Abstract
Dysphagia is associated with poor quality of life, and pneumonia due to aspiration is life-threatening. Cervical ossification of the anterior longitudinal ligament (C-OALL) is one of the causes of dysphagia, and we report two cases in which dysphagia improved after surgery. Case 1: A 76-year-old man had C-OALL of greater than 16 mm and dysphagia and developed myelopathy. A fall resulted in upper and lower limb insufficiency paralysis, and posterior decompression fixation was performed. Pressure on the pharynx by C-OALL remained, but dysphagia improved. Improvement in this case was considered to be due to the loss of intervertebral mobility. Case 2: A 62-year-old man developed dysphagia 6 years ago. It gradually exacerbated, and the C-OALL increased. Laryngeal fiberscope and swallowing angiography revealed that the pharyngeal cavity was compressed and narrowed anteriorly due to ossification. Resection of the ossification was performed, and the patient’s symptoms improved. Direct decompression was successful in this case. Several evaluation methods for dysphagia have been reported, including screening tests, endoscopy, contrast studies, and radiological evaluation. In case 1, extensive ossification was improved by posterior fixation, albeit incidentally, whereas in case 2, a patient with extensive ossification exhibited symptoms. It is necessary to examine the cervical mobility, extent and morphology of ossification, and timing of surgery stenosis to determine the risk factors and treatment options, including rehabilitation.
1. Introduction
Dysphagia makes eating difficult. The quality of life deteriorates, and pneumonia due to aspiration is life-threatening [1]. Diffuse idiopathic skeletal hyperostosis (DISH) [2,3] and cervical ossification of the anterior longitudinal ligament (C-OALL), one class of DISH, are causes of dysphagia. DISH lesions present in approximately 9.1–16.9% of patients over 60 years of age; dysphagia is observed in 0.1–6% of DISH patients [4,5]. The incidence of dysphagia among patients diagnosed with C-OALL is not low, with Resnick reporting it to be 17–28% [2]. Dysphagia due to C-OALL is caused by pressure on the hypopharyngeal esophagus due to increased ossification and edema of the larynx and esophagus caused by inflammation around the ossification foci and degeneration of the nerves [6,7]. Conservative treatment is usually selected [8,9], but surgery may be performed if there is no improvement. Usually, resection of C-OALL is selected [10,11,12], but it is not clear whether resection of the ossification foci alone can improve swallowing, as the thickness of the ossification or timing of surgery does not necessarily correlate with dysphagia [13]. In this study, we experienced two cases in which the dysphagia improved with a different technique despite similar swallowing evaluations, including X-rays. In case 1, different from previous reports, posterior fixation incidentally improved dysphagia without anterior resection, and in case 2, dysphagia improved after ossification resection. The difference between the two cases is the presence of intervertebral mobility and trauma. There is still a controversial research area reporting intervertebral mobility and timing of surgery, and we report these cases. Informed consent for this case report was obtained from the patients through scanned in their medical records.
2. Material and Methods
2.1. Radiological Evaluations
We measured radiological parameters on the lateral cervical radiograph as follows: the thickness of C-OALL, the occipito-C2 angle (O-C2A: angle between McGregor’s line and lower endplate of C2) [14,15], and the pharyngeal inlet angle (PIA) between McGregor’s line and the line that links the center of the C1 anterior arch and the apex of the cervical sagittal curvature [16]. A significant reduction in O-C2A was correlated with a decrease in oropharyngeal space and postoperative dysphagia [14,15]. Keneyama et al. considered that the dysphagia should be predicted at the condition of PIA < 90°, where the apex of the midcervical sagittal curvature protruded anterior to the swallow line (S-line): S-line (−). However, no patients experienced dysphagia as long as PIA was 90°, where the apex of the midcervical curvature stayed posterior to the S-line: S-line (+) [16] (Figure 1).

Figure 1.
(a) Occipito-C2 angle (O-C2A: angle between McGregor’s line and lower endplate of C2). (b) Pharyngeal inlet angle (PIA) is defined as the angle between McGregor’s line and the line that links the center of the C1 anterior arch and the apex of the cervical sagittal curvature. The swallow line (S-line) (−) is PIA < 90°. (c) The swallow line (S-line) (+) is PIA > 90°.
2.2. Screening Test
We used the 30 mL water swallowing test [17]. Participants sat in upright posture and drank 30 mL of room temperature water from a cup. According to the time spent to drink water and the presence or absence of coughing, the results included the following five levels:
Level I, drinking water successfully without interruption or coughing;
Level II, drinking water with interruption two times and with coughing;
Level III, drinking water without interruption but with coughing;
Level IV, drinking water with interruption over two times and with coughing;
Level V, coughing frequently and cannot drink the water successfully.
2.3. Practical Assessment Tool
We used the Food Intake LEVEL Scale [18], a 10-point observer-rating scale, to measure the severity of dysphagia. Dysphagia outcome and severity scale [19,20], a simple, easy-to-use, 7-point scale developed to systematically rate the functional severity of dysphasia based on objective assessment and make recommendations for diet level, independence level, and type of nutrition.
2.4. Clinical Otorhinolaryngology Assessment
We asked the otorhinolaryngology physician to perform video endoscopy (VE) and a swallowing contrast study (VF; videofluorography).
2.5. Clinical Presentations
Case 1: A 76-year-old man presented to otorhinolaryngology and gastroenterology departments 3 years ago because he had little difficulty in swallowing food. Endoscopy demonstrated compression of the dorsal side of the pharynx and esophagus. The patient was seen by an orthopedic surgeon, who diagnosed C-OALL and performed follow-up. Gradually, numbness and dyslexia in the upper limbs developed, and dysphagia was felt to have been exacerbated. The patient’s ability to walk was preserved. The patient was treated conservatively, but he fell and hit his head at home after drinking. He became paraplegic and was hospitalized for surgery due to the diagnosis of cervical spinal cord injury without bone injury.
Assessment on admission: hypovolemia and leakage. A urethral balloon was placed. Sensory disturbance: both shoulders, inguinal and subparesthesia, tendon reflexes; decreased biceps tendon reflexes, bilateral enhancement below the triceps tendon, Hoffmann’s reflex; −/+ Babinski, foot clonus −/−, manual muscle test of upper and lower extremities; deltoid 4/4, biceps 4/4, triceps 5/5, wrist dorsiflexion 3/3, palmar flexion 3/3, finger extension 3/3, lower limbs all 4/4.
A physical therapist (PT), occupational therapist (OT), and speech language hearing therapist (ST) intervened from the day after admission. The patient wore a cervical collar and was not restricted to bed rest. He could not hold a spoon but could eat regular meals with assistance after approximately 1 h of noting the difficulty in swallowing. The oral function was expected, and the jaw opened to a width of 3 fingers. There was slight tooth loss. Tongue movement did not differ between the left and right sides, and coughing was possible. On the 30 mL water swallowing test, 5 and 10 mL were ingested from a syringe, and swallowing was possible without swelling or hoarseness (LEVEL II). For safety reasons, his postoperative soup and water were thickened. Moreover, VE was conducted, but VF was not conducted for bed rest to spinal cord injury (Figure 2). His swallowing function was as follows: Food Intake LEVEL Scale: 8, and dysphagia severity scale: 6.
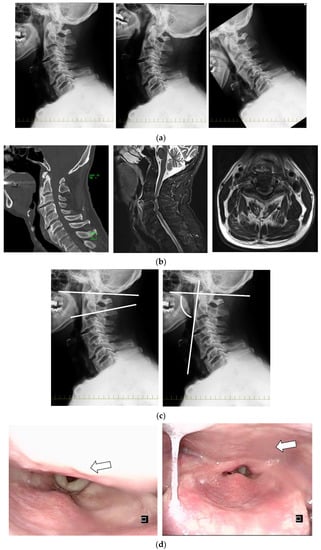
Figure 2.
Case 1. (a) Functional cervical spine X-ray (figures from left to right show middle, extension, and flexion). (b) CT showed no fracture, and MRI showed compression of the cervical spinal cord due to thickening of the ligamentum flavum and suspected damage to the intervertebral disc and anterior longitudinal ligament. MRI showed stenosis at the level of the pharynx due to 16.3 mm OALL at C3/4 and spinal cord compression. (c) Figures from left to right show an O-C2 angle of 25° and a pharyngeal inlet angle (PIA) of 76°, and the S-line was negative. (d) The patient was immobilized in the middle position, and video endoscopy (VE) revealed protrusion of the pharynx due to ossification (arrow), but swallowing was normal.
Radiological evaluation: the ossification size of C-OALL at C3/4 was 16.8 mm. Intervertebral mobility was noted at C3/4 and 5/6. C3/4 had 5° of intervertebral mobility with 4° of extension in retroflexion and −1° in flexion. The O-C2 angle was 25°. The pharyngeal inlet angle (PIA) was 76°. The S-line was negative. The C2-7 angle was 16° (Figure 2).
Posthospitalization day 4: Posterior decompression fixation of the 3rd and 6th cervical vertebrae was performed. Intraoperatively, the patient was immobilized in the intermediate position using a Mayfield holder. The postoperative ossification size was unchanged; the C3/4 intervertebral angle was fixed at 4° lordosis; the O-C2 angle, PIA angle, and S-line were negative; and the C2-7 angle was unchanged (Figure 3).
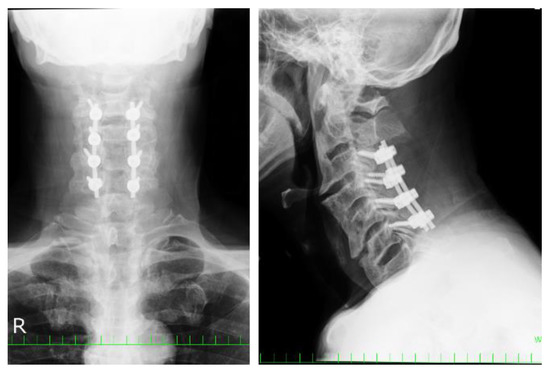
Figure 3.
Posterior decompression fixation of the 3rd–6th cervical vertebrae was performed in case 1.
Postoperative day 1: resumption of eating; postoperative day 2: getting out of bed. On postoperative day 4, the patient was able to eat staple food, whole porridge, and side dishes in a sitting position, and he was able to eat on his own using a spoon. There was no subjective difficulty in swallowing when meat was previously served with soft greens in bite-size pieces. The 30 mL water swallowing test was improved LEVEL I. His swallowing function was as follows: Food Intake LEVEL Scale: 9, and dysphagia severity scale: 6. Three weeks postoperatively, he was able to walk with a Lofstrand crutch cane and ascend and descend stairs with light assistance, although he was unsteady on his feet. He was transferred to another hospital for 3 weeks and 5 days postoperatively.
Case 2: A 62-year-old man noted a slight difficulty in swallowing 6 years ago. He was referred to the otorhinolaryngology department. He had no difficulty in passing food but had findings of pharyngeal compression. He was seen by an orthopedic surgeon, who noted C-OALL; he had difficulty eating solid foods and decreased appetite since 1 year ago. X-ray findings revealed increased ossification and were referred to us.
Evaluation on admission: there were no symptoms of nerve disturbance. There were no complaints other than dysphagia. The oral function was expected, and the jaw opened to a width of 3 fingers. The tooth was intact. Tongue movement did not differ between the left and right sides, and coughing was possible. On the 30 mL water swallowing test, he was drinking water successfully without interruption or coughing (LEVEL I). His swallowing function was as follows: Food Intake LEVEL Scale: 8, and dysphagia severity scale: 6.
Radiological evaluation: the extent of ossification increased from 6.1 mm (Figure 4a) to 11.6 mm over 6 years at C3/4, and the shape was globular type. Intervertebral mobility was noted at C3/4, with a total of 3° of intervertebral mobility in C3/4 with 2° of kyphosis in retroflection and −1° of forwarding flexion. The O-C2 angle was 30°, the PIA angle was 80°, the S-line was negative, and the C2-7 angle was 12° (Figure 4b). Otorhinolaryngological findings: VE and VF demonstrated that the pharyngeal cavity was compressed and narrowed anteriorly due to ossification, which was further exacerbated by flexion of the cervical spine (Figure 4d).
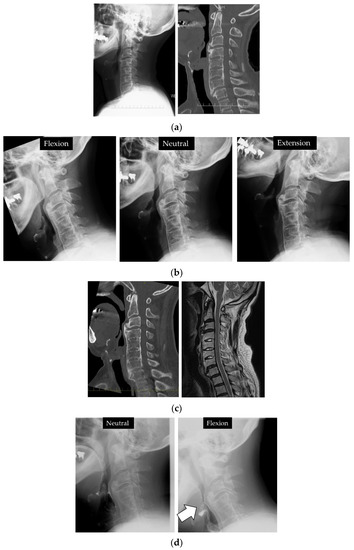
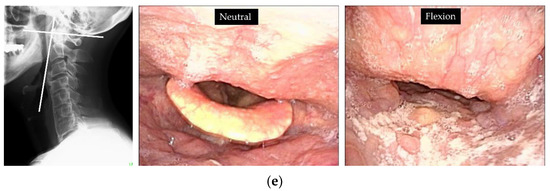
Figure 4.
Case 2. C3/4 OALL increased from 6.8 mm (a) to 11.6 mm (b) since the first X-ray and CT 6 years ago. (c) No evidence of spinal cord compression on MRI. (d) VF showed narrowing of the pharyngeal cavity in the area of ossification, which was further exacerbated by flexion (arrow). (e) The S-line was negative. VE: the pharyngeal cavity was compressed and narrowed anteriorly due to ossification, which was further exacerbated by flexion.
Posthospitalization course: C3/4 OALL was resected under general anesthesia. The ossification below C4 persisted and limited cervical mobility, but no dissection was performed without evidence of pharyngeal compression. Postoperatively, C3/4 OALL improved to 1 mm (Figure 5).
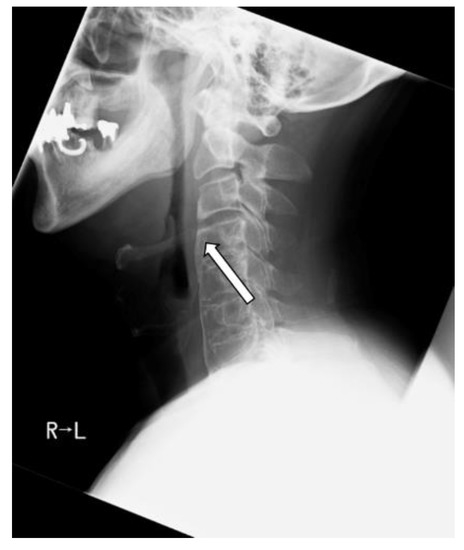
Figure 5.
Postoperatively, the ossification was reduced by resection (arrow).
The patient was able to get out of bed the day after surgery, and the ST intervened. The patient had an inducible swallowing reflex, and an ice massage was performed with no swelling or wet hoarseness. Postoperative day 3: he was able to eat 80% (compared with preoperative days) of main meals and side dishes in 15 min. He continued oral intake after that. On the 5th postoperative day, he was able to eat regular food without discomfort in the pharynx. He was discharged on the 10th postoperative day. Food-type-based dysphagia disappeared after 1 month postoperatively. The 30 mL water swallowing test was LEVEL I. His swallowing function was as follows: Food Intake LEVEL Scale: 10, and dysphagia severity scale: 7.
3. Discussion
Dysphagia is a significant risk factor for the development of choking and aspiration pneumonia [1]. The pathogenesis of dysphagia in C-OALL includes physical compression of the swallowing organs, inflammation of the larynx and esophagus, edema and degeneration, restriction of movement due to friction between the ossification and the cricothyroid cartilage, obstruction of the collapsing epiglottis due to ossification, degeneration of the surrounding nerves, and swelling of the posterior pharyngeal wall [21,22].
Dysphagia is diagnosed by questionnaires, such as the Eating Assessment Tool [23], screening tests, the 30 mL water swallowing test [17], clinical assessment by VE, and VF. In case 1, the patient was evaluated using the 30 mL water drinking test and VE only due to cervical spinal cord injury. Pressure drainage of the pharynx was noted. In case 2, the 30 mL water swallow test results were expected, but he was diagnosed with compression and dysphagia by both VE and VF.
In the report on the relationship between dysphagia and the thickness of C-OALL, the average thickness was more significant than 10.0–13.5 mm, and the morphology was often globular type [24,25]. On the other hand, there did not appear to be a direct correlation between the size and the severity of dysphagia [26]. The relationship between dysphagia and radiographic alignment in other cervical spine diseases has also been reported to assess dysphagia in OALL [16]. Tian et al. found the O-C2 angle irrelevant in cervical spine surgery because dysphagia in C-OALL occurs below the C2 vertebrae. On the other hand, they reported a 5° decrease in the C2-7 angle associated with the exacerbation of postoperative dysphagia [27]. However, there were patients with a greater extent of C-OALL without dysphagia, and the cutoff of the thickness of OALL and the alignment of the surgical procedure for C-OALL with dysphasia is unclear. The S-line in the two cases was negative, and we may need to consider a new index that takes alignment, thickness or morphology of C-OALL, and intervertebral mobility into account.
Regarding the treatment of C-OALL, there are reports of improvement by conservative treatment [8,9,28]. Aspiration pneumonia, depression due to loss of appetite, and weight loss for dysphasia require surgical treatment, although there have been reports of dysphagia occurring in 5.6%, hoarseness in 5.2%, transient sore throat in 4.8%, worsening of pre-existing myelopathy in 3%, and graft extrusion in 1.7% [29]. On the other hand, successful relief of dysphagia was obtained in 89% of patients after comparatively early surgery. Failure to relieve dysphagia was associated with an increased length of symptoms preoperatively [13]. Lofrese et al. reported that especially in the elderly, timely bone resection appeared crucial, even with mild dysphagia, in the presence of a long-lasting clinical history [10]. As an operation method, anterior cervical osteophytectomy (resection of C-OALL) is highly effective. The most common type of surgery is surgical resection of the ossification layer [10,25,30,31,32]. Reports of additional posterior fixation seem to be limited to cases with Parkinson’s disease and maintaining alignment [33]. Recurrence is a problem after resectioning the ossification layer alone, with recurrence in two out of seven cases reported at 10 years [34]. Lofrese et al. reported that one and two recurrences at clinical and radiological follow-up were registered 18–30 months after surgery in elderly patients and in the short range [10]. Kaur reported that the patient was 63 years old and had a recurrence 2 years after osteophytectomy. On the other hand, some papers showed that dysphagia did not recur for a long time. Hoeh et al. reported that at the final follow-up (23 ± 8 months) for six patients, the radiographic examinations showed no pathological regrowth, and the patients reported no recurrence of dysphagia [30]. Urrutia reported that postoperative radiographs demonstrated complete removal of osteophytes for five patients. At the final follow-up, ranging from 1 to 9 years (average of 59.8 months, median of 53 months), no patients reported a recurrence of dysphagia. The final radiographic examination demonstrated minimal regrowth of the osteophytes [31]. Further investigation is needed to determine what may be a factor in recurrence.
The reasons for the improvement of dysphagia in our cases are as follows: In case 1, although physical pressure on the swallowing organs remained, the reduced mobility of the cervical vertebrae resulted in reduced friction between the ossification and the larynx and pharynx, the obstruction of the epiglottis collapsing over the glottis was eliminated, and the pharynx was no longer in a compressed flexion position on preoperative X-ray. In case 2, the physical pressure was no longer present. The improvement was due to the decompression of the organs involved in the swallowing function. However, in case 1, it was difficult to assess whether posterior fixation improved swallowing by preoperative assessment. Preoperatively, the patient and his family were informed that if dysphagia was exacerbated, resection of the ossification would be performed. In these two cases, both patients underwent surgery relatively early after becoming aware of dysphagia and before it got serious, as well as similar laboratory tests. This may lead to an improved condition about the results. Fixation was performed in case 1, but attention should be paid to whether ossification increases in extent or dysphagia recurs in case 2 due to reossification.
4. Conclusions
We reported two cases of dysphagia due to C-OALL at the C3/4 level with an initial symptom of dysphagia. In case 1, the patient was treated posteriorly due to spinal cord injury, and dysphagia improved with fixation of a mobile responsible lesion despite the extensive ossification. The patient in case 2 improved after resection of the ossification, as previously reported. Although the present study results are short term and need to be carefully evaluated to determine how ossification changes during long-term follow-up, early surgical treatment of possible causative conditions may be effective.
Author Contributions
Conceptualization, N.N., H.O., and K.S.; data curation, T.K., K.Y., and T.S.; methodology, H.S., Y.I., M.F.; project administration, N.N.; resources, N.N.; supervision, T.S.; writing—original draft, N.N.; writing—review and editing, N.N. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Yamaguchi University Graduate School of Medicine.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the paper.
Acknowledgments
Medical English Service (Kyoto, Japan) provided professional English language editing of the manuscript for this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, T.; Zhao, Y.; Guo, A. Association of swallowing problems with frailty in Chinese hospitalized older patients. Int. J. Nurs. Sci. 2020, 7, 408–412. [Google Scholar] [CrossRef]
- Forestier, J.; Lagier, R. Ankylosing hyperostosis of the spine. Clin Orthop. Relat. Res. 1971, 74, 65–83. [Google Scholar] [CrossRef]
- Resnick, D.; Niwayama, G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976, 119, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Sarzi-Puttini, P.; Atzeni, F.; Olivieri, I.; Pappone, N.; Verlaan, J.J.; Buskila, D. Extraspinal manifestations of diffuse idiopathic skeletal hyperostosis. Rheumatology 2009, 48, 1478–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmucha, S.T.; Cravens, R.B., Jr. DISH syndrome and its role in dysphagia. Otolaryngol. Head Neck Surg. 1994, 110, 431–436. [Google Scholar] [CrossRef]
- Presutti, L.; Alicandri-Ciufelli, M.; Piccinini, A.; Trebbi, M.; Marchioni, D.; Ghidini, A.; Ruberto, M. Forestier disease: Single-center surgical experience and brief literature review. Ann. Otol. Rhinol. Laryngol. 2010, 119, 602–608. [Google Scholar] [CrossRef]
- Carlson, M.L.; Archibald, D.J.; Graner, D.E.; Kasperbauer, J.L. Surgical management of dysphagia and airway obstruction in patients with prominent ventral cervical osteophytes. Dysphagia 2011, 26, 34–40. [Google Scholar] [CrossRef]
- Eviatar, E.; Harell, M. Diffuse idiopathic skeletal hyperostosis with dysphagia (a review). J. Laryngol. Otol. 1987, 101, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.P.; van Royen, B.J.; David, E.F.; Mahieu, H.F. Anterior cervical osteophytes resulting in severe dysphagia and aspiration: Two case reports and literature review. J. Laryngol. Otol. 2009, 123, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Lofrese, G.; Scerrati, A.; Balsano, M.; Bassani, R.; Cappuccio, M.; Cavallo, M.A.; Cofano, F.; Cultrera, F.; De Iure, F.; Biase, F.D.; et al. Surgical Treatment of Diffuse Idiopathic Skeletal Hyperostosis (DISH) Involving the Cervical Spine: Technical Nuances and Outcome of a Multicenter Experience. Global Spine J. 2021, 2192568220988272. [Google Scholar] [CrossRef]
- Park, B.J.; Gold, C.J.; Piscopo, A.; Schwickerath, L.; Bathla, G.; Chieng, L.O.; Yamaguchi, S.; Hitchon, P.W. Outcomes and complications of surgical treatment of anterior osteophytes causing dysphagia: Single center experience. Clin. Neurol. Neurosurg. 2021, 207, 106814. [Google Scholar] [CrossRef]
- Cherfane, P.; Smaily, H.; Khalaf, M.G.; Ghaoui, N.; Melkane, A.E. Otolaryngologic manifestations of diffuse idiopathic skeletal hyperostosis (Forestier’s disease): A systematic review of the literature. Joint Bone Spine 2021, 88, 105218. [Google Scholar] [CrossRef]
- Gendreau, J.L.; Sheaffer, K.; Bennett, J.; Abraham, M.; Patel, N.V.; Herschman, Y.; Ruh, N.; Lindley, J.G. Timing of Surgical Intervention for Dysphagia in Patients With Diffuse Idiopathic Skeletal Hyperostosis: A Systematic Review and Meta-Analysis. Clin. Spine Surg. 2021, 34, 220–227. [Google Scholar] [CrossRef]
- Miyata, M.; Neo, M.; Fujibayashi, S.; Ito, H.; Takemoto, M.; Nakamura, T. O-C2 angle as a predictor of dyspnea and/or dysphagia after occipitocervical fusion. Spine 2009, 34, 184–188. [Google Scholar] [CrossRef]
- Yoshida, M.; Neo, M.; Fujibayashi, S.; Nakamura, T. Upper-airway obstruction after short posterior occipitocervical fusion in a flexed position. Spine 2007, 32, E267–E270. [Google Scholar] [CrossRef] [PubMed]
- Kaneyama, S.; Sumi, M.; Takabatake, M.; Kasahara, K.; Kanemura, A.; Hirata, H.; Darden, B.V. The Prediction and Prevention of Dysphagia After Occipitospinal Fusion by Use of the S-line (Swallowing Line). Spine 2017, 42, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Pouderoux, P.; Jacquot, J.M.; Royer, E.; Finiels, H. Deglutition disorders in the elderly. Evaluation methods. Presse Med. 2001, 30, 1635–1644. [Google Scholar] [PubMed]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom. Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Tohara, H.; Palmer, J.B.; Reynolds, K.; Kuhlemeier, K.V.; Palmer, S. Dysphagia severity scale]. Kokubyo Gakkai Zasshi 2003, 70, 242–248. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, K.H.; Purdy, M.; Falk, J.; Gallo, L. The Dysphagia Outcome and Severity Scale. Dysphagia 1999, 14, 139–145. [Google Scholar] [CrossRef]
- McCafferty, R.R.; Harrison, M.J.; Tamas, L.B.; Larkins, M.V. Ossification of the anterior longitudinal ligament and Forestier’s disease: An analysis of seven cases. J. Neurosurg. 1995, 83, 13–17. [Google Scholar] [CrossRef]
- Oppenlander, M.E.; Orringer, D.A.; La Marca, F.; McGillicuddy, J.E.; Sullivan, S.E.; Chandler, W.F.; Park, P. Dysphagia due to anterior cervical hyperosteophytosis. Surg. Neurol. 2009, 72, 266–270. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mizuno, J.; Nakagawa, H. Clinical and radiological analysis of ossification of the anterior longitudinal ligament causing dysphagia and hoarseness. Neurosurgery 2006, 58, 913–919; discussion 913–919. [Google Scholar] [CrossRef]
- Von der Hoeh, N.H.; Voelker, A.; Jarvers, J.S.; Gulow, J.; Heyde, C.E. Results after the surgical treatment of anterior cervical hyperostosis causing dysphagia. Eur. Spine J. 2015, 24 (Suppl. 4), S489–S493. [Google Scholar] [CrossRef] [PubMed]
- Seidler, T.O.; Pèrez Alvarez, J.C.; Wonneberger, K.; Hacki, T. Dysphagia caused by ventral osteophytes of the cervical spine: Clinical and radiographic findings. Eur. Arch. Otorhinolaryngol. 2009, 266, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Yu, J. The role of C2-C7 and O-C2 angle in the development of dysphagia after cervical spine surgery. Dysphagia 2013, 28, 131–138. [Google Scholar] [CrossRef]
- Goh, P.Y.; Dobson, M.; Iseli, T.; Maartens, N.F. Forestier’s disease presenting with dysphagia and dysphonia. J. Clin. Neurosci. 2010, 17, 1336–1338. [Google Scholar] [CrossRef]
- Spanu, G.; Marchionni, M.; Adinolfi, D.; Knerich, R. Complications following anterior cervical spine surgery for disc diseases: An analysis of ten years experience. Chir. Organi. Mov. 2005, 90, 229–240. [Google Scholar] [PubMed]
- Urrutia, J.; Bono, C.M. Long-term results of surgical treatment of dysphagia secondary to cervical diffuse idiopathic skeletal hyperostosis. Spine J. 2009, 9, e13–e17. [Google Scholar] [CrossRef] [PubMed]
- Lui Jonathan, Y.C.; Sayal, P.; Prezerakos, G.; Russo, V.; Choi, D.; Casey, A.T.H. The surgical management of dysphagia secondary to diffuse idiopathic skeletal hyperostosis. Clin. Neurol. Neurosurg. 2018, 167, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Virk, J.S. Dysphagia due to DISH-related anterior osteophytes: DISHphagia!! BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egerter, A.C.; Kim, E.S.; Lee, D.J.; Liu, J.J.; Cadena, G.; Panchal, R.R.; Kim, K.D. Dysphagia Secondary to Anterior Osteophytes of the Cervical Spine. Global Spine J. 2015, 5, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sugiyama, S.; Hosoe, H.; Iinuma, N.; Suzuki, Y.; Shimizu, K. Postsurgical recurrence of osteophytes causing dysphagia in patients with diffuse idiopathic skeletal hyperostosis. Eur. Spine J. 2009, 18, 1652–1658. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).