Comparison of the Kinematics Following Gait Perturbation in Individuals Who Did or Did Not Undergo Total Knee Replacement
Abstract
1. Introduction
2. Materials and Methods
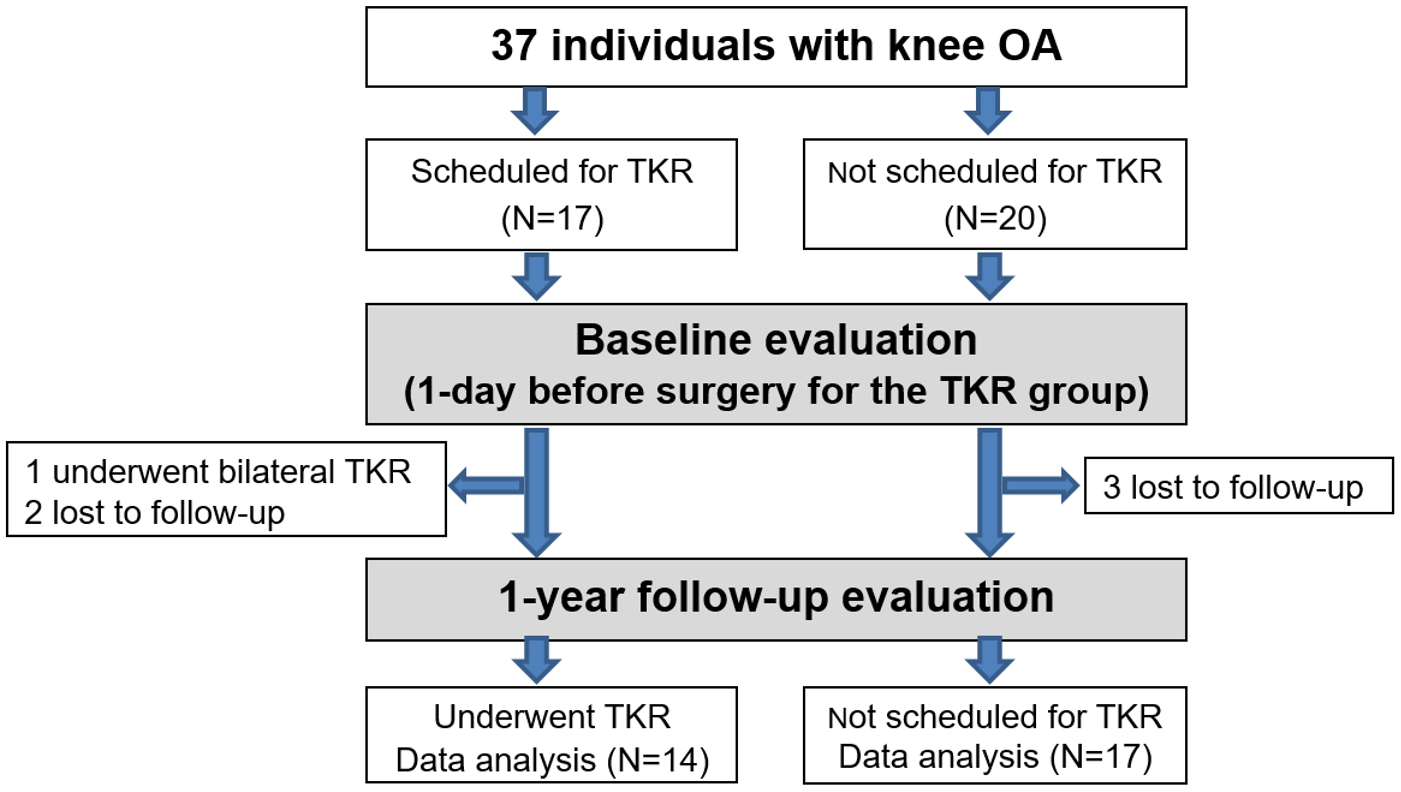
2.1. Population
2.2. Tools
2.3. Protocol
2.4. Post-Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Andriacchi, T.P.; Favre, J.; Erhart-Hledik, J.C.; Chu, C.R. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann. Biomed. Eng. 2015, 43, 376–387. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. YJOCA 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Favre, J.; Jolles, B.M. Gait analysis of patients with knee osteoarthritis highlights a pathological mechanical pathway and provides a basis for therapeutic interventions. EFORT Open Rev. 2016, 1. [Google Scholar] [CrossRef]
- Henriksen, M.; Graven-Nielsen, T.; Aaboe, J.; Andriacchi, T.P. Gait changes in patients with knee osteoarthritis are replicated by experimental knee pain. Arthritis Care Res. 2010, 62, 501–509. [Google Scholar] [CrossRef]
- Baert, I.A.C.; Mahmoudian, A.; Nieuwenhuys, A.; Jonkers, I.; Staes, F.; Luyten, F.P.; Truijen, S.; Verschueren, S.M.P. Proprioceptive accuracy in women with early and established knee osteoarthritis and its relation to functional ability, postural control, and muscle strength. Clin. Rheumatol. 2013, 32, 1365–1374. [Google Scholar] [CrossRef]
- Van Der Esch, M.; Steultjens, M.; Harlaar, J.; Knol, D.; Lems, W.; Dekker, J. Joint proprioception, muscle strength, and functional ability in patients with osteoarthritis of the knee. Arthritis Care Res. 2007, 57, 787–793. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; He, H.C. Correlations between joint proprioception, muscle strength, and functional ability in patients with knee osteoarthritis. J. Sichuan Univ. Med. Sci. Ed. 2015, 46, 880–884. [Google Scholar]
- Arden, N.K.; Crozier, S.; Smith, H.; Anderson, F.; Edwards, C.; Raphael, H.; Cooper, C. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Care Res. 2006, 55, 610–615. [Google Scholar] [CrossRef]
- Scott, R.D. Fall Incidence and Risk Factors in Patients after Total Knee Arthroplasty, 2nd ed.; Saunders: Philadelphia, PA, USA, 2006; Volume 132. [Google Scholar]
- Di, G.; Frattura, L.; Filardo, G.; Giunchi, D.; Fusco, A.; Zaffagnini, S.; Candrian, C. Risk of falls in patients with knee osteoarthritis undergoing total knee arthroplasty: A systematic review and best evidence synthesis. J. Orthop. 2018, 15, 903–908. [Google Scholar] [CrossRef]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Chan, A.C.M.; Jehu, D.A.; Pang, M.Y.C. Falls after total knee arthroplasty: Frequency, circumstances, and associated factors-a prospective cohort study. Phys. Ther. 2018, 98, 767–778. [Google Scholar] [CrossRef]
- Hill, K.; Schwarz, J.; Flicker, L.; Carroll, S. Falls among healthy, community-dwelling, older women: A prospective study of frequency, circumstances, consequences and prediction accuracy. Aust. N. Zealand J. Public Health 1999, 23, 41–48. [Google Scholar] [CrossRef]
- Hamacher, D.; Singh, N.B.; Van Dieen, J.H.; Heller, M.O.; Taylor, W.R. Kinematic measures for assessing gait stability in elderly individuals: A systematic review. J. R. Soc. Interface 2011, 8, 1682–1698. [Google Scholar] [CrossRef]
- Levinger, P.; Lai, D.T.H.; Menz, H.B.; Morrow, A.D.; Feller, J.A.; Bartlett, J.R.; Bergman, N.R.; Begg, R. Swing limb mechanics and minimum toe clearance in people with knee osteoarthritis. Gait Posture 2012, 35, 277–281. [Google Scholar] [CrossRef]
- Van Dieën, J.H.; Pijnappels, M.; Bobbert, M.F. Age-related intrinsic limitations in preventing a trip and regaining balance after a trip. Saf. Sci. 2005, 43, 437–453. [Google Scholar] [CrossRef]
- Krasovsky, T.; Baniña, M.C.; Hacmon, R.; Feldman, A.G.; Lamontagne, A.; Levin, M.F. Stability of gait and interlimb coordination in older adults. J. Neurophysiol. 2012, 107, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.N.; Wolford, M.L.; Bercovitz, A. Hospitalization for Total Knee Replacement Among Inpatients Aged 45 and Over; NCHS: Hyattsville, MD, USA, 2015; Volume 210, pp. 1–8. [Google Scholar]
- Şentürk, İ.; Kanatlı, U.; Ataoğlu, B.; Esen, E. Gait Analysis after Total Knee Arthroplasty: Comparison of Pre- and Postoperative Characteristics, 2nd ed.; Saunders: Philadelphia, PA, USA, 2006; Volume 42. [Google Scholar]
- Mandeville, D.; Osternig, L.R.; Chou, L.S. The effect of total knee replacement on dynamic support of the body during walking and stair ascent. Clin. Biomech. 2007, 22, 787–794. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mizner, R.L.; Ramsey, D.K.; Snyder-Mackler, L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin. Biomech. 2008, 23, 320–328. [Google Scholar] [CrossRef]
- Bade, M.J.; Kohrt, W.M.; Stevens-Lapsley, J.E. Outcomes before and after total knee arthroplasty compared to healthy adults. J. Orthop. Sports Phys. Ther. 2010, 40, 559–567. [Google Scholar] [CrossRef]
- Elkarif, V.; Kandel, L.; Rand, D.; Schwartz, I.; Greenberg, A.; Portnoy, S. Kinematics following gait perturbation in adults with knee osteoarthritis: Scheduled versus not scheduled for knee arthroplasty. Gait Posture 2020, 81, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Elkarif, V.; Kandel, L.; Rand, D.; Schwartz, I.; Greenberg, A.; Portnoy, S. Musculoskeletal Science and Practice Muscle activity while ambulating on stairs and slopes: A comparison between individuals scheduled and not scheduled for knee arthroplasty and healthy controls. Musculoskelet. Sci. Pr. 2021, 52, 102346. [Google Scholar] [CrossRef] [PubMed]
- Gage, W.H.; Frank, J.S.; Prentice, S.D.; Stevenson, P. Organization of postural responses following a rotational support surface perturbation, after TKA: Sagittal plane rotations. Gait Posture 2007, 25, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Swanik, C.B.; Reisman, D.S.; Rudolph, K.S. Individuals with medial knee osteoarthritis show neuromuscular adaptation when perturbed during walking despite functional and structural impairments. J. Appl. Physiol. 2014, 116, 13–23. [Google Scholar] [CrossRef][Green Version]
- Krasovsky, T.; Lamontagne, A.; Feldman, A.G.; Levin, M.F. Effects of walking speed on gait stability and interlimb coordination in younger and older adults. Gait Posture 2014, 39, 378–385. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. Control of rapid limb movements for balance recovery: Age-related changes and implications for fall prevention. Age Ageing 2006, 35, ii12–ii18. [Google Scholar] [CrossRef]
- Mandeville, D.; Osternig, L.R.; Chou, L.S. The effect of total knee replacement surgery on gait stability. Gait Posture 2008, 27, 103–109. [Google Scholar] [CrossRef]
- Lugade, V.; Lin, V.; Chou, L.S. Center of mass and base of support interaction during gait. Gait Posture 2011, 33, 406–411. [Google Scholar] [CrossRef]
- Gage, W.H.; Frank, J.S.; Prentice, S.D.; Stevenson, P. Postural responses following a rotational support surface perturbation, following knee joint replacement: Frontal plane rotations. Gait Posture 2008, 27, 286–293. [Google Scholar] [CrossRef]
- Han, L.; Yang, F. Strength or power, which is more important to prevent slip-related falls? Hum. Mov. Sci. 2015, 44, 192–200. [Google Scholar] [CrossRef]
- Kiss, R.M.; Bejek, Z.; Szendrői, M. Variability of gait parameters in patients with total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1252–1260. [Google Scholar] [CrossRef]
- Skinner, H.B.; Barrack, R.L.; Cook, S.D. Age related decline in proprioception. Clin. Orthop. Relat. Res. 1984, 184, 208–211. [Google Scholar] [CrossRef]
- Arnold, C.M.; Faulkner, R.A. The history of falls and the association of the timed up and go test to falls and near-falls in older adults with hip osteoarthritis. BMC Geriatr. 2007, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.; Anwer, S.; Brismée, J.M. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1–3 knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Danoff, J.R.; Goel, R.; Sutton, R.; Maltenfort, M.G.; Austin, M.S. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J. Arthroplast. 2018, 33, S71–S75.e2. [Google Scholar] [CrossRef]
- Beard, D.J.; Harris, K.; Dawson, J.; Doll, H.; Murray, D.W.; Carr, A.J.; Price, A.J. Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. J. Clin. Epidemiol. 2015, 68, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Powell, L.E.; Myers, A.M. The activities-specific balance confidence (ABC) scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50A, M28–M34. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Glenney, S.S. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2014, 20, 295–300. [Google Scholar] [CrossRef]
- Sawada, Y.; Akagi, M.; Hamanishi, C.; Asada, S.; Mori, S.; Maruo, Y.; Fukuda, K. Perioperative changes in proprioception after total knee arthroplasty and identification of factors affecting it. Rigakuryoho Kagaku 2008, 23, 279–283. [Google Scholar] [CrossRef][Green Version]
- Mandeville, D.S.; Osternig, L.R.; Lantz, B.A.; Mohler, C.G.; Chou, L.S. A multivariate statistical ranking of clinical and gait measures before and after total knee replacement. Gait Posture 2009, 30, 197–200. [Google Scholar] [CrossRef]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Flören, M.; Skwara, A.; Tibesku, C.O. Quantitative gait analysis in unconstrained total knee arthroplasty patients. Int. J. Rehabil. Res. 2002, 25, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.H.; Lee, J.; Lee, J.; Park, J.; Han, H.; Lee, M.C. Effects of knee osteoarthritis on hip and ankle gait mechanics. Adv. Orthop. 2019, 2019, 9757369. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.A.; Flowers, P.; Bade, M.; Cheuy, V.; Stevens-Lapsley, J.; Snyder-Mackler, L. Stiff knee gait may increase risk of second total knee arthroplasty. J. Orthop. Res. 2019, 37, 397–402. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Separating the effects of age and walking speed on gait variability. Gait Posture 2008, 27, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.S.; Gobb, A.G.; Bentley, G. Joint proprioception in normal, osteoarthritic and replaced knees. J. Bone Jt. Surg. B 1991, 73, 53–56. [Google Scholar] [CrossRef]
- Yakhdani, H.R.F.; Bafghi, H.A.; Meijer, O.G.; Bruijn, S.M.; Dikkenberg, N.V.D.; Stibbe, A.B.; Van Royen, B.J.; Van Dieën, J.H. Stability and variability of knee kinematics during gait in knee osteoarthritis before and after replacement surgery. Clin. Biomech. 2010, 25, 230–236. [Google Scholar] [CrossRef] [PubMed]

| Variable | NTKR (n = 17) | TKR (n = 14) | p | t | |
|---|---|---|---|---|---|
| Sex | 7 male; 10 female | 4 male; 10 female | 0.473 | −0.718 | |
| Age (years) | 68.3 (7.3) | 70.8 (6.4) | 0.586 | −0.997 | |
| BMI (kg/m2) | 29.1 (4.7) | 30.8 (4.4) | 0.687 | −0.994 | |
| Injured knee | 6 left; 11 right | 8 left; 6 right | 0.231 | −1.197 | |
| Functional measurements | OXFORD (0–48) | −6.9 (29.1) | −31.2 (46.5) | 0.164 | 1.774 |
| ABC (0–100%) | −4.9 (38.4) | 1.7 (38.3) | 0.845 | −0.471 | |
| TUG (s) | −11.6 (17.6) | −12.6 (27.8) | 0.078 | 0.116 | |
| VAS of pain | Knee OA limb | 0.4 (2.6) | −3.8 (3.3) | 0.281 | 3.984 |
| Knee CL limb | 1.4 (3.7)) | 1.0 (4.1) | 0.986 | 0.276 | |
| Passive Range of Motion (°) | Hip flexion OA limb * | 0.0 (0.0–0.0) | 0.0 (0.0–2.5) | 0.404 | −0.843 |
| Hip flexion CL limb * | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.598 | −0.527 | |
| Hip extension injured limb * | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.420 | −0.807 | |
| Hip extension CL limb * | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.485 | −0.699 | |
| Knee flexion OA limb | 0.0 (13.0) | 4.6 (16.3) | 0.296 | −0.882 | |
| Knee flexion CL limb * | 0.0 (−10.0–0.0) | 0.0 (−5.0–5.0) | 0.290 | −1.057 | |
| Knee extension OA limb * | −2.5 (0.0–2.5) | 0.0 (0.0–1.3) | 0.414 | −0.817 | |
| Knee extension CL limb * | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.163 | −1.395 | |
| Parameter | NTKR (n = 17) | TKR (n = 14) | p | t | |
|---|---|---|---|---|---|
| Spatial–temporal parameters | Velocity (m/s) * | 5.8 (−5.4–20.2) | 17.8 (9.9–44.8) | 0.104 | −1.625 |
| Cadence (steps/min) | 1.4 (6.9) | 2.8 (10.3) | 0.152 | −0.409 | |
| Swing duration (s) of CL limb | −2.4 (7.2) | −1.0 (10.6) | 0.038 | −0.373 | |
| Swing duration (s) of OA limb * | −5.3 (−10.0–3.0) | −4.6 (−8.0–8.5) | 0.368 | −0.900 | |
| Symmetry index step length | −33.8 (41.9) | −19.2 (64.3) | 0.092 | −0.660 | |
| Symmetry index of double support * | 21.2 (−35.6–244.6) | −28.9 (−43.2–120.5) | 0.161 | −1.401 | |
| Kinematics | Pelvic maximal posterior tilt | −35.7 (97.7) | −148.9 (196.9) | 0.032 | 1.733 |
| OA hip range of flexion–extension * | 62.1 (9.0–234.8) | 48.1 (4.4–120.5) | 0.651 | −0.453 | |
| CL hip range of flexion–extension | 3.3 (27.3) | −23.0 (50.3) | 0.041 | 1.760 | |
| OA knee range of flexion–extension | 8.0 (22.5) | 18.0 (46.2) | 0.006 | −0.721 | |
| CL knee range of flexion–extension * | 36.6 (−3.5–370.8) | 39.7 (−8.4–322.0) | 0.806 | −0.245 | |
| Parameter | NTKR (n= 17) | TKR (n = 14) | p | t |
|---|---|---|---|---|
| Contralateral limb tripped | ||||
| Stance duration | 91.4 (76.1–102.3) | 91.1 (74.6–126.0) | 0.312 | 1.030 |
| Step length | 19.4 (4.8–38.6) | 22.2 (9.8–38.2) | 0.188 | −1.356 |
| Base width | 89.1 (75.6–146.2) | 130.6 (98.5–160.6) | 0.989 | 0.014 |
| CL hip maximal extension * | −89.9 (−110.1–(−37.4)) | −106.5 (−157.8–(−88.4)) | 0.323 | −0.989 |
| OA hip maximal extension | 19.4 (161.9) | −234.8 (252.7) | 0.031 | −2.937 |
| CL knee maximal flexion * | −9.5 (−49.4–53.8) | −18.7 (−843.7–29.6) | 0.569 | −0.570 |
| OA knee maximal flexion | −3.0 (34.2) | −11.9 (43.7) | 0.244 | 0.563 |
| Injured limb tripped | ||||
| Stance duration | 77.9 (73.6–116.9) | 85.3 (75.8–139.9) | 0.219 | −1.260 |
| Step length | 15.7 (6.5–77.1) | 41.3 (16.5–169.6) | 0.069 | 1.996 |
| Base width | 110.8 (66.7–142.2) | 97.2 (76.3–142.7) | 0.167 | −1.430 |
| CL knee range of flexion–extension * | 101.6 (11.7–408.2) | 80.2 (5.0–443.2) | 0.958 | −0.053 |
| OA knee range of flexion–extension | −7.8 (41.3) | −5.9 (39.9) | 0.011 | −0.988 |
| Maximal OA ankle plantar flexion | −34.0 (36.5) | −35.9 (17.2) | 0.049 | −0.157 |
| Maximal CL ankle plantar flexion * | 4.1 (−19.7–61.3) | 6.7 (−9.0–89.9) | 0.770 | −0.293 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkarif, V.; Kandel, L.; Rand, D.; Schwartz, I.; Greenberg, A.; Gurion, R.; Portnoy, S. Comparison of the Kinematics Following Gait Perturbation in Individuals Who Did or Did Not Undergo Total Knee Replacement. Appl. Sci. 2021, 11, 7453. https://doi.org/10.3390/app11167453
Elkarif V, Kandel L, Rand D, Schwartz I, Greenberg A, Gurion R, Portnoy S. Comparison of the Kinematics Following Gait Perturbation in Individuals Who Did or Did Not Undergo Total Knee Replacement. Applied Sciences. 2021; 11(16):7453. https://doi.org/10.3390/app11167453
Chicago/Turabian StyleElkarif, Vicktoria, Leonid Kandel, Debbie Rand, Isabella Schwartz, Alexander Greenberg, Rivkin Gurion, and Sigal Portnoy. 2021. "Comparison of the Kinematics Following Gait Perturbation in Individuals Who Did or Did Not Undergo Total Knee Replacement" Applied Sciences 11, no. 16: 7453. https://doi.org/10.3390/app11167453
APA StyleElkarif, V., Kandel, L., Rand, D., Schwartz, I., Greenberg, A., Gurion, R., & Portnoy, S. (2021). Comparison of the Kinematics Following Gait Perturbation in Individuals Who Did or Did Not Undergo Total Knee Replacement. Applied Sciences, 11(16), 7453. https://doi.org/10.3390/app11167453







