A Review of the Vibration Arthrography Technique Applied to the Knee Diagnostics
Abstract
1. Introduction
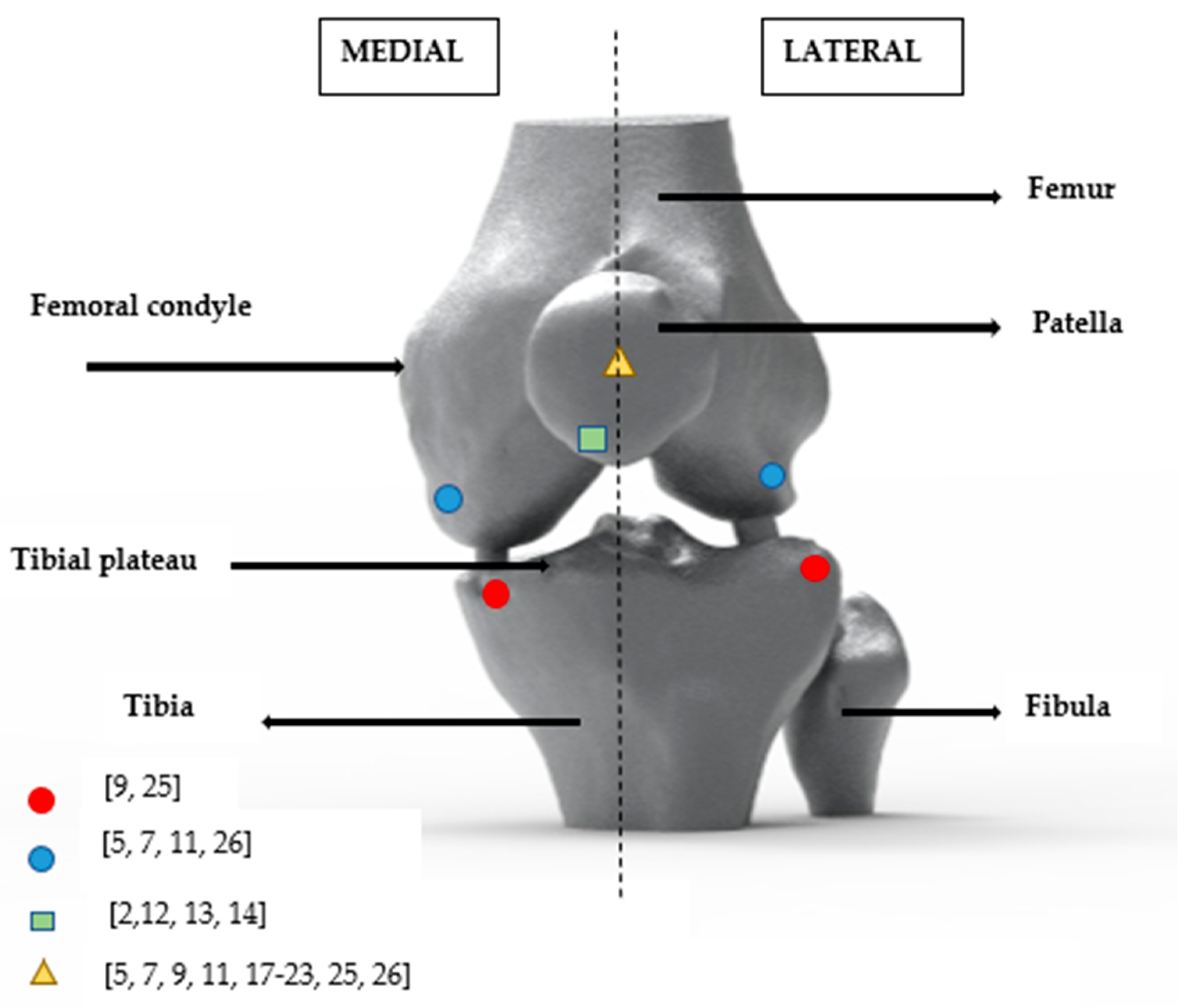
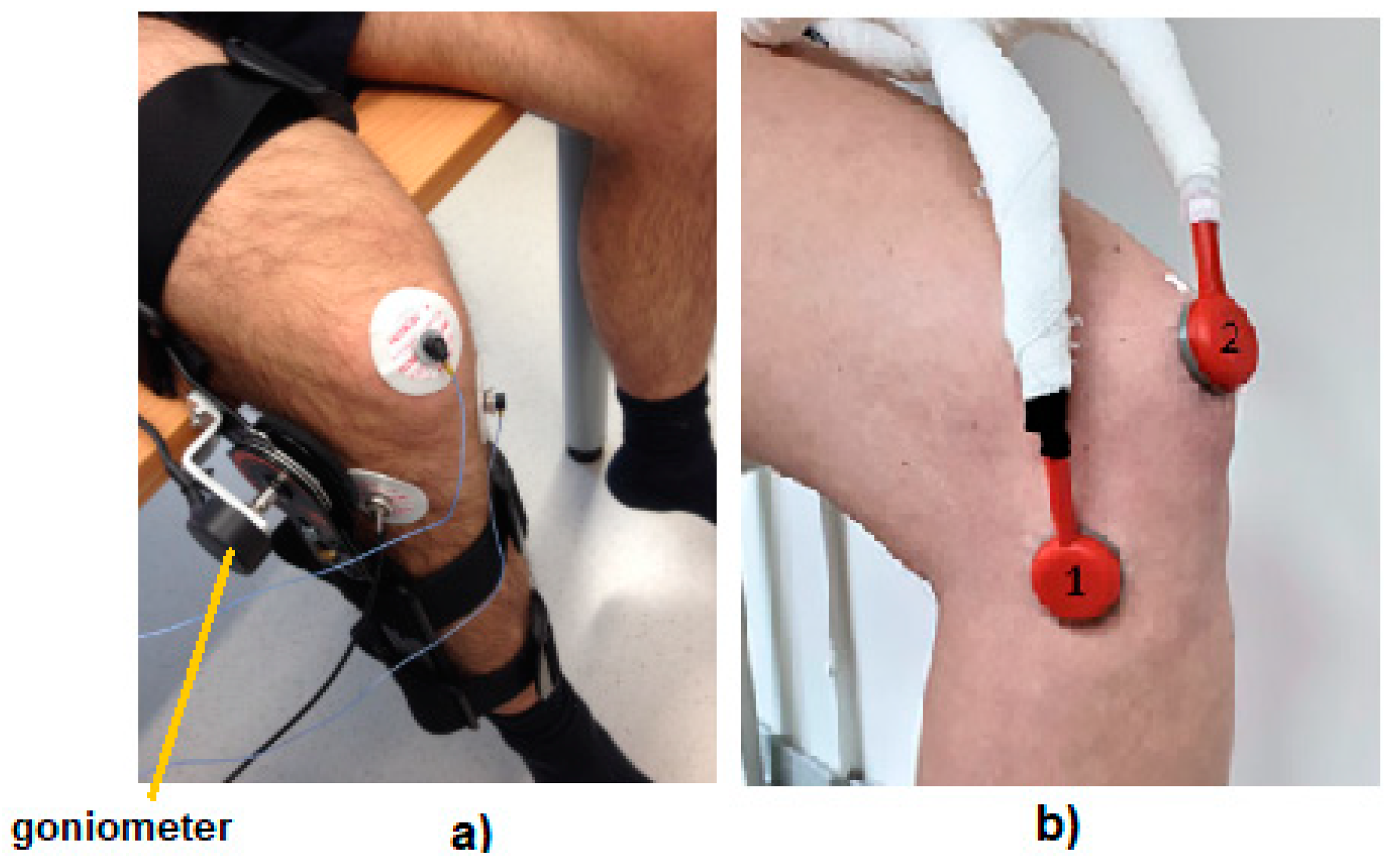
2. Experimental Methods
3. Signal Analysis
3.1. Signal Preprocessing
3.2. Signal Processing
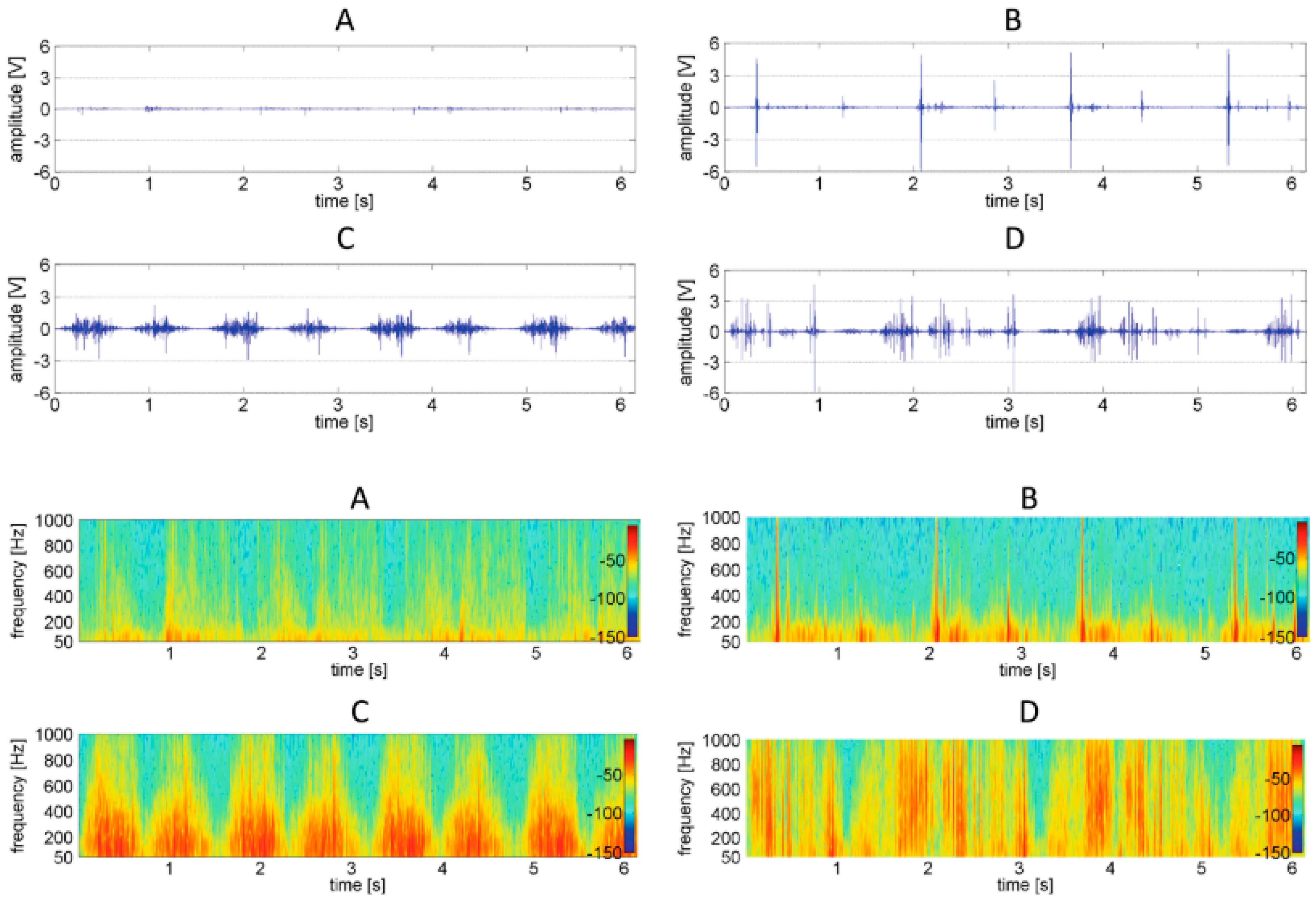
3.2.1. Time Domain Analysis
3.2.2. Frequency Domain Analysis
3.2.3. Time-Frequency Domain Analysis
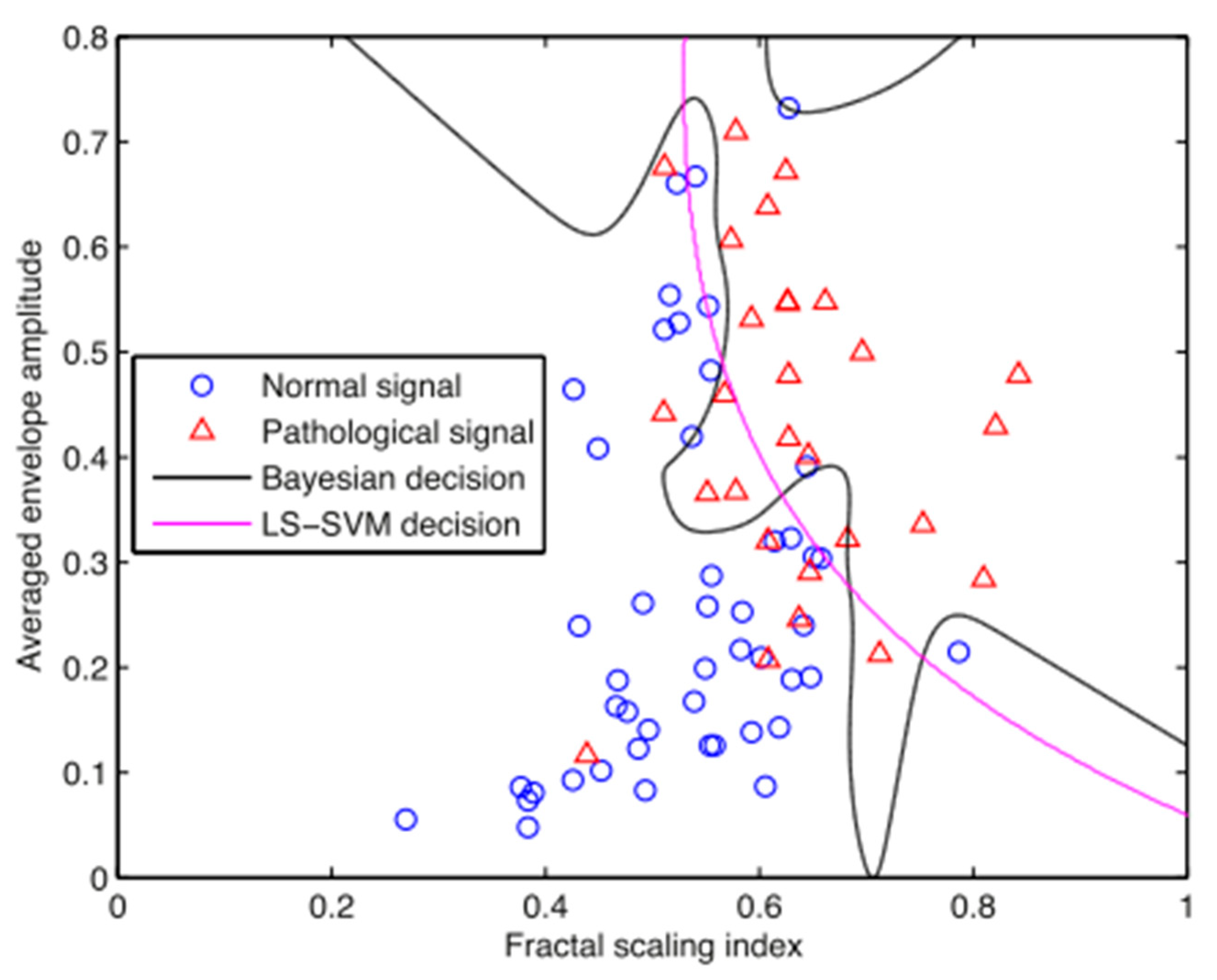
3.3. Classification
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marieb, E.; Hoehn, K. Anatomie et Physiologie Humaines; Pearson Education: Paris, France, 2005. [Google Scholar]
- Moreira, D.B.F. Classification of Knee Arthropathy with Accelerometer-Based Vibroarthrography. Master Thesis, Uni Porto, Porto, Portugal, 2015. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Wu, Y. Knee Joint Vibroarthrographic Signal Processing and Analysis; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lee, M.; et Chow, K. Ultrasound of the Knee. Semin. Musculoskelet. Radiol. 2007, 11, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Naredo, E.; Acebes, C.; Moller, I.; Canillas, F.; de Agustin, J.J.; de Miguel, E.; Filippucci, E.; Iagnocco, A.; Moragues, C.; Tuneu, R.; et al. Ultrasound Validity in the Measurement of Knee Cartilage Thickness. Ann. Rheum. Dis. 2009, 68, 1322–1327. [Google Scholar] [CrossRef]
- McCoy, G.F.; McCrea, J.D.; Beverland, D.E.; Kernohan, W.G.; Mollan, R.A. Vibration Arthrography as a Diagnostic Aid in Diseases of the Knee. A Preliminary Report. J. Bone Jt. Surg. 1987, 69B, 288–293. [Google Scholar] [CrossRef]
- Walters, C.F. The value of joint auscultation. Clin. Lab. Notes. Lancet 1929, 1, 920–921. [Google Scholar] [CrossRef]
- Kernohan, W.G.; Beverland, D.E.; McCoy, G.F.; Hamilton, A.; Watson, P.; Mollan, R. Vibration Arthrometry. Acta Orthop. Scand. 1990, 61, 70–79. [Google Scholar] [CrossRef]
- Hersek, S.; Baran Pouyan, M.; Teague, C.N.; Sawka, M.N.; Millard-Stafford, M.L.; Kogler, G.F.; Wolkoff, P.; Inan, O.T. Acoustical Emission Analysis by Unsupervised Graph Mining: A Novel Biomarker of Knee Health Status. IEEE Trans. Biomed. Eng. 2018, 65, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Kalo, K.; Niederer, D.; Sus, R.; Sohrabi, K.; Groß, V.; Vogt, L. Reliability of Vibroarthrography to Assess Knee Joint Sounds in Motion. Sensors 2020, 20, 1998. [Google Scholar] [CrossRef]
- Teague, C.N.; Hersek, S.; Toreyin, H.; Millard-Stafford, M.L.; Jones, M.L.; Kogler, G.F.; Sawka, M.N.; Inan, O.T. Novel Methods for Sensing Acoustical Emissions from the Knee for Wearable Joint Health Assessment. IEEE Trans. Biomed. Eng. 2016, 63, 1581–1590. [Google Scholar] [CrossRef]
- Madeleine, P.; Andersen, R.E.; Larsen, J.B.; Arendt-Nielsen, L.; Samani, A. Wireless Multichannel Vibroarthrographic Recordings for the Assessment of Knee Osteoarthritis during Three Activities of Daily Living. Clin. Biomech. 2020, 72, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, B.; Prior, J.; Shark, L.-K.; Selfe, J.; Cole, P.; Goodacre, J. Exploratory Study of a Non-Invasive Method Based on Acoustic Emission for Assessing the Dynamic Integrity of Knee Joints. Med. Eng. Phys. 2009, 31, 1013–1022. [Google Scholar] [CrossRef]
- Noor, M.A. Development of a Non-Invasive Bio-Acoustics Measurement System for Assessing Articular Cartilage Knee Joint Problem. J. Teknol. 2014. [Google Scholar] [CrossRef][Green Version]
- Kim, K.S.; Seo, J.H.; Kang, J.U.; Song, C.G. An Enhanced Algorithm for Knee Joint Sound Classification Using Feature Extraction Based on Time-Frequency Analysis. Comput. Methods Progr. Biomed. 2009, 94, 198–206. [Google Scholar] [CrossRef]
- Bączkowicz, D.; Majorczyk, E. Joint Motion Quality in Vibroacoustic Signal Analysis for Patients with Patellofemoral Joint Disorders. BMC Musculoskelet. Disord. 2014, 15, 426. [Google Scholar] [CrossRef]
- Barr, D.A.; Kernohan, W.G.; Mollan, R.A.B. An Investigation of the Short-Term Effect of Exercise on the Knee Joint by Means of Computer-Assisted Auscultation. Adv. Eng. Softw. 1994, 21, 27–35. [Google Scholar] [CrossRef]
- Reddy, N.P.; Rothschild, B.M.; Verrall, E.; Joshi, A. Noninvasive Measurement of Acceleration at the Knee Joint in Patients with Rheumatoid Arthritis and Spondyloarthropathy of the Knee. Ann. Biomed. Eng. 2001, 29, 1106–1111. [Google Scholar] [CrossRef]
- Cai, S.; Wu, Y.; Xiang, N.; Zhong, Z.; He, J.; Shi, L.; Xu, F. Detrending Knee Joint Vibration Signals with a Cascade Moving Average Filter. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: San Diego, CA, USA, 2012; pp. 4357–4360. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, S.; Zheng, F.; Cai, S.; Lu, M.; Wu, M. Removal of Artifacts in Knee Joint Vibroarthrographic Signals Using Ensemble Empirical Mode Decomposition and Detrended Fluctuation Analysis. Physiol. Meas. 2014, 35, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Rangayyan, R.M.; Wu, Y.F. Screening of Knee-Joint Vibroarthrographic Signals Using Statistical Parameters and Radial Basis Functions. Med. Biol. Eng. Comput. 2008, 46, 223–232. [Google Scholar] [CrossRef]
- Rangayyan, R.M.; Wu, Y. Analysis of Vibroarthrographic Signals with Features Related to Signal Variability and Radial-Basis Functions. Ann. Biomed. Eng. 2009, 37, 156–163. [Google Scholar] [CrossRef]
- Cai, S.; Yang, S.; Zheng, F.; Lu, M.; Wu, Y.; Krishnan, S. Knee Joint Vibration Signal Analysis with Matching Pursuit Decomposition and Dynamic Weighted Classifier Fusion. Comput. Math. Methods Med. 2013, 2013, 904267. [Google Scholar] [CrossRef]
- Rangayyan, R.M.; Oloumi, F.; Wu, Y.; Cai, S. Fractal Analysis of Knee-Joint Vibroarthrographic Signals via Power Spectral Analysis. Biomed. Signal Process. Control 2013, 8, 23–29. [Google Scholar] [CrossRef]
- Krishnan, S.; Rangayyan, R.M.; Bell, G.D.; Frank, C.B.; Ladly, K.O. Adaptive Filtering, Modelling and Classification of Knee Joint Vibroarthrographic Signals for Non-Invasive Diagnosis of Articular Cartilage Pathology. Med. Biol. Eng. Comput. 1997, 35, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Befrui, N.; Elsner, J.; Flesser, A.; Huvanandana, J.; Jarrousse, O.; Le, T.N.; Müller, M.; Schulze, W.H.W.; Taing, S.; Weidert, S. Vibroarthrography for Early Detection of Knee Osteoarthritis Using Normalized Frequency Features. Med. Biol. Eng. Comput. 2018, 56, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Bolus, N.B.; Jeong, H.K.; Whittingslow, D.C.; Inan, O.T. A Glove-Based Form Factor for Collecting Joint Acoustic Emissions: Design and Validation. Sensors 2019, 19, 2683. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Bolus, N.B.; Erturk, A.; Inan, O.T. Vibration Characterization of the Human Knee Joint in Audible Frequencies. Sensors 2020, 20, 4138. [Google Scholar] [CrossRef] [PubMed]
- Olowiana, E.; Selkow, N.; Laudner, K.; Puciato, D.; Baczkowitz, D. Vibroarthograpic analysis of patellofemoral joint arthrokinematics during squats with increasing external loads. BMC Sports Sci. Med. Rehabil. 2020, 12, 51. [Google Scholar] [CrossRef]
- Kalo, K.; Niederer, D.; Stief, F.; Wurzberger, L.; Drongelen, S.; Meurer, A.; Vogt, L. Validity of and recommendations for knee joint acoustic assessments during different movement conditions. J. Biomech. 2020, 109, 109939. [Google Scholar] [CrossRef]
- Duhamel, P.; Vetterli, M. Fast Fourier transform: A tutorial review and state of the art. Signal Process. 1990, 19, 259–299. [Google Scholar] [CrossRef]
- Rhif, M.; Abbes, A.B.; Farh, R.F.; Martinez, B.; Sang, Y. Wavelet transform application for/in non-stationary time-series analysis: A review. Appl. Sci. 2019, 9, 1345. [Google Scholar] [CrossRef]
- Tuan, C.-C.; Lu, C.-H.; Wu, Y.-C.; Chen, M.-C.; Chi, S.-W.; Lee, T.-F.; Yeh, W.-L. Developmental Screening System for Patient Vibration Signals with Knee Disorder. Appl. Sci. 2019, 9, 908. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lee, T.-F.; Lin, S.-Y.; Wu, L.-F.; Wang, H.-Y.; Chang, L.; Wu, J.-M.; Jiang, J.-C.; Tuan, C.-C.; Hong, M.-F.; et al. Non-Invasive Knee Osteoarthritis Diagnosis via Vibroarthrographic Signal Analysis. J. Inf. Hiding Multimed. Signal Process. 2014, 5, 497–507. [Google Scholar]
- Yang, S.; Cai, S.; Zheng, F.; Wu, Y.; Liu, K.; Wu, M.; Zou, Q.; Chen, J. Representation of Fluctuation Features in Pathological Knee Joint Vibroarthrographic Signals Using Kernel Density Modeling Method. Med. Eng. Phys. 2014, 36, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, K.; Li, H.; Wang, C. Modelling and Analysis on Biomechanical Dynamic Characteristics of Knee Flexion Movement under Squatting. Sci. World J. 2014, 2014, 321080. [Google Scholar] [CrossRef]
- Erdemir, A. Open Knee: Open Source Modeling and Simulation in Knee Biomechanics. J. Knee Surg. 2016, 29, 107–116. [Google Scholar] [CrossRef]
- Madeti, B.K.; Rao, C.S. Biomechanics of knee joint–A review. Front. Mech. Eng. 2015, 10, 176–186. [Google Scholar] [CrossRef]
- Beverland, D.E.; Kernohan, G.; McCoy, G.F.; Mollan, R.A.B. What Is Physiological Patellofemoral Crepitus? Med. Biol. Eng. Comput. 1985, 23, 1249–1250. [Google Scholar]



| Technique | Advantages | Limitations |
|---|---|---|
| X-Ray imaging | Inexpensive Fast | Indirect measurements because structures such as cartilage do not appear |
| Arthroscopy | Gold standard Low-risk assessment | Non-suitable for repeated assessment: invasive and requires anaesthesia (incisions made to the knee) |
| Ultrasound | Real-time imaging Low cost soft tissues and structures detected | Limited by the sound’s properties: ultrasounds cannot penetrate bones, some parts of the knee are hidden from sight |
| MRI | 3D imaging, accurate quantitative measurements of articular cartilage morphology Enables the detection of cartilage, menisci, ligaments, etc. | Expensive Complex Long acquisition times |
| Authors of Research Study | Population | Processing Technique: Extracted Features | Aim of the Study and Results |
|---|---|---|---|
| Reddy et al. (2001) [19] | 11 spondyloarthropathy—11 rheumatoid arthritic knees | FD analysis: (Discrete Fourier Transform): Mean power of the power spectrum in the frequency range of 100–500 Hz | Differentiate spondyloarthropathy and rheumatoid arthritic signals |
| Rangayyan et al. (2008) [22] | 51 H—38 with knee joint pathology | TD analysis: FF (extension/flexion), S, K, E | Classification of normal and abnormal knees |
| Rangayyan et al. (2009) [23] | 51 H—38 with knee joint pathology | TD analysis: TC, VMS | Classification of normal and abnormal knees |
| Mascaro et al. (2009) [14] | 11 H—10 OA | TD analysis (segmentation): amplitude, duration FD analysis (Fourier Transform): peak frequency | Provide a visual tool for differentiate healthy and OA knees |
| Kim et al. (2009) [16] | 20 H—11 OA | TFD analysis: EP, ESP, FP, FSP | Classification of normal and OA knees (accuracy of 91.4%) |
| Baczkowicz (2014) [17] | 64 H—86 knees with disorders | TD analysis: VMS and amplitude TFD analysis (STFT), partial sum of the power spectrum | Compare the impact of chondromalacia, lateral patellar compression syndrome and OA on knee joint sounds |
| Moreira (2015) [2] | 19 H—20 OA | TD analysis (segmentation): S, K, E, TC TFD analysis (Wavelet Transform) | Classification of normal and OA knees using a k-NN classifier (accuracy of 89%) |
| Befrui et al. (2018) [27] | 30 H—39 OA | TD analysis: segmentation Frequency domain: partial sum of the power spectrum | Classification of normal and OA knees using an SVM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Tocqueville, S.; Marjin, M.; Ruzek, M. A Review of the Vibration Arthrography Technique Applied to the Knee Diagnostics. Appl. Sci. 2021, 11, 7337. https://doi.org/10.3390/app11167337
de Tocqueville S, Marjin M, Ruzek M. A Review of the Vibration Arthrography Technique Applied to the Knee Diagnostics. Applied Sciences. 2021; 11(16):7337. https://doi.org/10.3390/app11167337
Chicago/Turabian Stylede Tocqueville, Sophie, Mihaela Marjin, and Michal Ruzek. 2021. "A Review of the Vibration Arthrography Technique Applied to the Knee Diagnostics" Applied Sciences 11, no. 16: 7337. https://doi.org/10.3390/app11167337
APA Stylede Tocqueville, S., Marjin, M., & Ruzek, M. (2021). A Review of the Vibration Arthrography Technique Applied to the Knee Diagnostics. Applied Sciences, 11(16), 7337. https://doi.org/10.3390/app11167337





