The Remediation Characteristics of Heavy Metals (Copper and Lead) on Applying Recycled Food Waste Ash and Electrokinetic Remediation Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
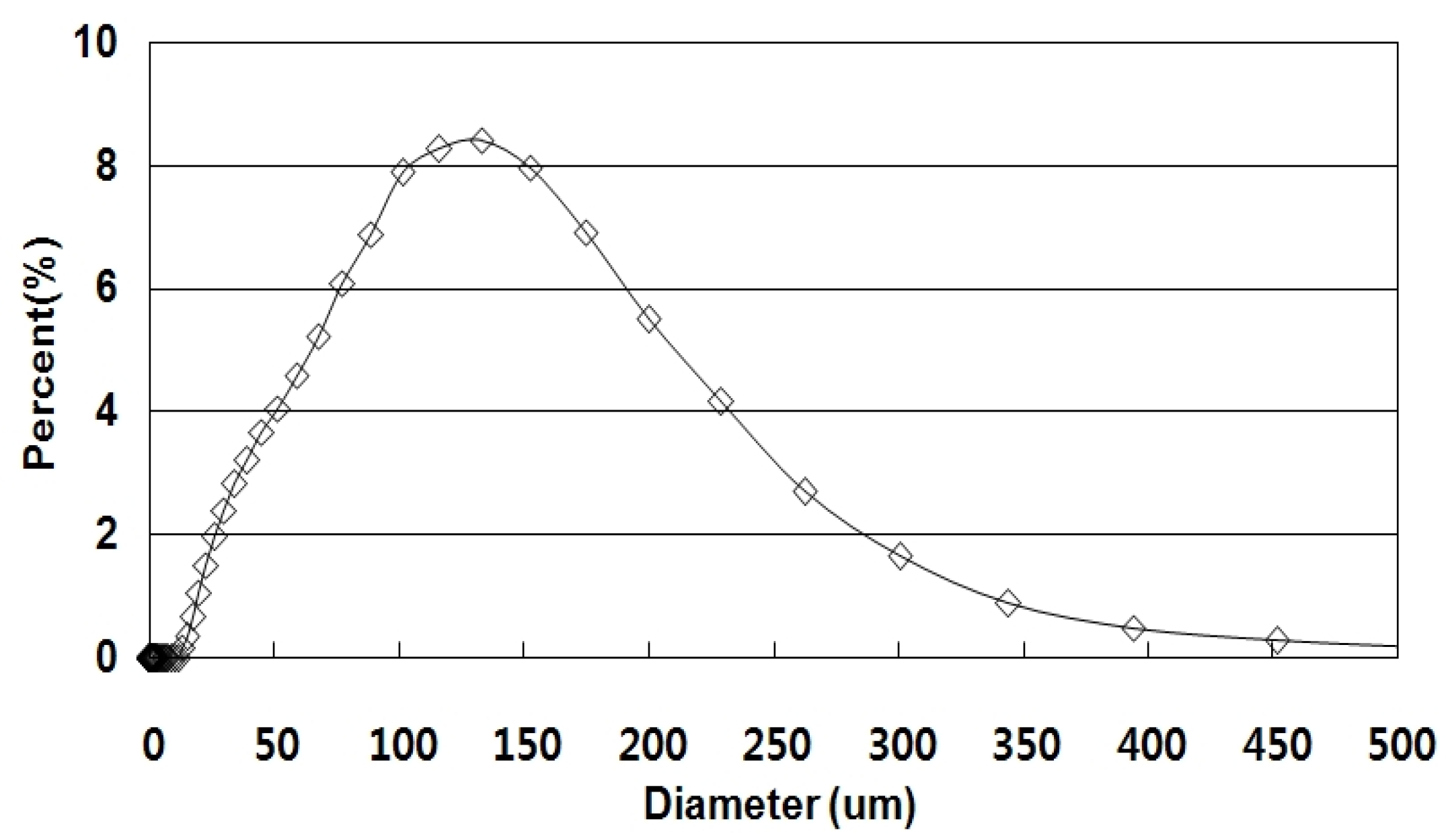
2.1.1. Soil Sample
2.1.2. Food Waste Ash
2.1.3. Improver
2.2. Experiments
2.2.1. Experimental Tools
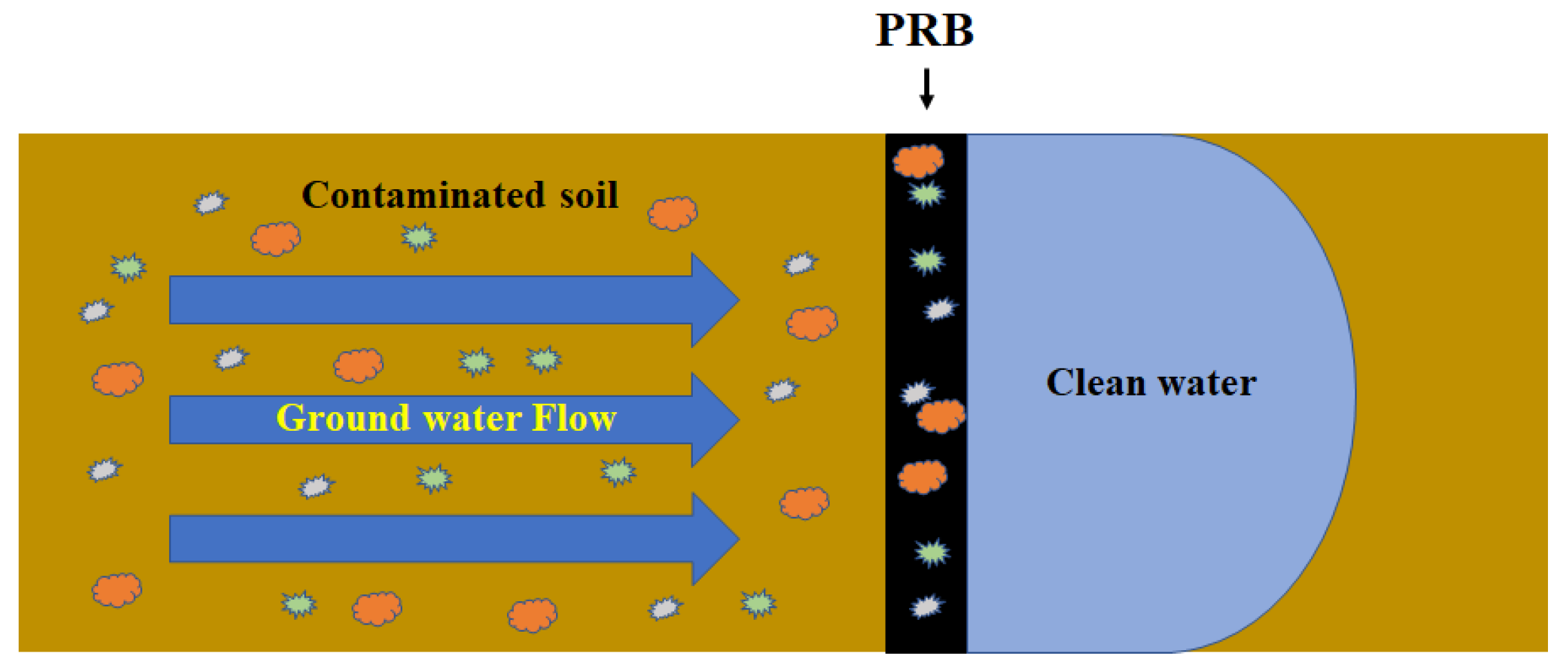
2.2.2. PRB
2.2.3. Test Method
3. Results
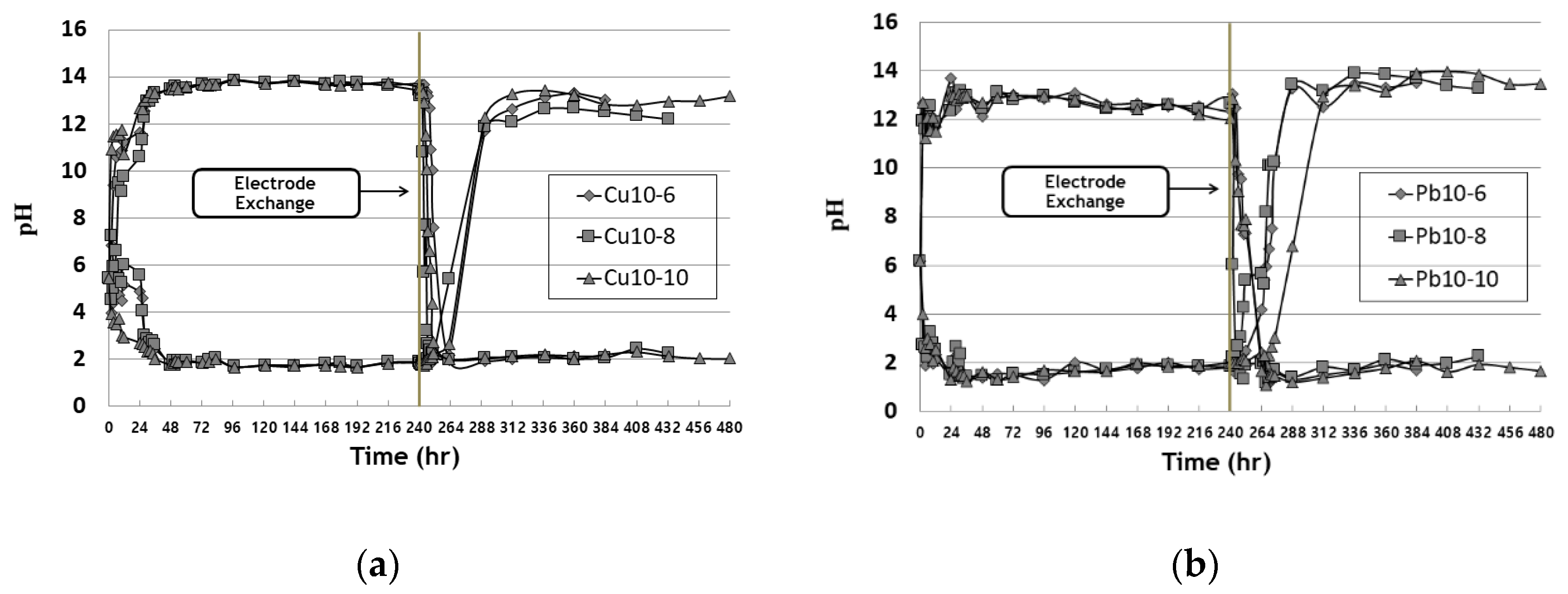
3.1. pH Change
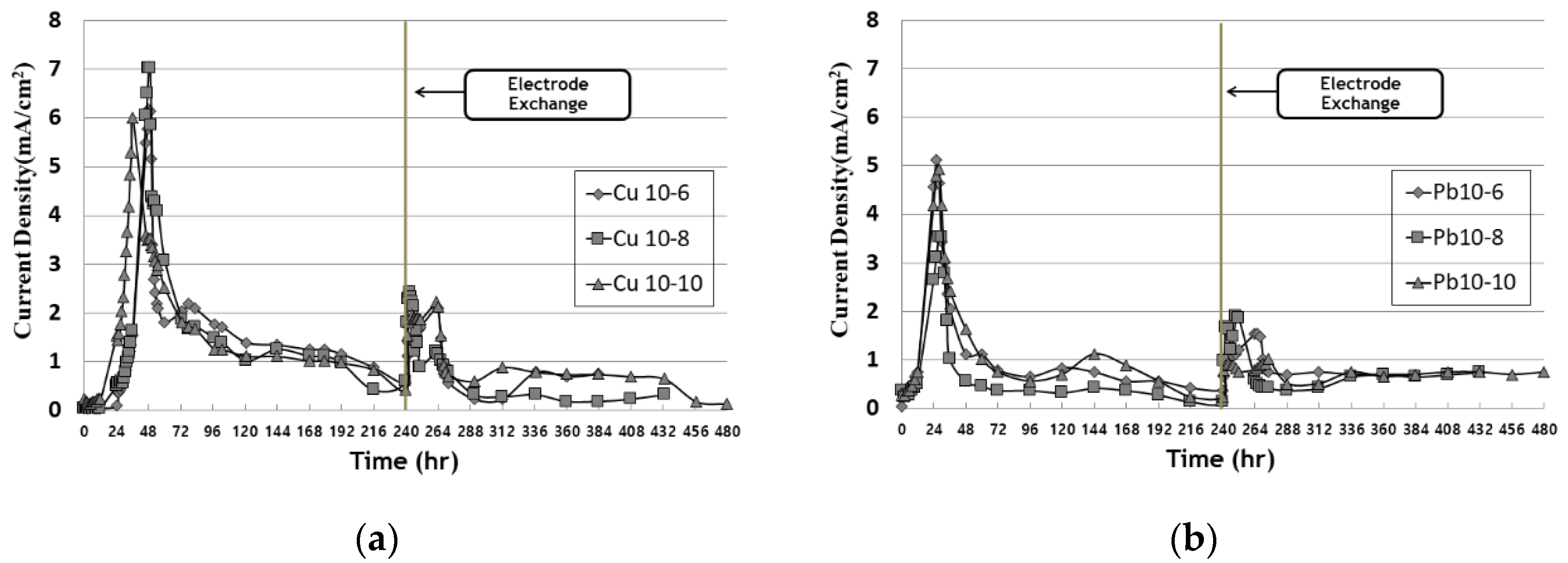
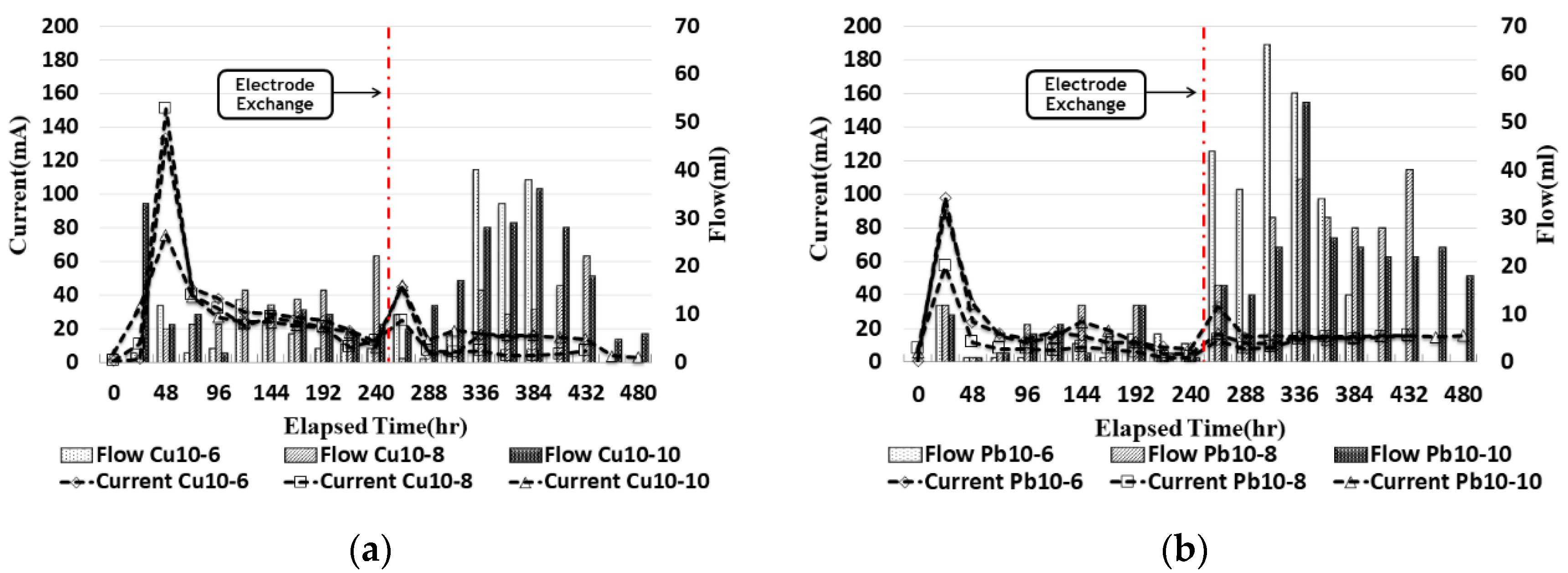
3.2. Current Density
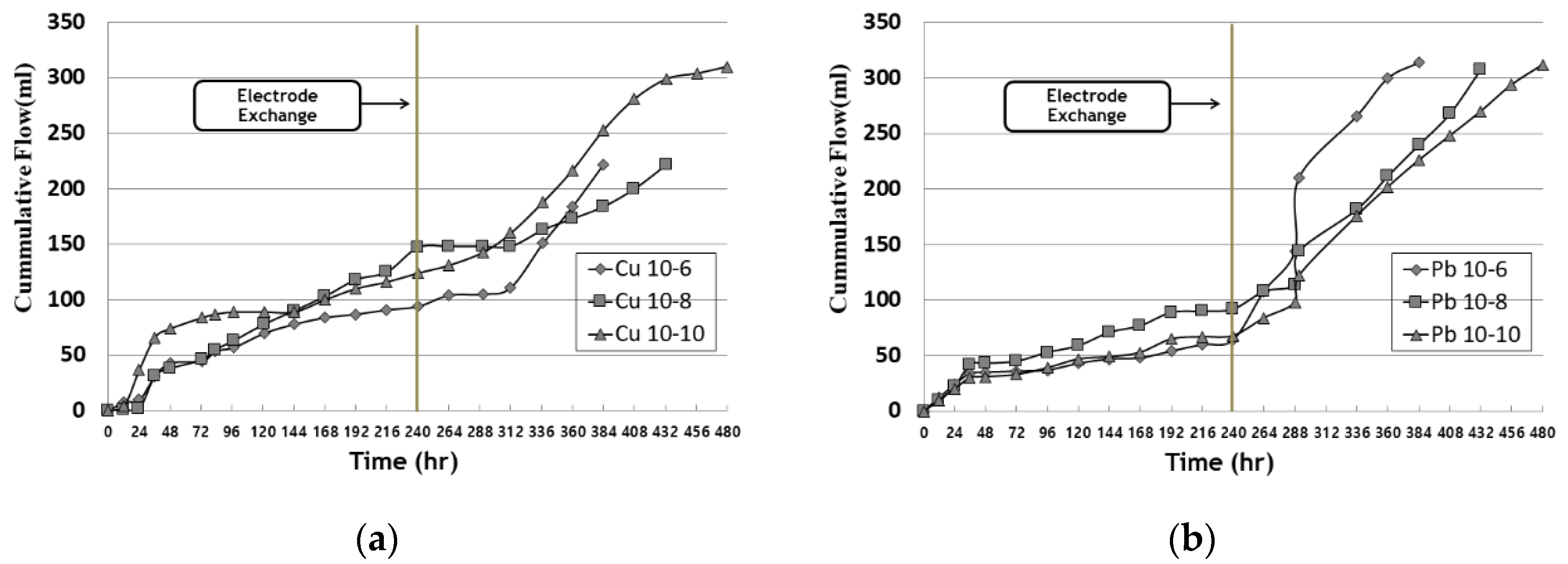
3.3. Outflow
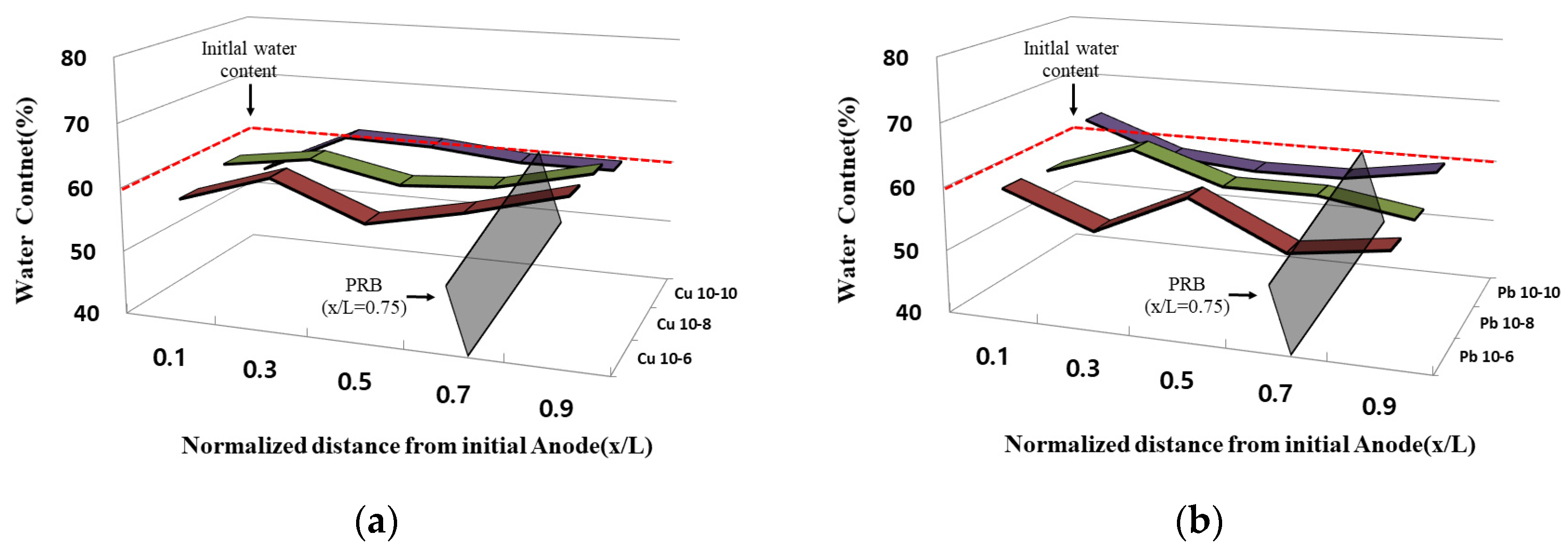
3.4. Water Content
3.5. pH and Residue of Heavy Metals in Samples after the End of the EK Experiment
3.6. Current and Outflow
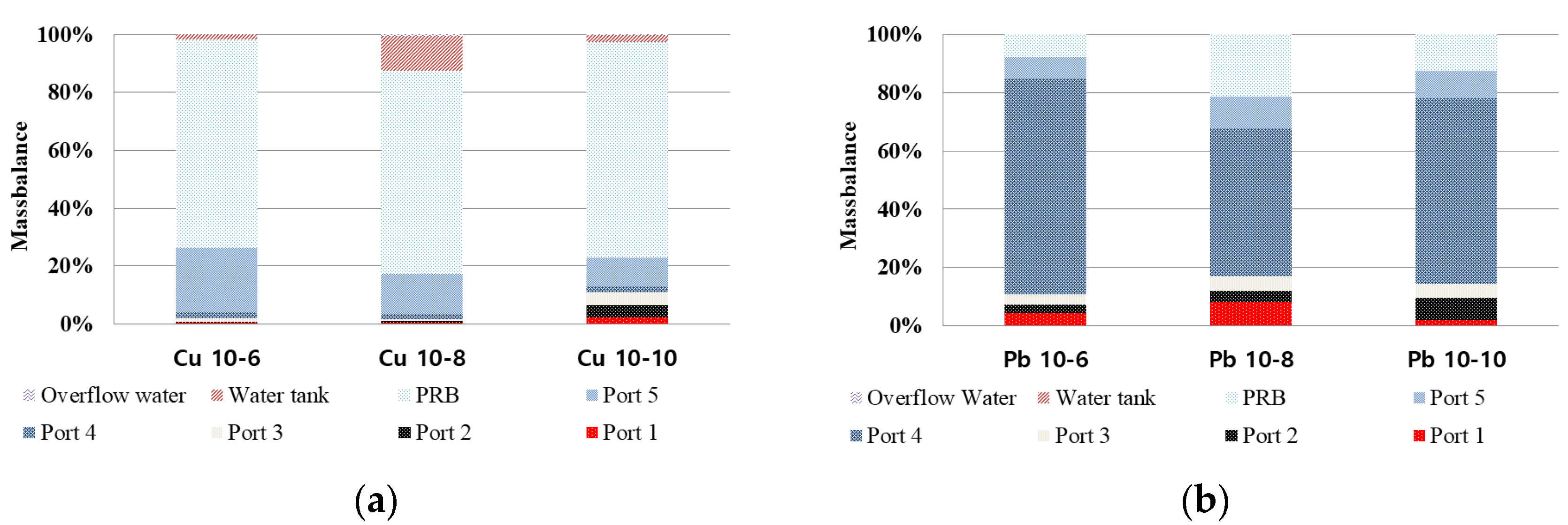
3.7. Mass Balance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb(ΙΙ) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 19585. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, Y.; Shih, K.; Li, B. Enhanced phosphorus availability and heavy metal removal by chlorination during sewage sludge pyrolysis. J. Hazard. Mater. 2019, 382, 121110. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Yan, X.; Wang, X.; Zhang, J.; Huang, J.; Zhao, J. Heavy metal chemical extraction from industrial and municipal mixed sludge by ultrasound-assisted citric acid. J. Ind. Eng. Chem. 2015, 27, 368–372. [Google Scholar] [CrossRef]
- Wu, Q.; Duan, G.; Cui, Y.; Sun, J. Removal of heavy metal species from industrial sludge with the aid of biodegradable iminodisuccinic acid as the chelating ligand. Environ. Sci. Pollut. Res. Int. 2015, 22, 1144–1150. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, Y.; Li, Q.; Sun, J. Effective removal of heavy metals from industrial sludge with the aid of a biodegradable chelating ligand GLDA. J. Hazard. Mater. 2015, 283, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.H.; Jeong, S.W. Effect of Water-Thoroughly-Rinsing in the Artificially Metal-Contaminated Soil Preparation on Final Soil Metal Concentrations. J. Korean Soc. Environ. Eng. 2011, 33, 670–676. [Google Scholar] [CrossRef][Green Version]
- Tang, J.; He, J.; Liu, T.; Xin, X.; Hu, H. Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 2017, 189, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. Soil Measurement Network and Soil Contamination Survey Results in 2005; Ministry of Environment: Sejong-si, Korea, 2005.
- Liu, H.; Li, J.; Zhao, M.; Li, Y.; Chen, Y. Remediation of oil-based drill cuttings using low-temperature thermal desorption: Performance and kinetics modeling. Chemosphere 2019, 235, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yuan, X.; Zhang, G.; Hu, J.; An, J.; Chen, T.; Wang, G. Stir bar sorptive extraction and automatic two-stage thermal desorption-gas chromatography-mass spectrometry for trace analysis of the byproducts from diphenyl carbonate synthesis. Microchem. J. 2020, 153, 104341. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int. Biodeterior. Biodegrad. 2017, 118, 73–81. [Google Scholar]
- Wang, F.; Shen, Z.; Liu, R.; Zhang, Y.; Xu, J.; Al-Tabbaa, A. GMCs stabilized/solidified Pb/Zn contaminated soil under different curing temperature: Physical and microstructural properties. Chemosphere 2020, 239, 124738. [Google Scholar] [CrossRef]
- Muddanna, M.H.; Baral, S.S. A comparative study of the extraction of metals from the spent fluid catalytic cracking catalyst using chemical leaching and bioleaching by Aspergillus niger. J. Environ. Chem. Eng. 2019, 7, 103335. [Google Scholar] [CrossRef]
- Cameselle, C. Enhancement of electro-osmotic flow during the electrokinetic treatment of A contaminated soil. Electrochim. Acta 2015, 181, 31–38. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Zhang, M.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y. Cr(VI) recovery from chromite ore processing residual using an enhanced electrokinetic process by bipolar membranes. J. Membr. Sci. 2018, 566, 190–196. [Google Scholar] [CrossRef]
- Fu, R.; Wen, D.; Xia, X.; Zhang, W.; Gu, Y. Electrokinetic remediation of chromium (Cr)-contaminated soil with citric acid (CA) and polyaspartic acid (PASP) as electrolytes. Chem. Eng. J. 2017, 316, 601–608. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Xin, X.; Hu, H.; Liu, T. Biosurfactants enhanced heavy metals removal from sludge in the electrokinetic treatment. Chem. Eng. J. 2018, 334, 2579–2592. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Malarbì, D.; Vagliasindi, F.G.A. Removal of mercury from marine sediments by the combined application of a biodegradable non-ionic surfactant and complexing agent in enhanced-electrokinetic treatment. Electrochim. Acta 2016, 222, 1569–1577. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Malarbì, D.; Maddalena, R.; Greco, V.; Vagliasindi, F.G.A. Remediation of Hg-contaminated marine sediments by simultaneous application of enhancing agents and microwave heating (MWH). Chem. Eng. J. 2017, 321, 1–10. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Malarbì, D.; Greco, V.; Vagliasindi, F.G.A. Surfactant and MGDA enhanced-Electrokinetic treatment for the simultaneous removal of mercury and PAHs from marine sediments. Sep. Purif. Technol. 2017, 175, 330–339. [Google Scholar] [CrossRef]
- Qiu, M.; He, C. Efficient removal of heavy metal ions by forward osmosis membrane with a polydopamine modified zeolitic imidazolate framework incorporated selective layer. J. Hazard. Mater. 2019, 367, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Mendez, E.; Perez, M.; Romero, O.; Beltran, E.D.; Castro, S. Effects of electrode material on the efficiency of hydrocarbon removal by an electrokinetic remediation process. Electrochim. Acta 2012, 86, 148–156. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawai, K.; Moribe, M.; Niinae, M. Recovery of Cr as Cr(III) from Cr(VI)-contaminated kaolinite clay by electrokinetics coupled with a permeable reactive barrier. J. Hazard Mater. 2014, 278, 297–303. [Google Scholar] [CrossRef]
- Yuan, L.; Li, H.; Xu, X.; Zhang, J.; Wang, N.; Yu, H. Electrokinetic remediation of heavy metals contaminated kaolin by a CNT-covered polyethylene terephthalateyarn cathode. Electrochim. Acta 2016, 213, 140–147. [Google Scholar] [CrossRef]
- Wang, L.; Huang, L.; Xia, H.; Li, H.; Li, X.; Liu, X. Application of a multielectrode system with polyaniline auxiliary electrodes for electrokinetic remediation of chromium-contaminated soil. Separ. Purif. Technol. 2019, 224, 106–112. [Google Scholar] [CrossRef]
- Alshawabkeh, A.N.; Gale, R.J.; Ozsu-Acar, E.; Bricka, R.M. Optimization of 2-D electrode configuration for electrokinetic remediation. J. Soil Contam. 2010, 8, 617–635. [Google Scholar] [CrossRef]
- Turer, D.; Genc, A. Assessing effect of electrode configuration on the efficiency of electrokinetic remediation by sequential extraction analysis. J. Hazard Mater. 2005, 119, 167–174. [Google Scholar] [CrossRef]
- Zhou, D.; Deng, C.; Cang, L.; Alshawabkeh, A.N. Electrokinetic remediation of a CueZn contaminated red soil by controlling the voltage and conditioning catholyte pH. Chemosphere 2005, 61, 519–527. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.H.; Park, S.W.; Ryu, B.G.; Bajargal, T. Electrolyte conditioning enhanced electrokinetic remediation of arsenic-contaminated mine tailing. J. Hazard Mater. 2009, 161, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.O.; Peng, C.S.; Abou-Shady, A. Simultaneous removal of cadmium from kaolin and catholyte during soil electrokinetic remediation. Desalination 2012, 300, 1–11. [Google Scholar]
- Ryu, B.G.; Park, G.; Yang, J.; Baek, K. Electrolyte conditioning for electrokinetic remediation of As, Cu, and Pb-contaminated soil. Separ. Purif. Technol. 2011, 79, 170–176. [Google Scholar] [CrossRef]
- Jelusic, M.; Vodnik, D.; Lestan, D. Revitalization of EDTA-remediated soil by fertilization and soil amendments. Ecol. Eng. 2014, 73, 429–438. [Google Scholar] [CrossRef]
- Jez, E.; Lestan, D. EDTA retention and emissions from remediated soil. Chemosphere 2016, 151, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Giannis, A.; Nikolaou, A.; Pentari, D.; Gidarakos, E. Chelating agent-assisted electrokinetic removal of cadmium, lead and copper from contaminated soils. Environ. Pollut. 2009, 157, 3379–3386. [Google Scholar] [CrossRef]
- Cappai, G.; Gioannis, G.D.; Muntoni, A.; Spiga, D.; Zijlstra, J. Combined use of a transformed red mud reactive barrier and electrokinetics for remediation of Cr/As contaminated soil. Chemosphere 2012, 86, 400–408. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Ramadan, B.S.; Sari, G.L.; Rosmalina, R.T.; Effendi, A.J. An overview of electrokinetic soil flushing and its effect on bioremediation of hydrocarbon contaminated soil. J. Environ. Manag. 2018, 218, 309–321. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Zhong, Q.; Xu, X.; Li, T.; Jia, Y.; Zhang, Y.; Peijnenburg, W.J.G.M.; Vijver, M.G. Effect of soil washing with biodegradable chelators on the toxicity of residual metals and soil biological properties. Sci. Total Environ. 2018, 625, 1021–1029. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, S.; Tong, Y.; Zhang, H.; Yang, H. Adaptive defensive mechanism of bioleaching microorganisms under extremely environmental acid stress: Advances and perspectives. Biotechnol. Adv. 2020, 42, 107580. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, J.; Huo, S.; Qi, S.; Gu, X.S. A real scale phytoremediation of multimetal contaminated e-waste recycling site with Eucalyptus globulus assisted by electrical fields. Chemosphere 2018, 201, 262–268. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metalsdconcepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Mao, X.; Han, F.X.; Shao, X.; Guo, K.; McComb, J.; Arslan, Z.; Zhang, Z. Electro-kinetic remediation coupled with phytoremediation to remove lead, arsenic and cesium from contaminated paddy soil. Ecotoxicol. Environ. Saf. 2016, 125, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R. Electrokinetic remediation of soils at complex contaminated sites: Technology status, challenges, and opportunities. In Coupled Phenomena in Environmental Geotechnics; CRC Press: Boca Raton, FL, USA, 2013; pp. 131–147. [Google Scholar]
- Dalloway, B.J. Heavy Metals in Soils; John Wiley & Sons Inc.: New York, NY, USA, 1990; p. 339. [Google Scholar]
- Lockhart, N.C. Electro-osmotic dewatering of clay-ΙΙΙ. Influence of clay type, exchangeable cations and electrode materials. Colloids Surf. 1983, 6, 253–269. [Google Scholar] [CrossRef]
- McMutry, D.C.; Elton, R.O. New approach to in-situ treatment of contaminated groundwaters. Environ. Prog. 1985, 4, 168–170. [Google Scholar] [CrossRef]
- Narasimhan, B.; Ranjan, R.S. Electrokinetic barrier to prevent subsurface contaminant migration: Theoretical model development and validation. J. Contam. Hydrol. 2000, 42, 1–17. [Google Scholar] [CrossRef]
- Zhu, N.M.; Qiang, L.; Guo, X.J.; Hui, Z.; Yu, D. Sequential extraction of anaerobic digestate sludge for the determination of partitioning of heavy metals. Ecotoxicol. Environ. Saf. 2014, 102, 18–24. [Google Scholar] [CrossRef]
- Zhu, N.-M.; Chen, M.; Guo, X.-J.; Hu, G.-Q.; Yu, D. Electrokinetic removal of Cu and Zn in anaerobic digestate: Interrelation between metal speciation and electrokinetic treatments. J. Hazard. Mater. 2015, 286, 118–126. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, D.; Wang, X. Removal of fluorine from red mud (bauxite residue) by electrokinetics. Electrochim. Acta 2017, 242, 300–306. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochem. 2009, 44, 296–301. [Google Scholar] [CrossRef]
- Yong, R.N.; Mohamed, A.M.O.; Warkentin, B.P. Principles of Contaminant Transport in Soils; Elsevier Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Zhang, P.; Jin, C.; Sun, Z.; Huang, G.; She, Z. Assessment of acid enhancement schemes for electrokinetic remediation of Cd/Pb contaminated soil. Water Air Soil Pollut. 2016, 227, 1–12. [Google Scholar] [CrossRef]
- Yang, J.S.; Man, J.K.; Choi, J.; Baek, K.; O’Loughlin, E.J. The transport behavior of As, Cu, Pb, and Zn during electrokinetic remediation of a contaminated soil using electrolyte conditioning. Chemosphere 2014, 117, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, S.; Santhanam, M.; Sundaram, M.; Curras, M.P. Electrokinetic remediation of inorganic and organic pollutants in textile effluent contaminated agricultural soil. Chemosphere 2014, 117, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.D.; Fu, R.B.; Zhang, W.; Gu, Y.Y. Enhanced electrokinetic remediation of heavy metals contaminated soils by stainless steel electrodes as well as the phenomenon and mechanism of electrode corrosion and crystallization. Huanjing Kexue 2017, 38, 1209–1217. [Google Scholar] [PubMed]
- Wang, J.; Huang, X.; Kao, J.; Stabnikova, O. Removal of heavy metals from kaolin using an upward electrokinetic soil remedial (UESR) technology. J. Hazard Mater. 2006, 136, 532–541. [Google Scholar] [CrossRef]


| Fixed Factor | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrode Gradient (V/cm) | Improver (M) | PRB Location (x/L) | PRB Thickness (cm) | Duration (Day) | |||||
| 1 | Acetic acid 0.05 | 0.75 | 1 | 10 | |||||
| Variable factor | |||||||||
| Pollutants | |||||||||
| Copper | Lead | ||||||||
| Duration after electrode exchange (Day) | |||||||||
| 6 | 8 | 10 | 6 | 8 | 10 | ||||
| Cu 10-6 | Cu 10-8 | Cu 10-10 | Pb 10-6 | Pb 10-8 | Pb 10-10 | ||||
| Test | Initial Amount of Pollutant (mg) | Residual in the Soil (mg) | PRB (mg) | Outflow (mg) | Water Tanks (mg) | Mass Balance (%) | Removal Rate (%) |
|---|---|---|---|---|---|---|---|
| Cu 10-6 | 180 | 47.24 | 129.75 | 0.16 | 2.82 | 99.99 | 73.76 |
| Cu 10-8 | 180 | 24.33 | 99.11 | 0.80 | 16.95 | 78.44 | 86.80 |
| Cu 10-10 | 180 | 40.03 | 129.78 | 0.69 | 3.87 | 96.87 | 77.76 |
| Pb 10-6 | 360 | 293.05 | 27.17 | 0.04 | 0.03 | 96.22 | 18.59 |
| Pb 10-8 | 360 | 205.19 | 65.35 | 0.07 | 0.05 | 84.37 | 43.00 |
| Pb 10-10 | 360 | 319.22 | 51.65 | 0.35 | 0.03 | 113.8 | 11.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Yun, J.-M.; Lee, J.-Y.; Hong, G.; Kim, J.-S.; Kim, D.; Han, J.-G. The Remediation Characteristics of Heavy Metals (Copper and Lead) on Applying Recycled Food Waste Ash and Electrokinetic Remediation Techniques. Appl. Sci. 2021, 11, 7437. https://doi.org/10.3390/app11167437
Lee S, Yun J-M, Lee J-Y, Hong G, Kim J-S, Kim D, Han J-G. The Remediation Characteristics of Heavy Metals (Copper and Lead) on Applying Recycled Food Waste Ash and Electrokinetic Remediation Techniques. Applied Sciences. 2021; 11(16):7437. https://doi.org/10.3390/app11167437
Chicago/Turabian StyleLee, Sounghyun, Jung-Mann Yun, Jong-Young Lee, Gigwon Hong, Ji-Sun Kim, Dongchan Kim, and Jung-Geun Han. 2021. "The Remediation Characteristics of Heavy Metals (Copper and Lead) on Applying Recycled Food Waste Ash and Electrokinetic Remediation Techniques" Applied Sciences 11, no. 16: 7437. https://doi.org/10.3390/app11167437
APA StyleLee, S., Yun, J.-M., Lee, J.-Y., Hong, G., Kim, J.-S., Kim, D., & Han, J.-G. (2021). The Remediation Characteristics of Heavy Metals (Copper and Lead) on Applying Recycled Food Waste Ash and Electrokinetic Remediation Techniques. Applied Sciences, 11(16), 7437. https://doi.org/10.3390/app11167437







