Abstract
Iron (Fe) deficiency causes great disturbances to plant growth, productivity and metabolism. This study investigated the effect of bicarbonate-induced Fe deficiency on Foeniculum vulgare (Mill) growth, nutrient uptake, the accumulation of secondary metabolites and the impact on bioactivities. When grown under indirect Fe deficiency conditions (+Fe +Bic), the plants decreased their total mass, an effect that was clearly evident in shoots (−28%). Instead, roots were the main organ affected regarding variations in the phenolic profile and their respective functionalities. Hydromethanolic extracts from bicarbonate-treated roots had a remarkable increase in the levels of phenolic compounds, both of flavonoids (isoquercetin and isorhamnetin) and phenolic acids (gallic acid, chlorogenic acid, syringic acid, ferulic acid, caffeic acid and trans-cinnamic acid), when compared to equivalent extracts from control plants. In addition, they exhibited higher scavenging abilities of DPPH•, NO•, RO2•, as well as inhibitory capacities towards the activity of lipoxygenase (LOX), xanthine oxidase (XO) and acetylcholinesterase (AChE). The overall results suggest that fennel species may modulate secondary metabolites metabolism to fight damages caused by iron deficiency.
1. Introduction
Iron (Fe) assumes a fundamental role in agriculture worldwide, since it is extremely necessary for the growth and development of plants [1,2]. Fe acts in several regulating life-sustaining processes, such as electron or oxygen transfer, photosynthesis and respiration functions [3]. On the other hand, plants frequently face difficulties in preserving Fe homeostasis, owing to the limited fraction of soluble Fe, particularly in calcareous soils. As an example, in Tunisia, the physico-chemical analysis of soil properties in the north revealed high concentrations of bicarbonate ions (HCO3−), which lead to the precipitation of Fe and a consequent severe limitation in plant development [4]. Fe deficiency causes chlorosis, which results from the proteolytic loss of photosynthetic components, such as PSI/PSII and the cyt b6/f complex [5].
Depending on their responses to Fe deficiency, higher plants are grouped as: (I) strategy I plants, characterized by (i) stimulation of rhizosphere acidification, (ii) accumulation of organic acids and/or (iii) secretion of chelating and reducing substances, mainly phenolic compounds; (II) strategy II plants (typically gramineous species), characterized by the extrusion of phytosiderophores, responsible for the mobilization of Fe from the rhizosphere [6]. Furthermore, in strategy I plants, exudation and/or accumulation of organic compounds such as flavins, organic acids and phenolic compounds play a crucial role in the mobilization of Fe2+ [7]. In fact, these compounds are considered as effective Fe chelators in the soil, by inducing the dissolution of ferric hydroxide, which is inaccessible to plants [8]. In this regard, polyphenols are particularly relevant, since, beside chelating properties, they simultaneously exhibit important antioxidant properties [9]. As anticipated by Naczk and Shahidi [10], these bioactive metabolites are synthesized in different parts of the plant, where they contribute to maintain reactive oxygen species (ROS) below toxic levels during abiotic/biotic stresses. Depending on the stress intensity and the plant efficiency to activate several mechanisms, the production of such metabolites can increase or decrease under Fe deficiency conditions [11].
Aromatic plants are claimed to have high polyphenol contents. Among them, fennel is widely cultivated in different countries of the Mediterranean (Tunisia, Italy and Spain) and especially used for food production. In fact, fennel condiment is extremely fragrant with a characteristic aniseed flavor [12] and it is used as an ingredient of foods, such as soups, salads and herbal infusions [13]. Its consumption has also been related to health benefits, a fact that is consistent with the usage of distinct parts of this plant (bulbs, young shoots, leaves, flowering stems, inflorescences and seeds) in household remedies for the treatment of digestive system complications, bronchitis, chronic cough and kidney stones [14,15]. The bioactive properties of fennel have been closely associated with polyphenols, which are particularly abundant in its shoots [14].
Possibly, the levels of such compounds can be raised, or de novo produced, when plants are subjected to different biotic and abiotic stresses, such as Fe deficiency [16]. Environmental constraints have, in general, an opposing effect on the yields of phenolic compounds, i.e., their increment is generally followed by the reduction of biomass production [17] and optimal polyphenol yields are expected to be obtained in stress-tolerant species [18,19].
Distinct works demonstrated the occurrence of variable responses of plant to Fe deficiency, depending on genotypes, particularly in pasture species, but little interest has been devoted to species with multi-variant proprieties subjected to metal deficiency [20].
Perception of the adaptation mechanism of plants with economic, medicinal and therapeutic virtues, such as Foeniculum vulgare, to calcareous conditions may provide a basis for their rational planting in alkali soil with an original program in the screening of new Fe-efficient genotypes. To deal with this issue, it is critical to evaluate the effect of low Fe availability on growth, mineral composition and photosynthetic activity of fennel and, secondly, to elucidate the secondary metabolism response, along with the impact on antioxidant efficiency.
2. Materials and Methods
2.1. Plant Material, Fe Scarcity Regime and Mineral Analysis
Fennel seeds were provided by “Baddar seeds Agency, Tunis, Tunisia”. Seeds were germinated in Petri dishes (15 seeds/box) with water-soaked filters. Ten-day-old seedlings were then hydroponically grown in Hoagland and Arnon nutrient solution for 20 days. The composition of the nutrient solution was 1.25 mM Ca(NO3)2, 1.25 mM KNO3, 0.5 mM MgSO4, 0.25 mM KH2PO4, 10 µM H3BO3, 1 µM MnSO4, 0.5 µM ZnSO4, 0.05 µM (NH4)6Mo7O24 and 0.4 µM CuSO4.
Plants were then divided in two lots, control (+Fe), with the addition of iron at 40 µM Fe (group 1), and (+Fe +Bic), in the presence of 40 µM Fe+, 20 mM NaHCO3+ and 0.5 gL−1 of CaCO3 (indirect iron deficiency, group 2). Iron was supplied in the form of Fe(III)-EDTA. Fennel plants were maintained under controlled climatic condition upheld in the growth chamber with a day/night photoperiod of 16/8 h, temperature (day/night) of 24/18 °C, photosynthetic photon flux density (PPFD) of 200 µmolm−2 s−1 and a relative humidity of 70%. The pH of the culture medium was adjusted to 6.0 for +Fe treatment and reached 8.2 in the +Fe +Bic treatment. CaCO3 was added to the nutrient solution to mimic the same conditions in calcareous soils. At harvest, shoots and roots were separated, rinsed three times with cold distilled water and blotted between two layers of filter paper. The dry weight (DW) was determined after 48 h of desiccation in a thermoventilated oven at 60 °C.
For mineral analysis, roots were cautiously washed once with 1% (v/v) HCl to facilitate extracellular Fe removing ensued by forcibly washing with distilled water [21]. The dried fennel organs (leaves and roots) were digested with a HNO3/HCIO4 solution (2.5:1, v/v) following the protocol of Houmani et al. [21]. Samples were analyzed for macro and micronutrient (Potassium (K), Iron (Fe), Zinc (Zn) and Copper Cu) by means of a Perkin-Elmer Analyst 100 Atomic Absorption Spectrophotometer (VARIAN 220 FS).
2.2. Photosynthetic Pigments
Chlorophyll was obtained from fresh leaves. For that, small leaf discs (200 mg) were grinded using a mortar and pestle, immersed in 5 mL of 80% acetone and left in the dark at 4 °C, for 72 h. Levels of chlorophyll a and b were determined following the methodology established by Lichtenthaler [22], through the measure of the optical density at 663 and 646 nm (UV-1800, Shimadzu, Kyoto, Japan).
2.3. Phenolic Compounds
The total phenolic content (TPC) of leaves and roots extracts (obtained with 1 g dry powder in 10 mL of 80% methanol, for 30 min) were estimated by the Folin–Ciocalteu reagent, as previously described by Saada et al. [23], and expressed as gallic acid equivalent (mg GAE)/dried weight of plant material (g DW). The total flavonoid content (TFC) in extracts from both plant organs were determined as previously reported by Wasli et al. [24] and expressed as milligram of catechin equivalent per gram of dried weight of plant material.
The individual phenolic compounds were identified by reversed-phase high performance liquid chromatography, using a HPLC system (consisting of a vacuum degasser, an autosampler and a binary pump with a maximum pressure of 400 bar; Agilent 1260, Agilent technologies, Waldbronn, Germany). The column was a reversed phase C18 analytical column of 4.6 mm × 100 mm and 3.5 μm particle size (Zorbax Eclipse XDB C18). The DAD detector was set to a scanning range of 200–400 nm and the chromatographic profile was recorded at 280 nm.
The injected volume was 2µL and the flow rate of mobile phase was 0.4 mL/min. The mobile phase consisted of a solvent A, corresponding to methanol, and a solvent B, composed of 0.1% formic acid. The gradient program was as follows: 0–5 min, 10–20% of A; 5–10 min, 20–30% of A; 10–15 min, 30–50% of A; 15–20 min, 50–70% of A; 20–25 min, 70–90% of A; 25–30 min, 90–50 % of A; 30–35 min, return to the initial conditions.
Identification of phenolic compounds was achieved by comparison of the retention time and the UV spectra with those of pure standards, injected under the same conditions. For that, five different concentrations (range of 1–100 µg/mL) of the standard compounds, caffeic acid, chlorogenic acid, ferulic acid, gallic acid, kaempferol, isoquercetin, quercetin, isorhamnetin, rutin, syringic acid and trans-cinnamic acid, were prepared in methanol. The calibration curve was constructed for each compound by plotting peak areas versus the concentrations. The summary of retention time, linear equations, squared correlation, limit of detection (LOD) and limit of quantification (LOQ) is presented in Supplementary Materials Table S1.
2.4. Antioxidant Activities
2.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity Assay
The ability to scavenge the DPPH radical was assessed following the general procedure of Catarino et al. [25] and the activity is expressed as IC50 (mg/mL), i.e., the extract dose required to reduce the absorbance at 517 nm by 50%.
2.4.2. Chemical Nitric Oxide (NO●) Quenching Assay
The NO● scavenging ability was measured as described by Catarino et al. [25], using 100 µL of sodium nitroprusside (3.33 mM) in PBS 100 mM (pH 7.4) and 100 µL of fennel extract solution (0.5–3 mg/mL). The reaction was started by adding 100 µl of the Griess reagent (0.5% sulphanilamide and 0.05% naphthyletylenediamine dihydrochloride in 2.5% H3PO4) to the mixture, following another 10 min, and subsequently measured using a microplate reader (Bio-Tek Instruments, nc; Winooski, VT, USA) at 562 nm [25]. The antiradical activity is expressed as IC50 (mg/mL).
2.4.3. Oxygen Radical Capacity (ORAC) Test
This assay was carried out following the procedures of Ou et al. [26], with slight modifications. The extract (15 µL) was added to 96-well microplates (black round bottom) and loaded with 75 µL of fluorescein solution to start the reaction. The reaction was initiated by the addition of 15 µL of 2,2′-azobis 2 methylpropionamidine (AAPH) to each well after incubation for 10 min at 37 ± 1 °C. The fluorescence (k ex.: 485 nm/em.: 530 nm) of fluorescein was recorded every minute after the addition of 375 mM of AAPH, for a total of 35 min. The results were determined by comparing the net areas under the fluorescein decay curves between the blank. Results are expressed in micromoles of Trolox equivalents (TE) per gram (µmol TE/g).
2.4.4. Ferric Reducing Power Assay (FRAP)
The analysis of FRAP followed the procedure of Wasli et al. [24]. A volume of 1 ml of fennel extracts was homogenized with 2.5 mL of phosphate buffer (0.2 mol L−1, pH 6.6) and 2.5 mL of potassium ferricyanide K3Fe(CN)6. After incubation at 50 °C for 25 min, 2.5 mL of TCA (10%) was mixed and the solution was centrifuged at 3000× g rpm for 10 min. Thereafter, 2.5 mL of upper layer was blended with 2.5 mL of deionized water, 0.5 mL of 0.1% of iron chloride (FeCl3) was added and absorbance was monitored at 700 nm. Results are expressed as EC50 value (mg/mL), i.e., the extract dose required to obtain an absorbance of 0.5.
2.4.5. Lipid Peroxidation Inhibition in the Presence of Thiobarbituric Acid Reactive Substances (TBARS)
The assay was carried out as previously described by Majdoub et al. [27]. A 250 µL aliquot of egg yolk homogenates (100 mg/mL) was mixed with 750 µL of acetic acid and 750 µL of TBA solution, dissolved in sodium dodecyl sulfate (11 mg/mL). The reaction mixture was then heated for 60 min in a water bath at 95 °C. After cooling, 250 µL of butanol was added to each tube, followed by vigorous vortexing and centrifugation for 10 min at 3000× g rpm. The upper organic layer absorbance was read at 532 nm against a blank (all reagents excluding the sample). Results are expressed as IC50 (concentration of extract that inhibits 50% of lipid peroxidation).
2.5. Enzymatic Inhibitory Ability
2.5.1. Lipoxygenase (LOX)
LOX activity was performed relying on the method recently of Majdoub et al. [27]. The reaction medium (total volume of 1 mL) contained 10 μL of fennel extract, plus 5 μL of enzyme solution (0.054 g/mL) and 50 μL of linoleic acid (0.001 M), in borate buffer (0.1 M, pH 9). The absorbance was read at 234 nm and the activity was estimated based on the following formula:
where Ac represents the absorbance of the control reaction and Ae is the absorbance of the sample extract. Results are expressed as IC50 values.
[(Ac − Ae/Ac) × 100] (3)
2.5.2. Xanthine Oxidase (XO)
This assay was conducted as reported by Pereira et al. [28]. The reaction medium was composed of 40 μL of fennel extract at distinct concentrations, 45 μL of sodium dihydrogen phosphate buffer (100 mM, pH 7.5) and 40 µL of enzyme solution. The reaction was started by the addition of xanthine (0.1 mM dissolved in buffer), after pre-incubation (5 min, 25 °C). Monitoring of absorbance was performed at 295 nm (10 min, 25 °C) and the percentage of inhibition was estimated through the equation
where mc and me represent the slope for control (no inhibitor) and the extract, respectively. The results are expressed as IC50 values (mg/mL).
% inhibition = mc − me mc × 100, (4)
2.5.3. Acetylcholinesterase (AChE)
This assay was performed following the method of El-Guendouz et al. [29], with minor modification. The reaction medium contained extract (60 µL of six different extract concentrations, 1–5 mg/mL), plus 425 μL of Tris-HCl buffer (0.1 M, pH 8) and 25 μL of enzyme (0.28 U/mL). After pre-incubation (15 min at room temperature), the reaction was started by the addition of substrate (75 μL of substrate). The reaction was stopped by the addition of 475 μL of 5,5′-dithiobis (2-nitrobenzoic acid) (0.059 g in 50 mL of buffer) and absorbance was read at 405 nm. The percentage of inhibition was calculated using the equation [(A0 − A1/A0) × 100]; the results are expressed as IC50 values.
2.6. Data Analysis
Data were analyzed by two-tailed unpaired t-test, for plant growth and levels of photosynthetic pigments, and one-way ANOVA, followed by Tukey’s post-hoc test for the remaining assays. The statistical tests were applied using GraphPad Prism, version 6, and the significance level was p < 0.05. Multivariate data analysis was carried out using principal component analysis (PCA). The Pearson correlation was performed using the XLSTAT program.
3. Results and Discussion
3.1. Effect of HCO3-Induced Fe Deficiency on Plant Growth and Levels of Photosynthetic Pigments and Nutrients
Fe is involved in crucial physiological and metabolic processes including growth, DNA synthesis, respiration and photosynthesis [1,2]. Fennel plants grown under bicarbonate treatment (i.e, indirect iron deficiency conditions) showed classical symptoms of Fe deficiency after 14 days of treatment. Indeed, the top leaves exhibited visible intercostal chlorosis (Figure 1) that indicates a survival strategy of the plant to such constraint. This is consistent with previous bibliographic data that describe interveinal chlorosis of young leaves as the most obvious visible symptom of Fe deficiency [30].
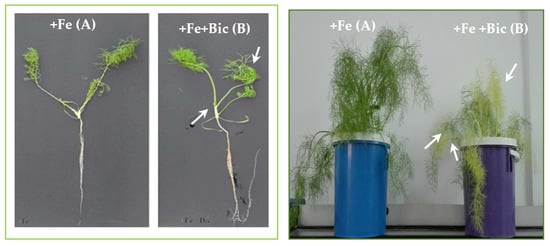
Figure 1.
Morphogenic changes in fennel plants cultivated (A) under Fe sufficiency (+Fe) or (B) under Fe deficiency (+Fe +Bic), after a period of cultivation of 14 days. Arrows indicate intercostal chlorosis.
Moreover, a decrease in the total content of chlorophylls in leaves of plants grown under Fe deficiency conditions was registered, when compared to the control. This resulted from a decrease in the levels of Chl a (−29%) and of Chl b (−39%), as presented in Figure 2. This reduction suggests a major role of Fe2+ in the biogenesis of chlorophyll precursors such as 5-aminolevulinic acid and protochlorophyllide [7].
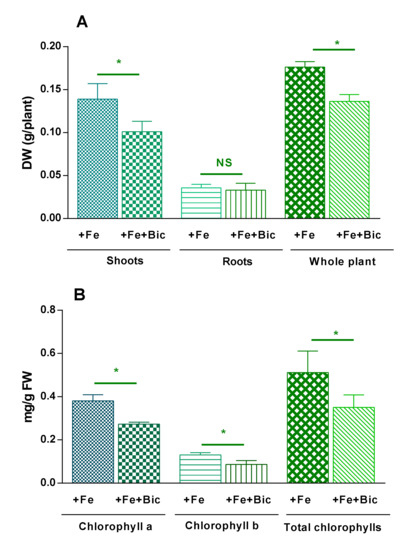
Figure 2.
Plant yields (A) and levels of photosynthetic pigment in leaves (B) of fennel plants grown under Fe sufficiency (+Fe) or under Fe deficiency conditions (+Fe + Bic). DW, dry weight; FW, fresh weight. Data represent the mean ± standard deviation of three independent assays. *represents significant differences at p < 0.05; NS represents no significant differences at p < 0.05.
The mean whole plant and shoot dry weights of Fe-deficient fennel plants were also reduced in relation to the control plants, contrary to root organs, which were able to uphold their growth (Figure 2). As previously described in Parietaria, in plants grown in calcareous media, the decrease in shoot growth represents an adaptive strategy of the plant to keep suitable levels of Fe and chlorophyll, as well as to preserve the photosynthetic activity in leaf tissues [31]. In turn, Dell’Orto et al. [32] noted that bicarbonate supply provoked the appearance of proteoid roots that are developed from secondary and tertiary lateral roots, to allow increasing the surface of contact between plant and soil (for the release of nutrient solubilizing compounds and for the uptake of nutrients from the rhizosphere). The authors commented that such cluster roots in plants grown in the presence of bicarbonate is not a specific response to Fe deficiency, but a reaction to the general condition of low nutrient availability [32]. In Sulla plants, when grown in a calcareous soil, a reduced shoot growth was found (but not that of roots), which confirms its strategy of increasing root uptake efficiency for nutrients that are not easily available under these conditions [33].
Fe starvation has been demonstrated to affect mineral element homeostasis [34]. As shown in Figure 3, Fe content was notably diminished under Fe deficiency conditions, in both treated organs (ca. −57% and −29%, for shoots and roots, respectively), as compared to control plants. At the same line, potassium content was considerably reduced in deficient shoots (−51%) and roots (−22%). In turn, zinc and copper exhibited an opposing tendency, with levels increases of about 19, 27, 24 and 36%, respectively, in bicarbonate-treated shoots and roots.

Figure 3.
Iron (Fe; A), potassium (K; B), zinc (Zn; C) and copper (Cu; D) contents in shoots and roots of fennel plants grown under Fe sufficiency (+Fe) or under Fe deficiency conditions (+Fe +Bic). DW, dry weight; FW, fresh weight. Data represent the mean ± standard deviation of three independent assays. Values in the same line with different superscripts (a, b) are significantly different at p < 0.05.
The reduced Fe content is explained as a primary reaction to the low amount of Fe to which the plants were exposed [34]. This is due to the very low solubility of iron oxides under alkaline pH conditions (buffered by the occurrence of HCO3- in these soils) [33,35].
In addition, Fe shortage is often associated with K+ starvation, although some authors claim that chlorotic leaves recurrently reduce K+ concentration [35]. In this context, Houmani et al. [21] reported a decreasing K+ acquisition by Sueda fruticosa plants subjected to Fe deficiency. Ionic antagonism and synergistic effect play a part in mineral element absorption. For example, insufficient Fe in cultured medium was shown to induce Cu uptake and its consequent accumulation in Commelina communis [36]. However, Marschner et al. [30] hypothesized that a decrease in K+ uptake, concomitant with an increase in Zn, could be attributed to the transporter sharing both elements. Ben Abdalah et al. [37] related the resistance of Hedysarum carnosum (Thelja isolate) to the increase in Zn contents.
3.2. Effect of HCO3-Induced Fe Deficiency on Phenolic Compounds
The antioxidant and chelating properties of phenolic compounds are crucial in the adaptive response to Fe deficiency [16]. For this reason, monitoring of total phenolic content (TPC) and total flavonoid content (TFC) was conducted in hydromethanolic extracts obtained from control and treated fennel plants. As shown in Figure 4, the levels of phenolic compounds were higher in leaves than in roots. Curiously, when submitted to Fe deficiency conditions, values of TPC and TFC decreased in leaves, compared to the control plants (from 3.93 ± 0.27 to 2.92 ± 0.21 mg GAE/g DWplant and from 2.14 ± 0.31 to 1.82 ± 0.04 mg CE/g DWplant, respectively), while the opposite trend was observed in roots (from 0.49 ± 0.02 to 1.07 ± 0.14 mg GAE g/DWplant and from 0.37 ± 0.08 to 0.88 ± 0.02 mg CE/g DWplant, respectively).
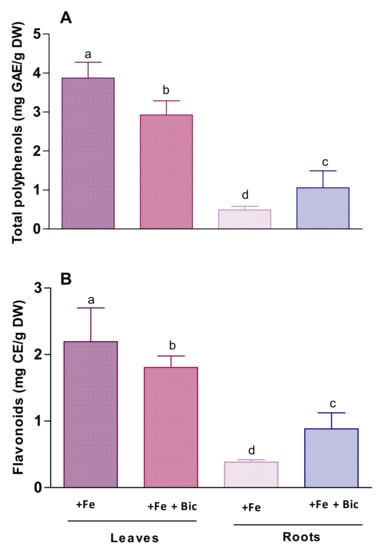
Figure 4.
Levels of total phenolic compounds (A) and of flavonoids (B) in leaves and roots of fennel plants grown under Fe sufficiency (+Fe) or under Fe deficiency conditions (+Fe +Bic). Data represent the mean ± standard deviation of three independent assays. Values with different superscripts are significantly different at p < 0.05.
RP-HPLC analysis was herein used to further understand the impact of iron deficiency on the levels of individual phenolic components for both organs. Eight phenolic compounds, already reported for this plant species [12], were identified by RP-HPLC analysis of the hydromethanolic extracts from leaves, namely, the gallic acid, chlorogenic acid, ferulic acid and trans-cinnamic acid, and the flavonoids isorhamnetin, rutin, kaempferol and quercetin. Fe starvation did not cause an appreciable qualitative modification on the chromatograms, but it changed the levels of the individual phenolic compounds (Supplementary Materials Figure S1). In particular, the amounts of gallic acid and kaempferol (i.e., the main identified hydroxybenzoic acid and flavonoid, respectively) were decreased by about 26% and 35%, respectively, whereas major hydroxycinnamic acids showed an opposite trend (increasing from 0.075 to 0.160 mg/g DW for chlorogenic acid and from 0.070 to 0.135 mg/g DW for trans-cinnamic acid) (Figure 5). Overall, it is of note that the phenolic acids pool (hydroxybenzoic plus hydroxycinnamic acids) was maintained, unlike that of flavonoids, that was significantly decreased, causing a total decrement in phenolic compounds (in agreement with the TPC and TFC results, in Figure 4).
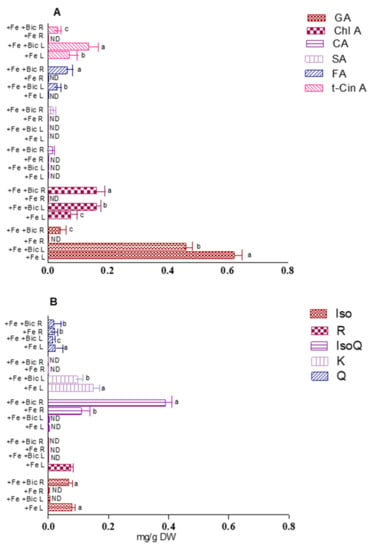
Figure 5.
Contents of individual phenolic acids (A) and flavonoids (B) (mg/g DW) in leaves (L) and roots (R) of fennel plants grown in the presence of Fe (+Fe) or in the presence of Fe plus bicarbonate (+Fe +Bic). GA, gallic acid; Chl A, chlorogenic acid; CA, caffeic acid; SA, syringic acid; FA, ferulic acid; t-Cin A, trans-hydroxycinnamic acid; Iso, isorhamnetin; R: rutin, IsoQ: isoquercetin; K, kaempferol; Q, quercetin; ND, non-detected. Data represent the mean ± standard deviation of three independent assays. Values with different superscripts are significantly different at p < 0.05.
In turn, in comparison to the control, the hydromethanolic extracts obtained from bicarbonate-treated root organs, showed a remarkable increase in the levels of phenolic compounds, including flavonoids (isoquercetin and isorhamnetin) and phenolic acids (gallic acid, chlorogenic acid, syringic acid, ferulic acid, caffeic acid and trans-cinnamic acid), compared to control plants. Notably, some of these compounds (e.g., isoquercetin and ferulic acid) were not detected in the extracts from the control plants, suggesting that, under Fe stress, roots may be able to synthesize new phenolic compounds (Figure S1 and Figure 5).
Phenolics represent the main classes of organic ligands exuded by strategy I plants under Fe-deficiency [38], due to their ability in the mobilizing of Fe in the rhizosphere by interfering two based mechanisms, including reduction and complexation [38,39]. According to Boyer et al. [40], caffeic acid, chlorogenic acid and ferulic acid uphold the reduction of Fe from ferrihydrite. Instead, Wasli et al. [24] hypothesized that the distribution of new biomass to root organ by reducing lignin synthesis via hydroxycinnamic acids is implicated in nutrient Fe acquisition, which could assure the exploration of soil and consequently improving Fe uptake. In turn, the accumulation of flavonoids in roots helps to regulate plant cell growth and differentiation, the activity of distinct protein kinases and the adjustment of peroxidation kinetics by the alteration of the lipid packing order, thus decreasing the fluidity of the membrane, which decrease free radicals diffusion and make peroxidative reactions difficult [20]. In this respect, Mira et al. [41] attested that quercetin and myricetin chelate Fe (II), after its reduction to Fe (III). Besides, Ismail et al. [42] demonstrated that rutin is able to extract large amounts of Fe, mainly through a reductive mechanism.
3.3. Effect of HCO3-Induced Fe Deficiency on Antioxidant Abilities
Plants need a balanced uptake, consumption and storage of mineral elements for proper ion homeostasis, this being distressed by nutritional imbalances in soils. Under Fe deficiency conditions, both leaf and root organs are subjected to ROS attack, partially due to the reduction of photosynthetic activity, which shows an over-reduction of the photosynthetic electron transport chain in leaves [33,43]. After the reduction of Fe (III) to Fe (II) by ferric chelate reductase, Fe(II) is re-oxidized in Fe-deprived root cells, generating a complex with citrate, Fe(III)-dicitrate. The occurrence of these two stable and inter-convertible forms of Fe accelerates potential electron transfer (or captured) to (or from) other species, to form free radicals. Accordingly, plant resistance to various stresses is associated with their antioxidant capacity and increased levels of antioxidants [24,43].
On the other hand, considering the multiple aspects of antioxidants and their reactivity, the application of a single method is, in general, considered to be a very limited approach to estimate the antioxidant properties of plant extracts, while the use of simultaneous tests is more appropriate for a real perspective [44]. In our study, the hydromethanolic extracts, obtained from leaves and from roots, were evaluated regarding their ability to trap free radicals, namely, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), nitric oxide (NO•) and peroxyl (RO2•), along with the capacity to reduce ferric Fe to ferrous form (FRAP assay) and to inhibit the malondialdehyde formation resulting from lipids oxidative damage, through TBARS assay.
As shown in Figure 6, the antioxidant potencies varied significantly among the plant organs. Indeed, in control plants, excepting for ORAC, the extracts obtained from leaves were more active than those from roots (as reflected by lower IC50 and EC50 values), possibly because of their richness in phenolic compounds. In general, bicarbonate supply did not affect the antioxidant potential of leaf extracts toward DPPH•, NO• and RO2• radicals. The only exception was observed in TBARS, for which the ability to hamper lipid peroxidation was even decreased in bicarbonate-treated plants, compared to those of the control condition. In turn, root extracts of fennel grown under iron deficiency conditions exerted higher scavenging ability towards the radicals DPPH and NO, as well as a higher potency to reduce ferric Fe and to inhibit lipid peroxidation. The application of linear regression analysis to establish relationships between the phenolic amounts with the antioxidant activities resulted in a high significant negative correlation between the IC50 values of DPPH (r = −0.95; r = −0.98) and the EC50 values of FRAP (r = −0.88; r = −0.93) with TPC/TFC, while lower or no correlation were observed in the case of NO• quenching activity (r = −0.54 for TPC; r = −0.64 for TFC) and TBARS (r values < 0.5), respectively ( Supplementary Materials Table S2). In general, these results suggest that both phenolic compounds and flavonoids are key contributors in DPPH and FRAP assays, while non-phenolic compounds may also have an important contribution on the remaining tests.
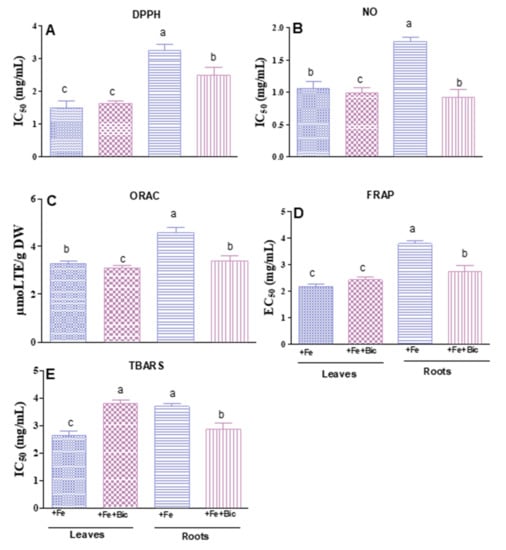
Figure 6.
Capacity of hydromethanolic extracts of fennel leaves (L) and roots (R) of fennel plants cultivated under Fe sufficiency (+Fe) or under Fe deficiency conditions (+Fe + Bic) to scavenge the radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH, A), nitric oxide (NO, B) and peroxyl (ORAC, C), to reduce ferric Fe to ferrous form (FRAP, D) and to inhibit lipids oxidative damage (TBARS, E). Data represent the mean ± standard deviation of three independent assays. Values with different superscripts are significantly different at p < 0.05.
Changes in the antioxidant activities of plants subjected to Fe-deficiency have been investigated [24,43], with variable results being reported. Recently, Wasli et al. [24] highlighted that, when grown under Fe deficiency, Anethum graveolens was able to increase the antioxidant capacity of the overall plant. In another study, Kabir et al. [45] described that Abelmoschus esculentus root extracts from the Fe-deficiency tolerant variety had higher DPPH scavenging ability than the control plants, which was significantly correlated with the levels of phenolic compounds. Moreover, the loss of the antioxidant potency of a sensitive variety of lettuce (Romaine), caused by an Fe deficiency treatment, was shown to be associated with the decrease in the amounts of flavonoids [46].
3.4. Effect of Bicarbonate-Induced Fe Deficiency on the Inhibitory Enzymatic Capacity
The effects of Fe deficiency growth on the capacity of extracts to decrease the activity of lipoxygenase (LOX), xanthine oxidase (XO) and acetylcholinesterase (AChE) was also evaluated to estimate their bioactive potential towards these enzymes. Note that LOXs isoforms are non-heme, non-sulfur iron containing dioxygenases that catalyze oxidation of polyunsaturated fatty acids (linoleic, linolenic and arachidonic acid) to generate hydroperoxides [47], which are widely distributed in plants, where they play several roles. On one hand, LOXs are used as a storage protein through the vegetative growth period. In addition, they are implicated in the mobilization of storage lipids during germination [48] and display a pivotal role in the generation of shielding components, such as jasmonates, divinyl ethers and leaf aldehydes, which helps shelter plants from insects and pathogens or during abiotic stress [49,50]. In turn, LOX reactions with unsaturated fatty acids (UFA) can produce off-flavors/off-odors and cause food spoilage.
The inhibitory potential towards LOX activity was distinct among the plant organs, being higher in bicarbonate-treated roots, as reflected by the lower IC50 values (Figure 7). The change in LOX activity in stressed-treated plants has been scarcely investigated. Yet, according to Majdoub et al. [27] and Rahman et al. [51], the propagation of lipid peroxidation in plants under biotic and abiotic stress, induced by higher lipolytic activity on the membrane, was associated with an increase in LOX activity. In addition, Bae et al. [52] reported a stimulation of the expression of LOX genes (LOX3 isoform) in ginseng seedlings submitted to water shortage, a fact that authors postulated to be an adaptative response of the plant to water deficit.
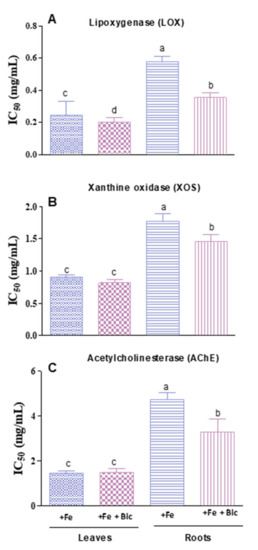
Figure 7.
Inhibitory activities of hydromethanolic extracts from leaves (L) and roots (R) of fennel plants cultivated under Fe sufficiency (+Fe) or under Fe deficiency (+Fe +Bic) conditions, towards lipoxygenase (LOX, A), xanthine oxidase (XOS, B) and acetylcholinesterase (AChE, C). Data represent the mean ± standard deviation of three independent assays. Values with different superscripts are significantly different (p < 0.05).
The hindering of XO activity under stress conditions could hamper the catalyzing of the oxidation of hypoxanthine to xanthine and the conversion of the latter to uric acid, producing O2•– and contributing to the increment of oxidative stress events [28,53]. Bicarbonate-treated roots exhibited an improved ability to inhibit the xanthine oxidase activity, in comparison with the roots of the control plants (+Fe). In turn, no significant variations were observed in leaves.
Presently, exploring for novel AChE inhibitors issued from natural sources with few side effects is required [54]. As far as we know, no works have been accounted concerning the impact of Fe scarcity on the ability of F. vulgare extracts to inhibit the activity of AChE, which prompted us to ponder over the efficiency of our samples for AChE inhibition. The gathered results show that fennel plants exerted inhibitory effect towards AChE, which, in roots, was increased in plants grown under Fe deficiency.
Trait biplot analysis of both treatments (+Fe and +Fe +Bic) correlating phenolic pools with the IC50 values of inhibitory enzyme activities (LOX, XO and AChE) revealed that the first two components (PCs) contributed by 99.02% to cumulative variance, with F1 axis and F2 axis explaining 93.3° and 5.71% of the total variance, respectively (Figure 8). Interestingly, the biplot and correlation matrix analysis (Figure 8; Table 1) proved strong correlation coefficients between TPC/TFC and the IC50 values of XO (−0.84; −0.79) and of AChE (−0.93; −0.98), which suggests a major role of phenolic pools in inhibiting these two enzymes in F. vulgare grown under Fe deficiency conditions. This, in part, supports the results of Majdoub et al. [27], who suggested that the AChE inhibitory effect observed in Zn-treated Pimpinella anisum leaves might be attributed to their increased amounts of non-enzymatic antioxidants.
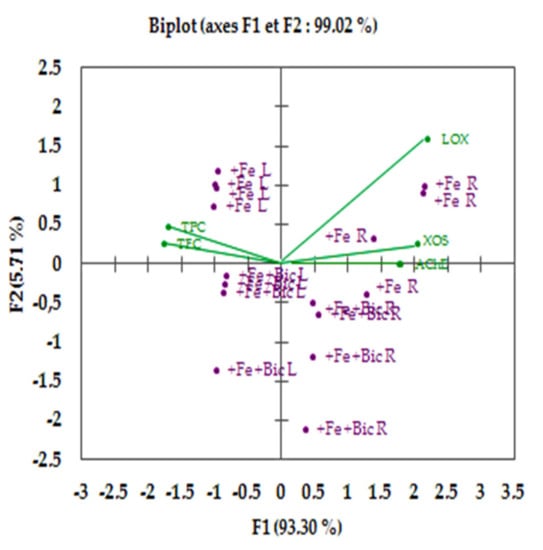
Figure 8.
Biplot for fennel plants grown in the presence of Fe (+Fe) or in the presence of Fe plus bicarbonate (+Fe +Bic) between inhibitory enzymes and total pool phenolics.

Table 1.
Correlation coefficients between total phenolic content (TPC)/total flavonoid content (TFC) and the IC50 values for LOX, XO and AchE activities.
4. Conclusions
In conclusion, this study reveals new insights regarding the mechanisms and processes involved in fennel Fe deficiency adaptation. When cultivated under Fe limiting conditions, F. vulgare shoot and whole plant biomass production significantly declined, whereas that of roots was not affected by such constraint. Moreover, lime-induced Fe deficiency resulted in a significant reduction of chlorophyll linked with an alteration in mineral homeostasis depending on plant organs, probably to support its nutrient use efficiency associated with the preservation of an adequate level of chlorophyll in leaves. On the other hand, a marked influence on the content of phenolic compounds in roots of plants subjected to iron deficiency was observed, a fact that seems to be associated with an improved antioxidant activity and capacity to inhibit the activity of lipoxygenase, xanthine oxidase and acetylcholinesterase. Further applications of molecular and proteomic techniques based on untargeted analysis of multiple genes or proteins could be useful to support the physiological and biochemical traits.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11157072/s1, Table S1: Retention time (RT), linear equations, squared correlation, limit of detection (LOD) and limit of quantification (LOQ) of standard compounds, Table S2: Correlation coefficients between Total Phenolic Content (TPC)/Total Flavonoid Content (TFC) and the IC50 values, in antioxidant activities, Figure S1: RP-HPLC chromatograms at 280 nm, from hydromethanolic extracts of leaves (A,B) and roots (C,D) of fennel plants grown under Fe sufficiency (A,C) or under Fe deficiency conditions (B,D). The peak numbers correspond to 1, gallic acid, 2, chlorogenic acid, 3, caffeic acid, 4, syringic acid, 5, ferulic acid, 6, trans-hydroxycinnamic acid, 7, isorhamnetin, 8, rutin, 9, isoquercetin, 10, kaempferol and 11, quercetin.
Author Contributions
H.W. contribution to conceptualization, experimentation, data curation, writing the original manuscript; N.J. contribution to supervision and writing review; M.S. contribution to investigation, data curation; R.K. contribution to resources; S.M.C. contribution to data curation, supervision, resources and writing review. All authors have read and agreed to the published version of the manuscript.
Funding
Thanks to the Tunisian Ministry of Higher Education and Scientific Research (LR15CBBC06). Thanks to the University of Aveiro, FCT/MEC for the financial support to the LAQV-REQUIMTE (UIDB/50006/2020), through national funds, and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Colangelo-Aksoy, E.; Jeong, I.S.; Koiwa, H. Loss of function of Arabidopsis C-terminal domain phosphatase-like1 activates iron deficiency responses at the transcriptional level. Plant Physiol. 2013, 161, 330–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ksouri, R.; Wided, M.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Toselli, M.; Marangoni, B.; Tagliavini, M. Iron content in vegetative and reproductive organs of nectarine trees in calcareous soils during the development of chlorosis. Eur. J. Agron. 2000, 13, 279–286. [Google Scholar] [CrossRef]
- Donnini, S.; Dell’Orto, M.; Zocchi, G. Oxidative stress responses and root lignification induced by Fe deficiency conditions in pear and quince genotypes. Tree Physiol. 2011, 31, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H.; Römheld, V. Strategies of plants for acquisition of iron. Plant Soil 1995, 165, 261–274. [Google Scholar] [CrossRef]
- Abadía, J.; López-Millán, A.F.; Rombolà, A.; Abadía, A. Organic acids and Fe deficiency: A review. Plant Soil 2002, 241, 75–86. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Zocchi, G. Metabolic changes in iron-stressed dicotyledonous plants. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Abadía, J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 359–370. [Google Scholar]
- Rawson, A.; Hossain, M.; Patras, A.; Tuohy, M.; Brunton, N. Effect of boiling and roasting on the polyacetylene and polyphenol content of fennel (Foeniculum vulgare) bulb. Food Res. Int. 2013, 50, 513–518. [Google Scholar] [CrossRef]
- Santayana, M.P.; Tardio, J.; Blanco, E.; Carvalho, A.M.; Lastra, J.J.; San-Miguel, E.; Morales, R. Traditional knowledge of wild edible plants used in the northwest of the Iberian Peninsula (Spain and Portugal): A comparative study. J. Ethnobiol. Ethnomed. 2007, 3, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. from Portugal. FCT 2009, 47, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrábida Natural Park (Portugal). J. Ethnopharmacol. 2013, 93, 183–195. [Google Scholar] [CrossRef]
- Tato, L.; De Nisi, P.; Donnini, S.; Zocchi, G. Low iron availability and phenolic metabolism in a wild plant species (Parietaria judaica L.). Plant Physiol. Biochem. 2013, 72, 145–153. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, B.; Wobus, U.; Weschke, W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant 2002, 109, 435–442. [Google Scholar] [CrossRef]
- Bettaieb-Rebey, I.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crop. Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Estaji, A.; Roosta, H.R.; Rezaei, S.A.; Hosseini, S.S.; Niknam, F. Morphological, physiological and phytochemical response of different Satureja hortensis L. accessions to salinity in a greenhouse experiment. J. Appl. Res. Med. Aromat. Plants 2018, 10, 25–33. [Google Scholar] [CrossRef]
- M’sehli, W.; Houmani, H.; Graziano, D.; Abdelly, C.; Gharsalli, M. Iron deficiency tolerance at leaf level in Medicago ciliaris Plants. Am. J. Plant Sci. 2014, 5, 2541–2553. [Google Scholar] [CrossRef] [Green Version]
- Houmani, H.; Jelali, N.; Abdelly, C.; Gharsalli, M. Mineral elements bioavailability in the halophyte species Suaeda fruticosa. J. Biol. Res. Thessalon. 2012, 17, 113–120. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Saada, M.; Falleh, H.; Jellali, I.; Snoussi, M.; Ksouri, R. Phenolic profile, biological activities and fraction analysis of the medicinal halophyte Retama raetam. S. Afr. J. Bot. 2014, 94, 114–121. [Google Scholar]
- Wasli, H.; Jelali, N.; Silva, A.M.S.; Ksouri, R.; Cardoso, S.M. Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (Anethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Ind. Crop. Prod. 2018, 126, 466–467. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum decoctions. Food Funct. 2017, 17, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescentprobe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Majdoub, N.; El-Guendouz, S.; Rezgui, M.; Carlier, J.; Costa, C.; Bettaieb-Ben Kaaba, L.; Miguel, M.G. Growth, photosynthetic pigments, phenolic content and biological activities of Foeniculum vulgare Mill., Anethum graveolens L. and Pimpinella anisum L. (Apiaceae) in response to zinc. Ind. Crop. Prod. 2017, 109, 627–636. [Google Scholar] [CrossRef]
- Pereira, O.R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis decoctions: Antioxidant activities and inhibition of carbohydrate and lipid metabolic enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [Green Version]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-acetylcholinesterase, antidiabetic, anti-inflammatory, antityrosinase and antixanthine oxidase activities of Moroccan propolis. Int. J. Food Sci. Tech. 2016, 51, 1762–1773. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.F.; Liu, Y.Z.; Du, W.; Fu, L.N.; Hussain, S.B.; Peng, S.A. Physiological and transcriptional analysis reveals pathways involved in iron deficiency chlorosis in fragrant citrus. Tree Genet. Genomes 2017, 85, 38–49. [Google Scholar] [CrossRef]
- Donnini, S.; De Nisi, P.; Gabotti, D.; Tato, L.; Zocchi, Z. Adaptive strategies of Parietaria diffusa (M.&K.) to calcareous habitat with limited iron availability. Plant Cell Environ. 2012, 35, 1171–1184. [Google Scholar]
- Dell’Orto, M.; De Nisi, P.; Pontiggia, A.; Zocchi, G. Fe deficiency responses in Parietaria diffusa: A calcicole plant. J. Plant Nutr. 2003, 26, 2057–2068. [Google Scholar] [CrossRef]
- Elkouni, A.; Rabhi, M.; Ivanov, A.G.; Krol, M.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Huner, N.P.A. Structural and functional integrity of Sulla carnosa photosynthetic apparatus under iron deficiency conditions. Plant. Biol. 2017, 20, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M.; Norvell, W.A. Growth and nutrient uptake by barley (Hordeum vulgare L. cv Herta): Studies using an N-(2- hydroxyethyl) ethylenedinitrilotriacetic acid-buffered nutrient solution technique. II. Role of zinc in the uptake and root leakage of mineral nutrients. Plant Physiol. 1993, 101, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelali, N.; Ben salah, I.; M’sehli, W.; Donnini, S.; Zocchi, G.; Gharsalli, M. Comparison of three pea cultivars (Pisum sativum) regarding their responses to direct and bicarbonate- induced iron deficiency. Sci. Hortic. 2011, 129, 548–553. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Tian, G.; Zheng, S. Fe deficiency induces Cu uptake and accumulation in Commelina communis. Plant. Sci. J. 2004, 166, 1371–1377. [Google Scholar] [CrossRef]
- Abdallah, H.B.; Mai, H.J.; Slatni, T.; Fink-Straube, C.; Abdelly, C.; Bauer, P. Natural variation in physiological responses of Tunisian Hedysarum carnosum under iron deficiency. Front. Plant Sci. 2018, 9, 1383. [Google Scholar] [CrossRef] [Green Version]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Nannipieri, P.; Torrent, J.; Martinoia, E.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Boyer, R.F.; Clark, H.M.; Sanchez, S. Solubilization of ferrihydrite iron by plant phenolics: A model for rhizosphere processes. J. Plant Nutr. 1989, 12, 581–592. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2008, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Maksimović, J.D.; Maksimović, V.; Shabala, L.; Živanović, B.D.; Yu, T.; Jacobsen, S.E.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2016, 43, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Jelali, N.; Doninni, S.; Dellorto, M.; Abdelly, C.; Gharsalli, M.; Zocchi, G. Implication of antioxidant defence in tolerance to Fe deficiency of pisum Sativum leaves. J. Appl. Biotechnol. Bioeng. 2017, 2, 1–9. [Google Scholar]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Evaluation of spectrophotometric methods for antioxidant compound measurement in relation to total antioxidant capacity in beverages. Food Chem. 2010, 120, 607–614. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.M.; Haider, S.A.; Paul, N.K. Mechanisms associated with differential tolerance to Fe deficiency in okra (Abelmoschus esculentus Moench). Env. Exp. Bot. 2015, 112, 16–26. [Google Scholar] [CrossRef]
- Msilini, N.; Oueslati, S.; Amdouni, T.; Chebbi, M.; Ksouri, R.; Lachaal, M.; Ouerghi, Z. Variability of phenolic content and antioxidant activity of two lettuce varieties under Fe deficiency. J. Sci. Agric. 2013, 93, 2016–2021. [Google Scholar] [CrossRef]
- Chedea, V.S.; Jisaka, M. Lipoxygenase and carotenoids: A co-oxidation story. Afr. J. Biotechnol. 2013, 12, 2786–2791. [Google Scholar]
- New comer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Pallavi, P.C.; Singh, A.K.; Singh, S.; Singh, N.K. In Silico Structural and Functional Insights into the Lipoxygenase Enzyme of Legume Cajanus Cajan. Int. J. Recent Innov. Trends Comput. Commun. 2014, 5, 87–91. [Google Scholar]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rahman, U.; Uddin, T.; Choudhary, G.; Iqbal, M. Discovery and molecular docking simulation of 7-hydroxy-6-methoxy- 2H-chromen-2-one as a LOX Inhibitor. Pak. J. Pharm. Sci. 2019, 32, 217–220. [Google Scholar]
- Bae, K.S.; Rahimi, S.; Kim, Y.J.; Renuka Devi, B.S.; Khorolragchaa, A.; Sukweenadhi, J.; Silva, J.; Myagmarjav, D.; Yang, D.C. Molecular characterization of lipoxygenase genes and their expression analysis against biotic and abiotic stresses in Panax ginseng. Eur. J. Plant Pathol. 2016, 145, 331–343. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Neto, R.T.; Silva, A.M.S.; Cardoso, S.M. Health-promoting effects of Thymus herba-barona, Thymus pseudolanuginosus, and Thymus caespititius decoctions. Int. J. Mol. Sci. 2017, 18, 1879. [Google Scholar] [CrossRef]
- Hasbal, G.; Yilmaz-Ozden, T.; Can, A. Antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz (wild service tree) fruits. JFDA 2014, 23, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).