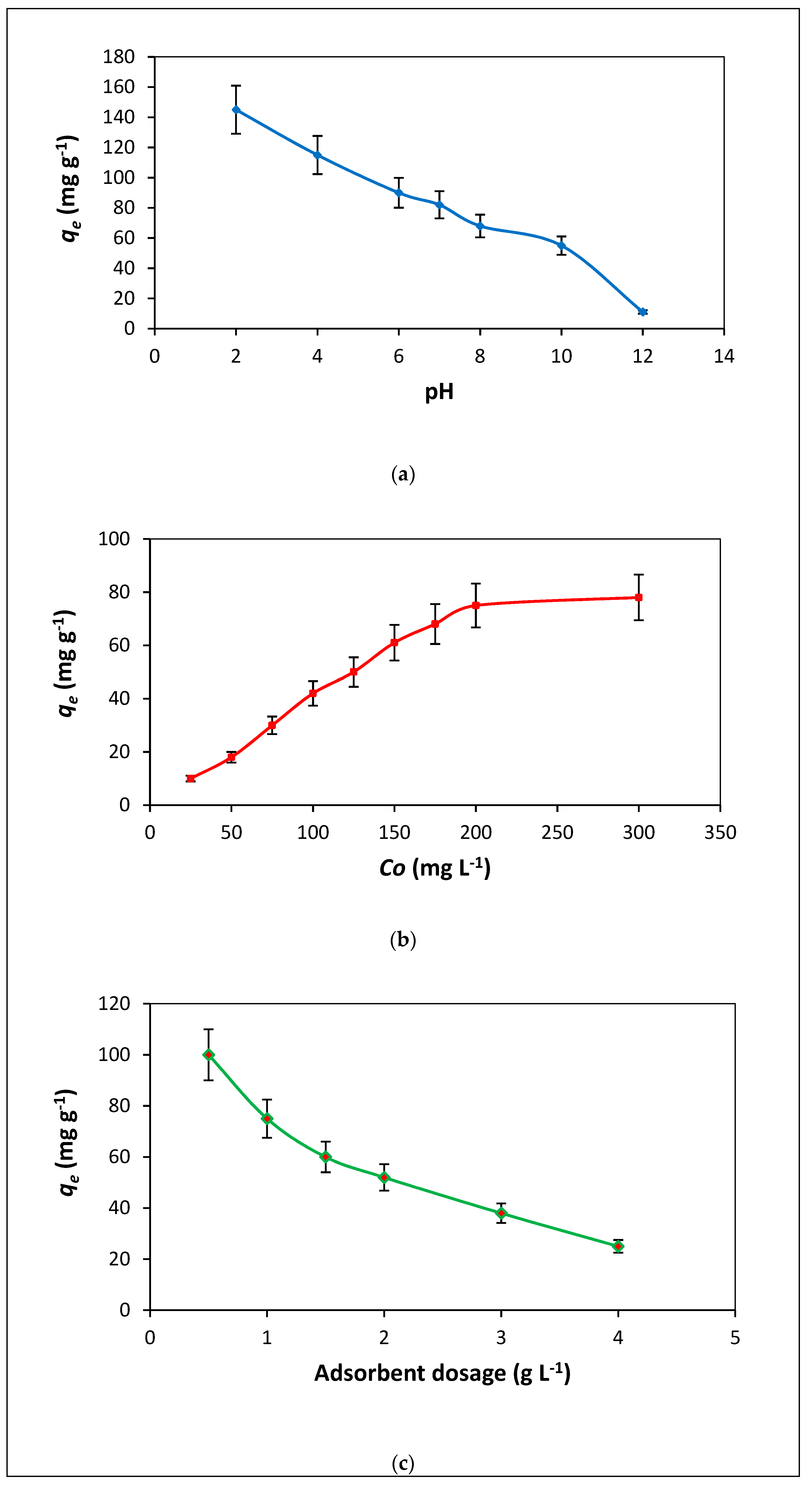
3.2. The Influence of Variables on CV Adsorption on NIFGS
3.2.1. Solution pH
3.2.2. Dye Concentration Influence
3.2.3. Adsorbent Dosage Influence
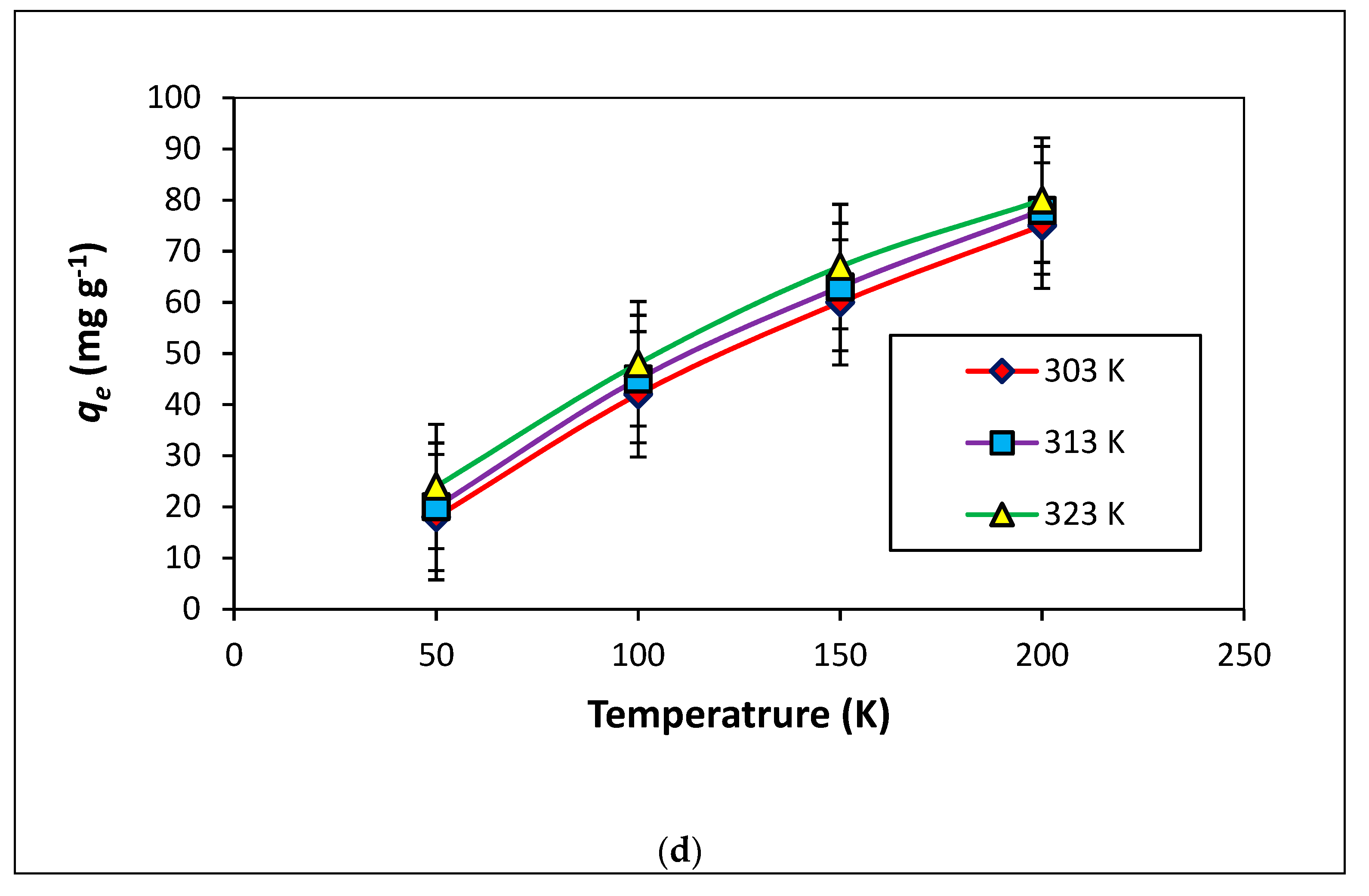
3.2.4. Temperature Influence
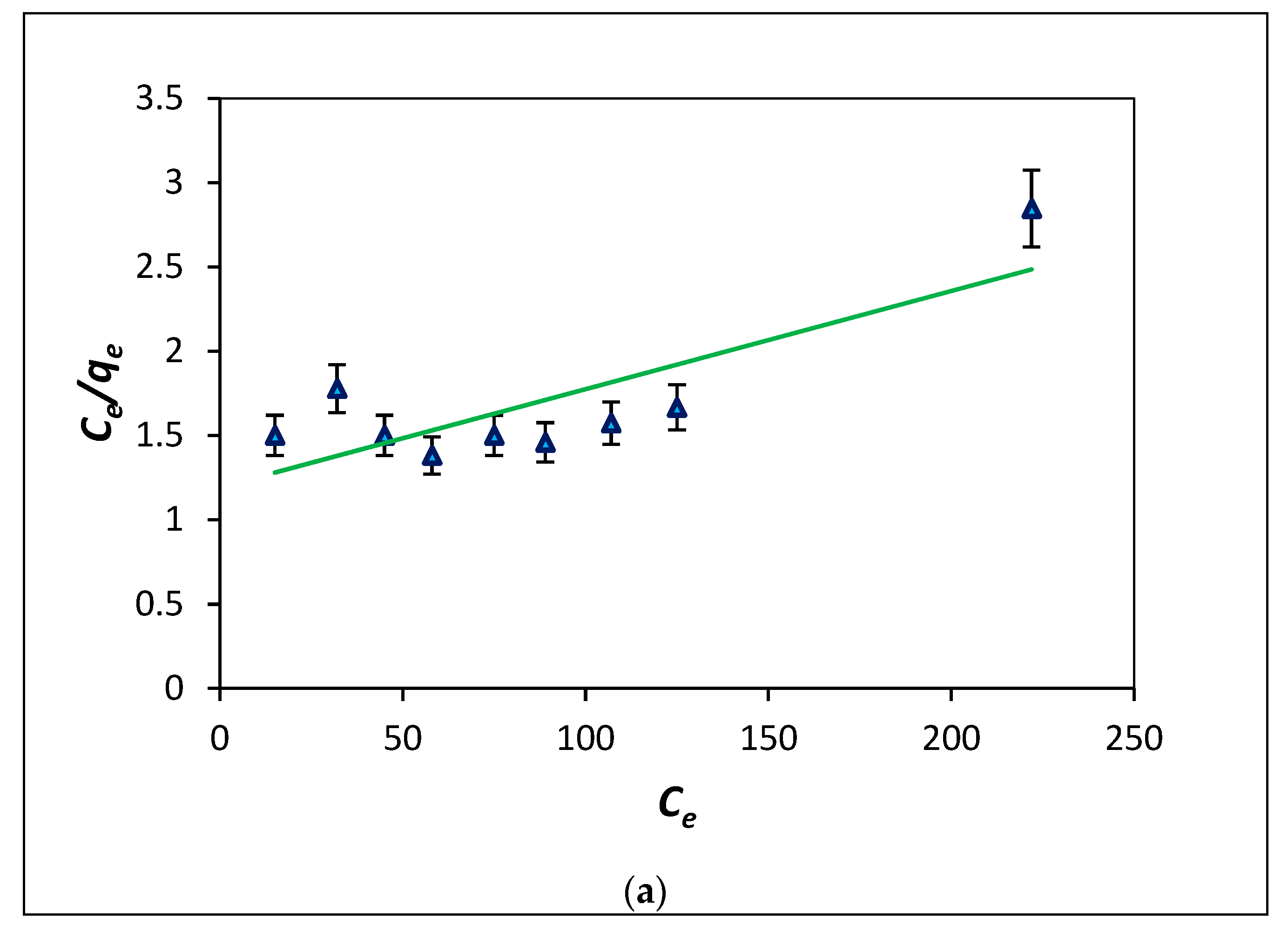
3.3. Adsorption Isotherms—Modelling Analysis
3.4. Adsorption Kinetics
3.5. Thermodynamics of the Adsorption Process
3.6. Process Optimization
3.7. Mechanism of Adsorption
- The progression of adsorption is a multistep activity;
- The factors that have credible influence on the process of adsorption are the solution acidic level (pH), the concentration of dye, the amount of adsorbent used and variation in temperature;
- Monolayer is a formation initiated when the CV mass transfer occurs onto NIFGS;
- The process of diffusion is likely to be a slow process;
- The strong adherence of CV dye onto NIFGS is probably by bonds established between dye N+ anions and hydrogen of cellulosic –OH group;
- Weak interaction is due to Van der Waals forces of attraction and strong electrostatic forces of attraction is because the –N+ cationic group and the –OH+ group negative charge of NIFGS contribute substantially to the adherence of the dye onto NIFGS.
3.8. Optimized Condition
3.8.1. Studies on Composites
Preparation of CV Dye-Adsorbed NIFGS
Preparation of the Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indian Brand Equity Foundation. Textile Industry & Market Growth in India. Available online: https://www.fibre2fashion.com/industry-article/2363/indian-textile-industry-an-overview (accessed on 22 January 2021).
- Indian Brand Equity Foundation. Indian Textiles and Apparel Industry. Available online: https://www.ibef.org/industry/textiles.aspx (accessed on 28 March 2021).
- The World’s Most Polluting Industries. Available online: https://www.worldatlas.com/articles/the-top-10-polluting-industries-in-the-world.html (accessed on 23 November 2020).
- Wu, G.H.; Wu, X.Y.; Wang, L.; Liu, S.Q.; Ding, X.; Cuc, S. Water footprint and carbon footprint reduction in textile’s waste recycling. Redactie 2015, 66, 85. [Google Scholar]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 4, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.A.; Kang, S.W.; Hunge, Y.M. Photocatalytic degradation of Rhodamine B using graphitic carbon nitride photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 4, 15577–15585. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenolA using titanium dioxide@ nanodiamond composites under UV light illumination. J. Colloid Interface Sci. 2021, 4, 1058–1066. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Tian, Z.; Tlili, I.; Kazi, S.; Goodarzi, M. Thermal evaluation of a heat pipe working with n-pentane-acetone and n-pentane-methanol binary mixtures. J. Therm. Anal. Calorim. 2020, 4, 2435–2445. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Goodarzi, M.; Arjomandi, M. Reforming of methanol with steam in a micro-reactor with Cu–SiO2 porous catalyst. Int. J. Hydrogen Energy 2019, 4, 19628–19639. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Goodarzi, M.; Arjomandi, M. Experimental investigation and performance optimisation of a catalytic reforming micro-reactor using response surface methodology. Energy Convers. Manag. 2019, 4, 111983. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R. Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphenenanoplatelets-methanol nano-suspension. Renew. Energy 2019, 4, 364–372. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Arya, H.; Saeedi, M.; Ahmadi, D. Flow boiling heat transfer to MgO-therminol 66 heat transfer fluid: Experimental assessment and correlation development. Appl. Therm. Eng. 2018, 4, 552–562. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; Jalali, E.; Sarafraz, M.M.; Akbari, O.A.; Karimipour, A.; Goodarzi, M.; Bach, Q.V. Effects of magnetic field on micro cross jet injection of dispersed nanoparticles in a microchannel. Int. J. Numer. Methods Heat Fluid Flow 2019, 4, 2683–2704. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Kiani, T.; Hormozi, F. Critical heat flux and pool boiling heat transfer analysis of synthesized zirconia aqueous nano-fluids. Int. Commun. Heat Mass Transf. 2016, 4, 75–83. [Google Scholar] [CrossRef]
- Peyghambarzadeh, S.M.; Sarafraz, M.M.; Vaeli, N.; Ameri, E.; Vatani, A.; Jamialahmadi, M. Forced convective and subcooled flow boiling heat transfer to pure water and n-heptane in an annular heat exchanger. Ann. Nucl. Energy 2013, 4, 401–410. [Google Scholar] [CrossRef]
- Li, Z.X.; Khaled, U.; Al-Rashed, A.A.; Goodarzi, M.; Sarafraz, M.M.; Meer, R. Heat transfer evaluation of a micro heat exchanger cooling with spherical carbon-acetone nanofluid. Int. J. Heat Mass Transf. 2020, 4, 119124. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Leon, A.S.; Tlili, I.; Alkanhal, T.A.; Tian, Z.; Goodarzi, M.; Arjomandi, M. Experimental investigation on thermal performance of a PV/T-PCM (photovoltaic/thermal) system cooling with a PCM and nanofluid. Energies 2019, 4, 2572. [Google Scholar] [CrossRef] [Green Version]
- Kale, V.; Hunge, Y.M.; Kamble, S.A.; Deshmukh, M.; Bhoraskar, S.V.; Mathe, V.L. Modification of energy level diagram of nano-crystalline ZnO by its composites with ZnWO4 suitable for sunlight assisted photo catalytic activity. Mater. Today Commun. 2021, 4, 102101. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H.; Fujishima, A.; Terashima, C. Nanoflakes-like nickel cobaltite as active electrode material for 4-nitrophenol reduction and supercapacitor applications. J. Hazard. Mater. 2021, 4, 126453. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interfaces 2021, 4, 101075. [Google Scholar] [CrossRef]
- Dávila-Jiménez, M.M.; Elizalde-González, M.P.; Peláez-Cid, A.A. Adsorption interaction between natural adsorbents and textile dyes in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2005, 4, 107–114. [Google Scholar] [CrossRef]
- Szyguła, A.; Guibal, E.; Ruiz, M.; Sastre, A.M. The removal of sulphonated azo-dyes by coagulation with chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2008, 4, 219–226. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. A novel sustainable design to develop polypropylene and unsaturated polyester resin polymer composites from waste of major polluting industries and investigation on their physicomechanical and wear properties. Polym. Compos. 2019, 4, 1142–1157. [Google Scholar] [CrossRef]
- Yakuth, S.A.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Nutraceutical industrial chillies stalk waste as a new adsorbent for the removal of Acid Violet 49 from water and textile industrial effluent: Adsorption isotherms and kinetic models. Desalin. Water Treat. 2019, 4, 94–112. [Google Scholar] [CrossRef] [Green Version]
- Papegowda, P.K.; Syed, A.A. Isotherm, kinetic and thermodynamic studies on the removal of methylene blue dye from aqueous solution using saw palmetto spent. Int. J. Environ. Res. 2017, 11, 91–98. [Google Scholar] [CrossRef]
- India Production of Horticulture Crops in India. CIEC. Available online: https://www.ceicdata.com/en/india/production-of-horticulture-crops-inindia/production-horticulture-crops-spices (accessed on 23 November 2020).
- Petropoulos, G.A. (Ed.) Fenugreek: The Genus Trigonella; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Dhaif-Allah, M.A.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Kinetic and isotherm modeling for acid blue 113 dye adsorption onto low-cost nutraceutical industrial fenugreek seed spent. Appl. Water Sci. 2020, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Tahir, S.S.; Rauf, N. Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 2006, 4, 1842–1848. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I Solids. J. Am. Chem. Soc. 1916, 4, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 4, 385471. [Google Scholar]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 4, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 4, 115–124. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. Adsorption of Acid Blue 113 from aqueous solution onto nutraceutical industrial coriander seed spent: Isotherm, kinetics, thermodynamics and modeling studies. Desalin. Water Treat. 2019, 4, 321–337. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. Valorization of Nutraceutical Industrial Coriander Seed Spent by the Process of Sustainable Adsorption System of Acid Black 52 from Aqueous Solution. Int. J. Environ. Res. 2019, 13, 639–659. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 4, 253–268. [Google Scholar] [CrossRef]
- Sulthana, R.; Taqui, S.N.; Zameer, F.; Syed, U.T.; Syed, A.A. Adsorption of ethidium bromide from aqueous solution onto nutraceutical industrial fennel seed spent: Kinetics and thermodynamics modeling studies. Int. J. Phytoremediat. 2018, 4, 1075–1086. [Google Scholar] [CrossRef]
- Taqui, S.N.; Yahya, R.; Hassan, A.; Nayak, N.; Syed, A.A. Development of sustainable dye adsorption system using nutraceutical industrial fennel seed spent—Studies using Congo red dye. Int. J. Phytoremediat. 2017, 4, 686–694. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 2007, 4, 388–396. [Google Scholar] [CrossRef]
- Dhaif-Allah, M.A.H.; Taqui, S.N.; Syed, U.T.; Syed, A.A. Development of sustainable acid blue 113 dye adsorption system using nutraceutical industrial Tribulus terrestris spent. SN Appl. Sci. 2019, 1, 330. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Khaled, U.; Al-Rashed, A.A.; Meer, R.; Goodarzi, M.; Sarafraz, M.M. Potential application of Response Surface Methodology (RSM) for the prediction and optimization of thermal conductivity of aqueous CuO (II) nanofluid: A statistical approach and experimental validation. Phys. A Stat. Mech. Its Appl. 2020, 4, 124353. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Safaei, M.R.; Goodarzi, M.; Alrashed, A.A.; Nguyen, T.K. New temperature, interfacial shell dependent dimensionless model for thermal conductivity of nanofluids. Int. J. Heat Mass Transf. 2017, 114, 207–210. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; D’Orazio, A.; Karimipour, A.; Goodarzi, M.; Bach, Q.V. A novel sensitivity analysis model of EANN for F-MWCNTs–Fe3O4/EG nanofluid thermal conductivity: Outputs predicted analytically instead of numerically to more accuracy and less costs. Phys. A Stat. Mech. Its Appl. 2019, 4, 406–415. [Google Scholar] [CrossRef]
- Ahmadi, A.A.; Arabbeiki, M.; Ali, H.M.; Goodarzi, M.; Safaei, M.R. Configuration and optimization of a minichannel using water–alumina nanofluid by non-dominated sorting genetic algorithm and response surface method. Nanomaterials 2020, 4, 901. [Google Scholar] [CrossRef]
- Peng, Y.; Parsian, A.; Khodadadi, H.; Akbari, M.; Ghani, K.; Goodarzi, M.; Bach, Q.V. Develop optimal network topology of artificial neural network (AONN) to predict the hybrid nanofluids thermal conductivity according to the empirical data of Al2O3–Cu nanoparticles dispersed in ethylene glycol. Phys. A Stat. Mech. Its Appl. 2020, 4, 124015. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mohseni-Gharyehsafa, B.; Ghazvini, M.; Goodarzi, M.; Jilte, R.D.; Kumar, R. Comparing various machine learning approaches in modeling the dynamic viscosity of CuO/water nanofluid. J. Therm. Anal. Calorim. 2020, 4, 2585–2599. [Google Scholar] [CrossRef]
- Giwa, S.O.; Sharifpur, M.; Goodarzi, M.; Alsulami, H.; Meyer, J.P. Influence of base fluid, temperature, and concentration on the thermophysical properties of hybrid nanofluids of alumina–ferrofluid: Experimental data, modeling through enhanced ANN, ANFIS, and curve fitting. J. Therm. Anal. Calorim. 2021, 143, 4149–4167. [Google Scholar] [CrossRef]

| Eq. No. | Equation | Description | Parameter |
|---|---|---|---|
| General Adsorption Studies | |||
| (1) | Adsorption capacity at equilibrium | qe: equilibrium adsorption capacity (mg L−1) qt: time t adsorption capacity (mg L−1) Co: initial concentration of adsorbent (mg L−1) Ce: equilibrium adsorbent concentration (mg L−1) Ct: time t adsorbent concentration V: adsorbate solution volume (L) W: adsorbent weight (g) | |
| (2) | Adsorption capacity at time t | ||
| (3) | Percentage removal efficiency (RE) | ||
| Adsorption isotherm studies | |||
| (4) | Langmuir isotherm [30] | Qm: monolayer adsorption capacity (mg g−1) Ka: adsorption constant of Langmuir isotherm (L mg−1) RL factor implies whether the adsorption is when (RL > 1): unfavourable (RL = 1): linear (0 < RL < 1): favourable and (RL = 0): irreversible | |
| (5) | Separation factor of Langmuir isotherm | ||
| (6) | Freundlich isotherm [31] | KF is adsorption constant of Freundlich isotherm (mg/g) nF: heterogeneity factor indicates the nature of adsorption is (nF < 1): chemisorption (nF = 1): linear or (nF > 1): physisorption | |
| Adsorption kinetic studies | |||
| (7) | Pseudo-first order Equation [32] | qt: time t adsorption capacity (mg L−1) qe: equilibrium adsorption capacity (mg L−1) k1: rate constant of pseudo-first order (s−1) k2: rate constant of pseudo-second order (mol−1 L−1 s−1) t: adsorption duration (s) | |
| (8) | Pseudo-second order Equation [33] | ||
| Adsorption thermodynamic studies | |||
| (9) | Standard Gibbs free energy | ΔG°: standard free energy (J mol−1) ΔH°: enthalpy change (J mol−1) ΔS°: entropy change (J mol−1 K−1) T: absolute temperature (K) R: ideal gas constant (J mol−1 K−1) : chemical equilibrium constant Co: initial adsorbent concentration (mg L−1) Ce: adsorbent concentration at equilibrium (mg L−1) | |
| (10) | Standard Gibbs free energy at chemical equilibrium | ||
| (11) | Thermodynamic equilibrium constant | ||
| (12) | Variant of standard Gibbs free energy | ||
| Initial Concentration [µg mL−1] | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | qe (mg g−1) | K1 (min−1) | R2 | |
| 50 | 3.22 | 0.054 | 0.99 | 19.12 | 48.77 | 0.97 |
| 100 | 4.52 | 0.045 | 0.98 | 44.24 | 237.25 | 0.98 |
| Dye Concentration | Temperature | Change in Free Energy ΔG° | Change in Entropy ΔS° | Change in Enthalpy ΔH° |
|---|---|---|---|---|
| [µg mL−1] | [K] | [kJ/mol] | [J/mol K] | [kJ/mol] |
| 50 | 303 | −2.52 | 23.28 | 9.91 |
| 313 | −2.65 | |||
| 323 | −2.77 | |||
| 100 | 303 | −3.26 | 43.35 | 13.79 |
| 313 | −3.46 | |||
| 323 | −3.64 | |||
| 200 | 303 | −4.07 | 63.28 | 17.82 |
| 313 | −4.29 | |||
| 323 | −4.53 |
| Sample | Summation of Squares | DoF | Square Mean | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 13,547.2 | 1 | 13,547.2 | 103.2 | <0.0001 |
| A | 113.6 | 1 | 113.6 | 0.9 | 0.3566 |
| B | 717.6 | 1 | 717.6 | 5.5 | 0.0233 |
| C | 1826.0 | 1 | 1826.0 | 13.9 | 0.0005 |
| D | 10,862.6 | 1 | 10,862.6 | 82.7 | <0.0001 |
| E | 878.9 | 1 | 878.9 | 6.7 | 0.0126 |
| AC | 20.1 | 1 | 20.1 | 0.2 | 0.6972 |
| BC | 1323.5 | 1 | 1323.5 | 10.1 | 0.0025 |
| A2 | 736.5 | 1 | 736.5 | 4.8 | 0.0322 |
| B2 | 100.7 | 1 | 100.7 | 0.7 | 0.4218 |
| C2 | 223.0 | 1 | 223.0 | 1.4 | 0.2333 |
| D2 | 2437.6 | 1 | 2437.6 | 15.8 | 0.0002 |
| E2 | 494.3 | 1 | 494.3 | 3.2 | 0.0777 |
| Residual | 6695.6 | 51 | 131.3 | ||
| Lack of fit | 5782.9 | 49 | 118.0 | 0.3 | 0.9724 |
| Total | 54,053.8 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taqui, S.N.; C.S., M.; Goodarzi, M.S.; Elkotb, M.A.; Khatoon, B.A.; Soudagar, M.E.M.; Koki, I.B.; Elfasakhany, A.; Khalifa, A.S.; Ali, M.A.; et al. Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent. Appl. Sci. 2021, 11, 7635. https://doi.org/10.3390/app11167635
Taqui SN, C.S. M, Goodarzi MS, Elkotb MA, Khatoon BA, Soudagar MEM, Koki IB, Elfasakhany A, Khalifa AS, Ali MA, et al. Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent. Applied Sciences. 2021; 11(16):7635. https://doi.org/10.3390/app11167635
Chicago/Turabian StyleTaqui, Syed Noeman, Mohan C.S., Mohammad Shahab Goodarzi, Mohamed Abdelghany Elkotb, Bibi Ahmadi Khatoon, Manzoore Elahi M. Soudagar, Isa Baba Koki, Ashraf Elfasakhany, Amany Salah Khalifa, Masood Ashraf Ali, and et al. 2021. "Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent" Applied Sciences 11, no. 16: 7635. https://doi.org/10.3390/app11167635
APA StyleTaqui, S. N., C.S., M., Goodarzi, M. S., Elkotb, M. A., Khatoon, B. A., Soudagar, M. E. M., Koki, I. B., Elfasakhany, A., Khalifa, A. S., Ali, M. A., Saifullah, Z., Siddiqui, M. I. H., Safaei, M. R., & Saleel, C. A. (2021). Sustainable Adsorption Method for the Remediation of Crystal Violet Dye Using Nutraceutical Industrial Fenugreek Seed Spent. Applied Sciences, 11(16), 7635. https://doi.org/10.3390/app11167635











