Abstract
The sorption of basic dyes onto magnetic nanosorbent is commonly used as a novel material to combat powdered activated carbon, which is difficult to handle and separate during water and wastewater treatment. This adsorption method is commonly implemented in water and wastewater treatment due to its low cost and high performance. To explore the feasibility of adsorption, six different nonlinear isotherm models were applied and introduced to evaluate the performance and adsorption mechanisms via Microsoft Excel, and they were then compared to those of MATLAB and OriginPro. The Langmuir best provided the sorption of methylene blue adsorbed for a two-parameter isotherm model. The three-parameter model Toth gave a goodness of fit indicating a heterogeneous sorbent surface. Error function analysis found that the Residual Sum of Squares Error, Chi-square, Coefficient of determination, Average Relative Error, Hybrid Fractional Error Function, Marquardt’s Percent Standard Deviation and Root Mean Square Error provided the best overall results. In comparison, it could be concluded that six isotherm models showed the confidence interval that is simply the best fit at all experimental data points provided by the three software tools. All error function results indicated that the Microsoft Excel Solver function spreadsheet method satisfied all the statistic measures to predict the real variance of the probability of experimental data for all six isotherm models of adsorption of basic dye removal. One added benefit of this Microsoft Excel software tool is the built-in function associated with the desired type of application, which designates the desired type of error/statistical functions not mentioned in this research to the adsorbent materials used.
1. Introduction
In a water treatment plant, it is a necessity to remove contaminants, such as organic and inorganic materials, which can act as precursors for the production of disinfection by-products. Various types of activated carbon are widely used for the treatment of contaminants in many environmental applications by an adsorption process because of the high specific surface area; the high degree of surface reactivity [1,2]; and the existence of specific surface chemistry, such as the presence of heteroatoms, including oxygen, nitrogen, hydrogen, sulfur and phosphorous- [3] and nitrogen-containing groups [4]. However, it is widely known that powdered activated carbon is extremely difficult to handle and separate from water and wastewater treatment plants. Recently, magnetic particles and magnetic nanosorbents were used to adsorb pollutants from water and air [5], but their applications are limited due to their small surface area and morphology structures. A magnetic field has previously been used to separate the adsorbent from aqueous solutions after the adsorption process [6]. Many researchers have used magnetite (Fe3O4) for adsorption from air and wastewater because magnetite has good performance and biocompatibility [7]. However, magnetite or magnetic particles can remove about 60% of natural organic matter from the water [8]. Magnetite can be combined with amorphous carbon (activated carbon) by an impregnation process to enhance the adsorption of various pollutants in an aqueous solution. For instance, Bastami and Entezari [9] studied and characterized a composite of activated carbon and iron oxide applied for the removal of p-nitrophenol from aqueous solutions. Moreover, Cheng and Gao [10] developed a magnetic nanosorbent when removing strontium ions from aqueous solutions. Wongcharee et al. modified magnetic nanosorbent prepared from macadamia nut shell for the removal of basic dye [11]. The results indicated that the magnetic nanosorbent was suitable for specific water and wastewater treatment application using the advanced software tools of OriginPro and MATLAB in the interpretation of the adsorption isotherm models.
Artificial dyes are being utilized in various applications such as food, paper, printing industries, cosmetics, textiles and dye houses in order to color products [12]. In general, dyes are synthetic organic pollutants composed of complex aromatic structures [13]. The discharge of colored wastewater causes significant harmful effects downstream, increasing the toxicity and chemical oxygen demand (COD) [12]. Methylene blue (MB) is considered to be one of the basic dyes that is commonly soluble in water and used by textile industries, which can cause eye and skin irritation; vomiting; nausea; and sympathetic effects, including blood changes [14]. The literature shows that patterns of the evacuation of contaminant dyes in water bodies and the development of outcome measure limitations have resulted in the utilization of adsorption processes amongst other suitable techniques [15,16].
Adsorption methods are applied in various water treatments depending on the condition of the water sources when eliminating colors, inorganic and organic pollutants, odor and oil [17] due to low capital and operation cost. For basic dye adsorption, the process is combined in two mechanism steps of adsorption and/or ion exchange, including being controlled by various parameters, such as pH, temperature, adsorbent and contact time [18]. A proper understanding of the mechanism of adsorption may involve chemisorption and physisorption or both in the sorption process [19], which indicate the adsorption equilibrium information. Several literature reports necessitate the interpretation of adsorption isotherms due to them being extremely important for the general improvement of adsorption mechanism pathways and process design for removing basic dyes in wastewater sources. Linear regression analysis has long been applied and developed to evaluate the adsorption isotherm parameters and statistical results, along with indicating the best fitting and theoretical assumptions of adsorption models [20]. However, linearization is still poorly defined for the distribution of error change for experimental data and the predicted adsorption isotherm. Therefore, nonlinear regression needs to address shortfalls based on the convergence criteria of the experimental data and the predicted adsorption isotherm as has become inevitable [21]. Residual Sum of Squares Error (ERRSQ/SSE) [22], Chi-square (χ2) [23], Average Relative Error (ARE) [24], Hybrid Fractional Error Function (HYBRID) [25], Marquardt’s Percent Standard Deviation (MPSD) [26] and Root Mean Square Error (RMSE, polynomial p: the number of terms) [27,28,29] can usually be utilized to predict the best fitting of isotherms to minimize the residual between the experimental data measurement and the mathematical isotherm model. These error functions are normally used for water and wastewater treatment applications to predict the adsorption capacity and adsorption mechanism via advanced software tools, such as MATLAB, OriginPro, Minitab and Sigma Plot. Even though advanced program software tools are not available for built-in error functions as mentioned above, Microsoft Excel is a readily available operation that is not difficult, as isotherm functions can be built owing to the availability of computer algorithms.
This research recognizes the importance of magnetic nanosorbent to deal with basic dyes in an aqueous solution. The objective of this study is to present a magnetic nanosorbent composite that increases the affinities for specific contaminants and allows the use of efficient batch reactors and resolves the challenge of separating the spent adsorbent from water using magnetic fields for the removal of basic dyes from water. Treatment performance and efficiencies were evaluated using adsorption isotherm models to clarify the involved mechanisms through the Microsoft Excel Solver spreadsheet-based program by nonlinear regression analyses to explore the theoretical assumptions. A comparison of parameter values for the isotherm models obtained by nonlinear fitting was also evaluated with advanced software tools, such as MATLAB and OriginPro (free trial).
2. Materials and Methods
Experimental data of isotherms (Table 1) were taken from a previous study using a magnetic nanosorbent for methylene blue adsorption [11]. The magnetic nanosorbent was derived from waste macadamia nut shell and magnetite nanoparticles. They were used for removing the basic dye with simple handling and separation during water and wastewater treatment in various applications and conditions. Batch sorption of isotherm studies was performed following the equations as shown below.
where qe (mg g−1) is the methylene adsorbed at equilibrium, C0 is an initial concentration, Ce is an equilibrium concentration of methylene blue (mg L−1), V (L) is the volume of the raw water used in a previous study (the synthetic solution was prepared from methylene blue) and m (g) is the mass of the prepared magnetic nanosorbent.

Table 1.
Experimental data of methylene blue adsorbed onto magnetic nanosorbent.
2.1. Isotherm Models
Numerous nonlinear regression approaches to isotherm parameter estimation have appeared in the literature. Two- and/or three-parameter isotherm models are normally applied to classify and characterize an adsorption mechanism process in various applications in water and wastewater treatment, even for specific pollution, such as gas phase or particles. Sorption isotherm models have described the information on the affinity between magnetic nanosorbent and methylene blue [18,23,30] for this case study. The experimental data as shown in Table 1 were computed using Langmuir [31], Freundlich [32], Temkin [33], Khan [34], Toth [35] and Liu [36] isotherm models as follows in Equations (2)–(7), respectively, via Microsoft Excel, MATLAB and OriginPro programs. Nonlinearized empirical models are expressed as follows:
2.2. Error Function Analysis
The error functions are an important statistical parameter used to measure the deviation of theoretically predicted isotherm parameters for evaluating the suitability of the nonlinearized empirical model onto experimental results. Various error functions have been established either empirically or based on theoretical considerations. To validate the best fitting of the adsorption isotherm of two- and three-parameter models as mentioned above, seven different statistical error functions, namely Residual Sum of Squares Error (ERRSQ/SSE) [22], Chi-square (χ2) [23], Coefficient of determination (R2), Average Relative Error (ARE) [24], Hybrid Fractional Error Function (HYBRID) [25], Marquardt’s Percent Standard Deviation (MPSD) [26] and Root Mean Square Error (RMSE, polynomial p: the number of terms) [27,28,29], were assessed to describe the adsorption characteristics of methylene blue adsorbed onto the magnetic nanosorbent. The error analysis equations are conveyed as follows:
where n is the number of experimental data points, p (polynomial model) is the number of parameters in each isotherm model, qe,cal (mg g−1) is the theoretically calculated adsorption capacity of methylene blue adsorbed at equilibrium and qe,exp (mg g−1) is the experimental capacity of methylene blue adsorbed at equilibrium.
2.3. Operational Software Tools
Microsoft Excel was used as a tool for the determination of nonlinear isotherm models and error functions via Windows 10 and Microsoft 365 2020 on PC. The experimental data were entered manually into an Excel sheet operating the graphical data of the isotherm models. Statistical analysis was also performed and compared to that of MATLAB and OriginPro via the formulated algorithm that follows [37,38,39]. The steps of the operation are shown in Figure 1.
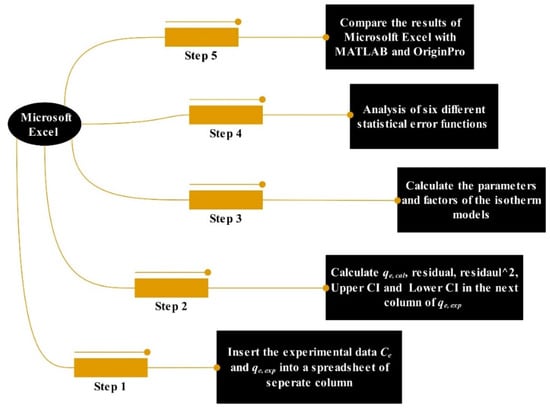
Figure 1.
Operation steps of software tools for predicting the experimental data.
The following step demonstrates how to solve the experimental data of basic dye (methylene blue as a model substrate) loaded onto magnetic nanosorbent in the Microsoft Excel spreadsheet using six isotherm models and seven statistical error functions as mentioned in Section 2.1 and Section 2.2, respectively. Table 2 shows the formulas of factors for the model, the statistical analysis results and the calculated excel codes/formulas. In Table 2, the Freundlich isotherm model was used as an example model to elucidate how to solve the isotherm model via the Microsoft Excel solver spreadsheet-based program. The other isotherm models as mentioned above in Section 2.1 can be applied using the procedures as described below.

Table 2.
Microsoft Excel Solver spreadsheet-based program for Freundlich isotherm model.
The Application of the Microsoft Excel Solver Spreadsheet Are as Follows
(a) Experimental data from Table 1 were entered into rows A1–A11 (Ce, mg L−1) and B1–B11 (qe, exp, mg g−1, Equation (1)) or were more than those values depending on the experimental data sets. Column C is the equilibrium capacity (qe,cal, mg g−1) of the cationic dye adsorbed onto the magnetic nanosorbent for the equilibrium adsorption capacity (Equation (3)) as shown in the Excel form of Figure 2 and Figure 3. Before calculating qe,cal values, the factors for the model in columns H2 (kf) and H3 (n) are very critical for nonlinear regression analysis of the isotherm models selected. Therefore, n and kf must be computed using residual^2 values in column E to calculate the sum of squared residuals (SSR, column I1, = SUM(E1:E11), 3860.74). In the next stage, the initial estimate parameter values of 0.5 and 1.5 (any values can be inserted instead of 0.5 and 1.5) were inserted into lows I2 and I3 before operating the algorithm, respectively. It can be seen that the qe, cal values (poorly defined, as shown in the right inset) did not parallel with the qe, exp values together with the residual values that may be shown in a different type of line as shown in Figure 2.
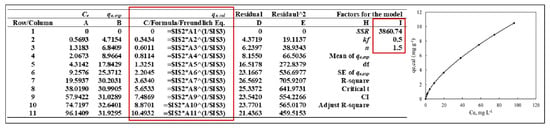
Figure 2.
The first stage to find the n and kf factors of the nonlinear isotherm model selected to solve the qe, cal parameter results.
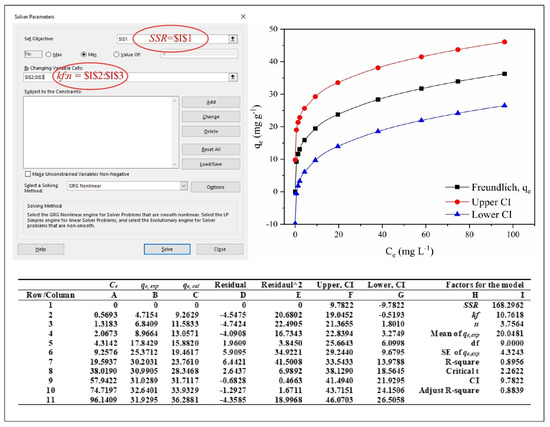
Figure 3.
Running nonlinear of Microsoft Excel Solver function for isotherm models to obtain recognized equilibrium adsorption values (qe, cal), order reaction (n) and Freundlich isotherm rate constant (kf) in Excel.
Hence, the Solver function from the Tools menu of Microsoft Excel was needed to fit the experimental data of the nonlinear isotherm model regression analysis as displayed in Figure 3.
(b) To manipulate the nonlinear isotherm model using the Microsoft Excel Solver function, open the Microsoft Excel Tools menu followed by Data, Solver and Solver parameters, respectively, as shown in Figure 3. The dialogue box shown in Figure 3 appears as the Solver parameters. To fit the nonlinear function of the Freundlich isotherm model, the Solver parameters of set objectives (SSR) and changing variable cells (kf and n) were required. SSR (column I1, 3860.74) was set up with the objective as $I$1, along with changing the variable cells of kf (column I2) and n (column I3) to $I$2:$I$3. Then, click the Solve button to formulate the algorithm for the Freundlich isotherm model that achieved the qe,cal data results, the fitted data curve and the lower CI and upper CI (up-right inset) in the Excel sheet as indicated in the bottom inset and up-right inset of Figure 3, respectively.
(c) To identify and describe the statistical results and isotherm model parameters for the controlling mechanism, MATLAB and OriginPro were used as tools for comparison with the Microsoft Excel Solver function spreadsheet-based program for nonlinear expressions of adsorption isotherm models onto magnetic nanosorbent. This research work only proposed and described the method of how to minimize the statical error functions and isotherm parameters via the Microsoft Excel Solver function. MATLAB and OriginPro procedure details are not shown here.
3. Results and Discussion
3.1. Authentication of Isotherm Model Data Using Microsoft Excel Solver Spreadsheet-Based Program
The experimental data of methylene blue adsorbed onto magnetic nanosorbent were evaluated and analyzed via the Microsoft Excel Solver spreadsheet-based program for the six different nonlinearized forms of Freundlich, Langmuir, Temkin, Liu, Toth and Khan models described above. The error functions (the goodness of fit) were also examined with the same time period to achieve the statistical values from the isotherm models, such as ERRSQ/SSE, χ2, R2, HYBRID, ARE, MPSD and RMSE. The values of the isotherm parameters and the regression statistics with formulas (factors for the model and statistical results) are shown in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7.

Table 3.
Microsoft Excel Solver spreadsheet-based program for Langmuir isotherm model.

Table 4.
Microsoft Excel Solver spreadsheet-based program for Temkin isotherm model.

Table 5.
Microsoft Excel Solver spreadsheet-based program for Liu isotherm model.

Table 6.
Microsoft Excel Solver spreadsheet-based program for Toth isotherm model.

Table 7.
Microsoft Excel Solver spreadsheet-based program for Khan isotherm model.
The nonlinear regression of isotherm models and the goodness of fit were built-in functions using the Microsoft Excel Solver spreadsheet-based program available in Microsoft Office. They were appropriate to describe the experimental data with accuracy to predict the best fit for the methylene blue adsorbed onto the magnetic nanosorbent material. These parameter values were evaluated with MATLAB and OriginPro and described under Section 3.2.
Within this framework, Table 2, Table 3 and Table 4 concern the prediction of two-parameter isotherm models, such as the Freundlich, Langmuir and Temkin models. The parameters for these isotherm models are kf (10.7618 (mg g−1) (mg L−1)1/n), n (3.7564), qm (34.4765 mg g−1), kl (0.2346 L mg−1), bt (6.0492 J mol−1) and kt (3.5884 L mol−1). qm is the maximum adsorption capacity of methylene blue adsorbed onto magnetic nanosorbent (mg g−1), and kl is the affinity constant or Langmuir isotherm rate constant (L mg−1) to the apparent energy of sorption. kf is the Freundlich isotherm rate constant (mg g−1) (mg L−1)1/n, and nf is the order of reaction or the heterogeneous factor surfaces (dimensionless) for the Freundlich model. bt is the Temkin isotherm constant (J mol−1), and kt is the Temkin isotherm rate constant (L mol−1). qe, cal in column C was computed as appropriate for the values of Ce data and the empirical equation of each model shown in Equations (2)–(4) and/or the formula array shown in Table 2, Table 3 and Table 4 (Langmuir = $I$3 * ($I$2 * (A/(1 + ($I$2*A)))), Freundlich = $I$2 * A^(1/$I$3) and Temkin = $I$2*LN($I$3*A)), respectively. It can be observed that the qe, cal values of each model and row were different and were affected by the statistical results, R-square and Adjust R-square together with residual values, upper CI, lower CI and SSR and parameter models also. From the perspective of ERRSQ/SSE, Chi-square, ARE, RMSE, HYBRID and MPSD, the built-in formula is shown in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7. It was found that the statistical results were different depending on the isotherm model function. Likewise, a comparison for the best fitting of two nonlinear parameters of the isotherm models demonstrated that the Langmuir model presented an R-square (0.9897) and Adjust R-square (0.9885) close to 1. ERRSQ/SSE (16.6590), Chi-square (1.2540), ARE (−2.1538), RMSE (1.2907), HYBRID (−2.3931) and MPSD (15.2297) values also indicated that all small values of significance were evidence for the acceptance of the nonlinear regression modeling. It could be confirmed that the adsorption of methylene blue or cationic dye onto the magnetic nanosorbent surface involves homogenous sites, with all active sites having equivalent energy, which had no more adsorption onto the magnetic nanosorbent and/or with no interaction among the adsorbed molecules [30,40]. However, the Langmuir model is not applicable for the explanation of the adsorption mechanism for the adsorption process; it just provides the information of the ability of adsorption and equilibrium processes as mentioned (qe, qm and kl) [41]. Therefore, three-parameter isotherm models (Table 5, Table 6 and Table 7) were required to explain the adsorption mechanism as described below.
Table 5, Table 6 and Table 7 provide a summary of the three-parameter isotherm model types for this task, including Liu, Toth and Khan models. The model parameters of qm, kg, ng, kth, nth, kk and ak are summarized followed by the statistical analysis results via the Microsoft Excel Solver spreadsheet-based program. The nonlinear regression values of three-parameter isotherm models were compared and presented in Table 5, Table 6 and Table 7 in columns I and K. Table 5, Table 6 and Table 7 summarize that for the magnetic nanosorbent, mathematically the Toth model provides a significant goodness of fit that was better than that of the Liu and Khan models. The Toth model was modified from the Langmuir isotherm model equation (homogeneous surface, n = 1, the Toth equation reduces to Langmuir model) that is useful to describe the heterogeneous systems with both a low- and high-end boundary of methylene blue concentration [42,43]. This model correlates and/or assumes an asymmetrical quasi-Gaussian site energy distribution [44]. The Liu and Khan models had a slightly reduced mathematical complexity; however, each of them showed an excellent fit. In this case, the Khan model also gave a goodness of fit but was unable to forecast the qm (48.6815 mg g−1, error 34%) as shown in Table 7. In terms of the overall statistical results, it was clear that the Toth model was the best fitting and close to the Liu model.
3.2. Comparison of Parameter Values for the Isotherm Models Obtained by Nonlinear Fitting
Usually, various advanced nonlinear regression programs are used to assess the standard error of the isotherm parameters. Those parameter values sometimes distort the forecasts for nonlinear isotherm functions, additive or symmetrical errors, and exact confidence intervals cannot be calculated. The nonlinear regression equations of isotherm models are formulated from the linear assumptions that constantly provide underestimates of the true uncertainty of asymptotic standard errors. Therefore, Microsoft Excel was used for calculating the isotherm parameters and statical results; however, it was complex and took a long time to operate for the built-in function to conduct the necessary evaluation and comparison. Consequently, an approach was implemented for this research in order to evaluate the standard error of the results obtained derived from the experimental and predicted data that were computed by the software tools as mentioned above.
Table 8 presents the comparison results for parameter values for the isotherm models obtained by nonlinear fitting via the Microsoft Excel Solver function with MATLAB and OriginPro. Virtually the same results can be noted for the magnetic nanosorbent material and that the Langmuir and Toth provided the best (and very good) statistics among the two-parameter and three-parameter isotherm models, respectively. It can be seen that the model parameters were close/similar for the three software tools of the Microsoft Excel Solver function with MATLAB and OriginPro. However, there are some statistical results and model parameters that are discussed below.

Table 8.
Comparison of isotherm parameters and error functions from Microsoft Excel Solver function with MATLAB and OriginPro.
It can be seen that the result of R-square (0.9384) of the Temkin isotherm model for MATLAB was different with Microsoft Excel (0.9553) and OriginPro (0.9553) due to the real natural logarithm function ln (experimental data, Ce) being defined only for Ce as more than zero; the natural logarithm of zero was undefined (log 0, it is not a real number) in MATLAB. For that reason, it might be that the number of degrees of freedom (df, maximum number of logically independent values) were reduced to 8 from the data sample set of qe and Ce (11, Table 1) as compared to Microsoft Excel (9) and OriginPro (9). Therefore, the relation to various forms of hypothesis testing in some statistical results was effected by the Adj R-square (0.9306) and RMSE (3.0001) as presented in Table 8.
In terms of the Khan isotherm model, the parameter and statistical results were similar for Microsoft Excel and OriginPro, while MATLAB showed differences in all results. It might be that the zero is undefined (It has no meaning) that cannot be proven without math for zero divided by any nonzero number [46]. On the other hand, zero does not have a multiplicative inverse under any circumstances [47] for the MATLAB software tool.
It can be concluded that the six isotherm models showed the confidence interval that was simply the best fit at all experimental data points using the three software tools. The results indicated that the Microsoft Excel Solver function spreadsheet method fulfilled all the statistic measures to predict the real variance of the probability of experimental data for all six isotherm models of adsorption of cationic dye removal. Nevertheless, whether it is a suitable program method for water and wastewater application depends on the user.
Figure 4 and Figure 5 illustrate the discussed isotherms for a visual confirmation of the results. Observation of the graphs confirms that magnetic nanosorbents fit the Langmuir and Toth isotherm models well. Moreover, the results confirm the arguments of the regression results of isotherm parameters and error functions from the Microsoft Excel Solver function with MATLAB and OriginPro programs as shown in Table 8. Considering these good fits to all isotherm models as shown in Figure 4 and Figure 5, it can be concluded that the Microsoft Excel Solver spreadsheet-based program has achieved its place among the tools in the product to express the parameter and statistical results of the isotherm models when providing the mechanism of the adsorption process in water and wastewater treatment or any specific pollutants needed in the treatment plant.
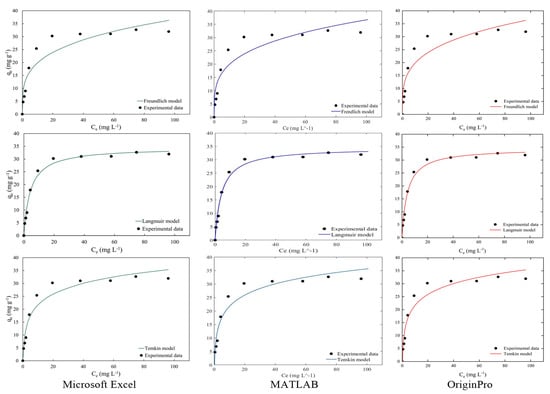
Figure 4.
The comparison of two-parameter isotherm plots from a Microsoft Excel-based program, MATLAB and OriginPro.
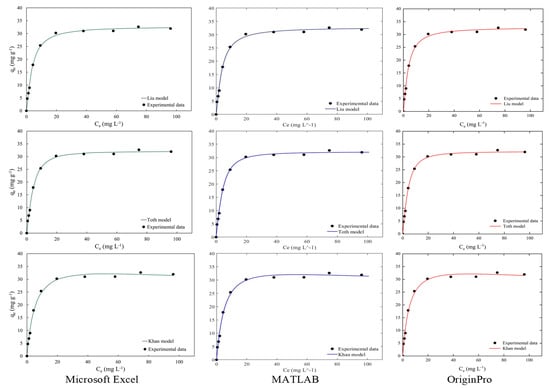
Figure 5.
The comparison of three-parameter isotherm plots from a Microsoft Excel-based program, MATLAB and OriginPro.
A comparison using the Microsoft Excel Solver spreadsheet-based program was also achieved for analyzing nonlinear isotherm models and error functions when designing an adsorption process system, such as adsorption of lead ions on powdered corn cobs [38], adsorption metals using biosorption in water and wastewater [37], adsorption of methylene blue onto rice husk [48] and biocomposite for phosphate ion removal [49].
4. Conclusions
Isotherm adsorption studies were conducted on magnetic nanosorbent, and methylene blue was chosen as a model substrate to evaluate the capacity of the adsorbent obtained, the controlling mechanism of the adsorption process and the relationship between the amounts of methylene blue adsorbed per unit mass. The complexity of nonlinear regression was applied for standardizing the experimental data through the Microsoft Excel program. It can be concluded that the characteristics of the methylene blue adsorbed onto the magnetic nanosorbent forms monolayer and multilayer physical types, which illustrated monolayer coverage (Langmuir model, qm = 34.48 mg g−1, R2 = 0.9897 close to 1) for a two-parameter model, followed by multilayer adsorption (Freundlich and Temkin model). The three-parameter model Toth (qm = 32.13 mg g−1 was close to experimental data and R2 = 0.9965 close to 1) provided a significant goodness of fit and was better than the Liu and Khan models, which indicated the heterogeneous systems had both a low- and high-end boundary of methylene blue concentration adsorbed onto the magnetic nanosorbent. When comparing the software tools, the results indicated that the Microsoft Excel Solver function spreadsheet method satisfied all the statistic and isotherm parameter measures to predict the adsorption of basic dye removal for all six isotherm models. However, it possesses a few negative points that need to be considered before devising the adsorption isotherm model, such as its time consuming nature, the precision of the isotherm model function and the sensible initial parameter of the experimental data set. It could be concluded that the predicted values of the parameter and the statistical analysis for the adsorption isotherm models are reasonable when predicting them for the user who is not an expert in advanced software tools.
Author Contributions
Conceptualization, methodology, validation, investigation and writing, S.W. and K.S.; formal analysis T.K.; resources, J.R., A.W. and C.S.; funding acquisition, S.W. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was financially supported by Mahasarakham University 2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank, acknowledge and express gratitude that this research project was financially supported by Mahasarakham University 2021 in providing an opportunity to pursue this research work. The authors would like to express their special thanks to the University of Southern Queensland for providing the use of experimental equipment, software tools and the opportunity to complete this wonderful research work. This research was also supported by a Unit of Excellent (UOE64001) from the University of Phayao.
Conflicts of Interest
The authors wish to confirm no conflicts of interest regarding the publication of this manuscript. This research did not receive any specific grant financial support from funding agencies in the public, commercial or not-for-profit sectors for this work that could have influenced its outcome.
Nomenclature
| ak | Khan isotherm exponent (dimensionless) |
| bt | Temkin isotherm constant (J mol−1) |
| C0 | Initial concentration of adsorbate (mg L−1) |
| Ce | Concentration of adsorbate at equilibrium (mg L−1) |
| Ke | Thermodynamic equilibrium constant (dimensionless) |
| kf | Freundlich isotherm rate constant (mg g−1) (mg L−1)1/n |
| kg | Liu isotherm rate constant (L mg−1) |
| kk | Khan isotherm rate constant (L mg−1) |
| kl | Langmuir isotherm rate constant (L mg−1) |
| kr | Rate constant of general order model [h−1 (g mg−1)n−1] |
| kt | Temkin isotherm rate constant (L mol−1) |
| kth | Toth isotherm rate constant (L mg−1) |
| n | Order of reaction |
| nf | Heterogeneity factor (dimensionless) |
| ng | Liu isotherm exponent (dimensionless) |
| nth | Toth isotherm exponent (dimensionless) |
| qe | Predicted mass of MB adsorbed at equilibrium (mg g−1) |
| qex | Mass of MB adsorbed at equilibrium (experimental) (mg g−1) |
| qm | Maximum adsorption capacity (mg g−1) |
| R2 | Nonlinear coefficient of determination |
| SSE | Sum of squared error (residuals) |
| χ2 | Chi-square test |
Abbreviation
| ERRSQ/SSE | Residual Sum of Squares Error |
| ARE | Average Relative Error |
| HYBRID | Hybrid Fractional Error Function |
| MPSD | Marquardt’s Percent Standard Deviation |
| RMSE | Root Mean Square Error |
| MB | Methylene blue |
References
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Hou, C.-H.; Hsi, H.-C.; Wu, J.-W. Optimization of highly microporous activated carbon preparation from Moso bamboo using central composite design approach. J. Taiwan Inst. Chem. Eng. 2015, 50, 266–275. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated Carbon Spheres for CO2 Adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Liu, Z.; Huang, Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Rios, R.V.R.A.; Fabris, J.D.; Sapag, K.; Garg, V.K.; Lago, R.M. Clay–iron oxide magnetic composites for the adsorption of contaminants in water. Appl. Clay Sci. 2003, 22, 169–177. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, S.; Liu, S.; Ma, L.P.; Sun, H.; Wang, S. Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Ng, M.; Kho, E.T.; Liu, S.; Lim, M.; Amal, R. Highly adsorptive and regenerative magnetic TiO2 for natural organic matter (NOM) removal in water. Chem. Eng. J. 2014, 246, 196–203. [Google Scholar] [CrossRef]
- Bastami, T.R.; Entezari, M.H. Activated carbon from carrot dross combined with magnetite nanoparticles for the efficient removal of p-nitrophenol from aqueous solution. Chem. Eng. J. 2012, 210, 510–519. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, Z.; Ma, W.; Sun, Q.; Wang, B.; Wang, X. Preparation of magnetic Fe 3 O 4 particles modified sawdust as the adsorbent to remove strontium ions. Chem. Eng. J. 2012, 209, 451–457. [Google Scholar] [CrossRef]
- Wongcharee, S.; Aravinthan, V.; Erdei, L.; Sanongraj, W. Use of macadamia nut shell residues as magnetic nanosorbents. Int. Biodeterior. Biodegrad. 2017, 124, 276–287. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Rida, K.; Bouraoui, S.; Hadnine, S. Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl. Clay Sci. 2013, 83–84, 99–105. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, Kinetics, and Thermodynamics of Methylene Blue Adsorption by Pine Tree Leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Ayawei, N.; Jnr, M.H.; Spiff, I. Rhizophora mangle waste as adsorbent for metal ions removal from aqueous solution. Eur. J. Sci. Res. 2005, 9, 21. [Google Scholar]
- Shooto, N.; Ayawei, N.; Wankasi, D.; Sikhwivhilu, L.; Dikio, E. Study on cobalt metal organic framework material as adsorbent for lead ions removal in aqueous solution. Asian J. Chem. 2016, 28, 277. [Google Scholar] [CrossRef]
- Ahiduzzaman, M.; Islam, A.S. Preparation of porous bio-char and activated carbon from rice husk by leaching ash and chemical activation. SpringerPlus 2016, 5, 1248. [Google Scholar] [CrossRef] [Green Version]
- Wongcharee, S.; Aravinthan, V. Application of mesoporous magnetic nanosorbent developed from macadamia nut shell residues for the removal of recalcitrant melanoidin and its fractions. Sep. Sci. Technol. 2020, 55, 1636–1649. [Google Scholar] [CrossRef]
- Chincholi, M.; Sagwekar, P.; Nagaria, C.; Kulkarni, S.; Dhokpande, S. Removal of dye by adsorption on various adsorbents: A review. Int. J. Sci. Eng. Technol. Res. 2014, 3, 835–840. [Google Scholar]
- Edgar, T.F.; Himmelblau, D.M.; Lasdon, L.S. Optimization of Chemical Processes; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kumar, K.V.; Sivanesan, S. Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: Comparison of linear and non-linear regression methods. J. Hazard. Mater. 2006, 136, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Pal, A. Statistical Analysis of Optimized Isotherm Model for Maxsorb III/Ethanol and Silica Gel/Water Pairs; School of Engineering Sciences, Kyushu University: Fukuoka, Japan, 2018. [Google Scholar]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Kapoor, A.; Yang, R. Correlation of equilibrium adsorption data of condensible vapours on porous adsorbents. Gas Sep. Purif. 1989, 3, 187–192. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Chan, L.; Cheung, W.; Allen, S.; McKay, G. Error analysis of adsorption isotherm models for acid dyes onto bamboo derived activated carbon. Chin. J. Chem. Eng. 2012, 20, 535–542. [Google Scholar] [CrossRef]
- Azqhandi, M.H.A.; Foroughi, M.; Yazdankish, E. A highly effective, recyclable, and novel host-guest nanocomposite for Triclosan removal: A comprehensive modeling and optimization-based adsorption study. J. Colloid Interface Sci. 2019, 551, 195–207. [Google Scholar] [CrossRef]
- Bruce, P.; Bruce, A. Practical Statistics for Data Scientists: 50 Essential Concepts; O’Reilly Media: Newton, MA, USA, 2017. [Google Scholar]
- Shah, K.J.; Gandhi, V. Advances in Wastewater Treatment II; Materials Research Forum LLC: Millersville, PA, USA, 2021. [Google Scholar]
- Wongcharee, S.; Aravinthan, V.; Erdei, L.; Sanongraj, W. Mesoporous activated carbon prepared from macadamia nut shell waste by carbon dioxide activation: Comparative characterisation and study of methylene blue removal from aqueous solution. Asia Pac. J. Chem. Eng. 2018, 13, e2179. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Giles, C.H. The History and Use of the Freundlich Adsorption Isotherm. J. Soc. Dye. Colour. 1973, 89, 287–291. [Google Scholar] [CrossRef]
- Temkin, M. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Khan, A.; Ataullah, R.; Al-Haddad, A. Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures. J. Colloid Interface Sci. 1997, 194, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Tóth, J. A uniform interpretation of gas/solid adsorption. J. Colloid Interface Sci. 1981, 79, 85–95. [Google Scholar] [CrossRef]
- Bergmann, C.P.; Machado, F.M. Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Hossain, M.; Ngo, H.; Guo, W. Introductory of Microsoft Excel SOLVER function-spreadsheet method for isotherm and kinetics modelling of metals biosorption in water and wastewater. J. Water Sustain. 2013, 3, 223–237. [Google Scholar]
- Adekunbi, E.; Babajide, J.; Oloyede, H.; Amoko, J.; Obijole, O.; Oke, I. Evaluation of Microsoft excel solver as a tool for adsorption kinetics determination. IFE J. Sci. 2019, 21, 169–183. [Google Scholar] [CrossRef]
- Srenscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wrόbel, R.J.; Michalkiewicz, B. Comparison of optimized isotherm models and error functions for carbon dioxide adsorption on activated carbon. J. Chem. Eng. Data 2015, 60, 3148–3158. [Google Scholar] [CrossRef]
- Suwannahong, K.; Wongcharee, S.; Kreanuarte, J.; Kreetachart, T. Pre-treatment of acetic acid from food processing wastewater using response surface methodology via Fenton oxidation process for sustainable water reuse. J. Sustain. Dev. Energy Water Environ. Syst. 2020. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.; Palanivelu, K.; Velan, M. Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Ho, Y.; Porter, J.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Liu, Y. Is the free energy change of adsorption correctly calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Aufmann, R.N.; Lockwood, J. Mathematics: Journey from Basic Mathematics through Intermediate Algebra; Cengage Learning: Boston, MA, USA, 2020. [Google Scholar]
- Namakando, C. Zeropsis I; Fultus Corporation: Palo Alto, CA, USA, 2004. [Google Scholar]
- Huang, Y.-T.; Lee, L.-C.; Shih, M.-C.; Huang, W.-T. Introductory of Excel Spreadsheet for comparative analysis of linearized expressions of Langmuir isotherm for methylene blue onto rice husk. Int. J. Sci. Res. Publ. 2019, 9. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Hayat, J.; Iqbal, M.; Noreen, S.; Nawaz, S. Biocomposite application for the phosphate ions removal in aqueous medium. J. Mater. Res. Technol. 2018, 7, 300–307. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).