Harvesting of Antimicrobial Peptides from Insect (Hermetia illucens) and Its Applications in the Food Packaging
Abstract
1. Introduction
- Improves food safety by preventing the development of resistant strains of microorganisms;
- Prohibited use of some of the antimicrobial agents due to toxicological reasons;
- Distinctive mode of action of antimicrobial peptides with effective results.
2. Antimicrobial Activity of Peptides Isolated from Insects
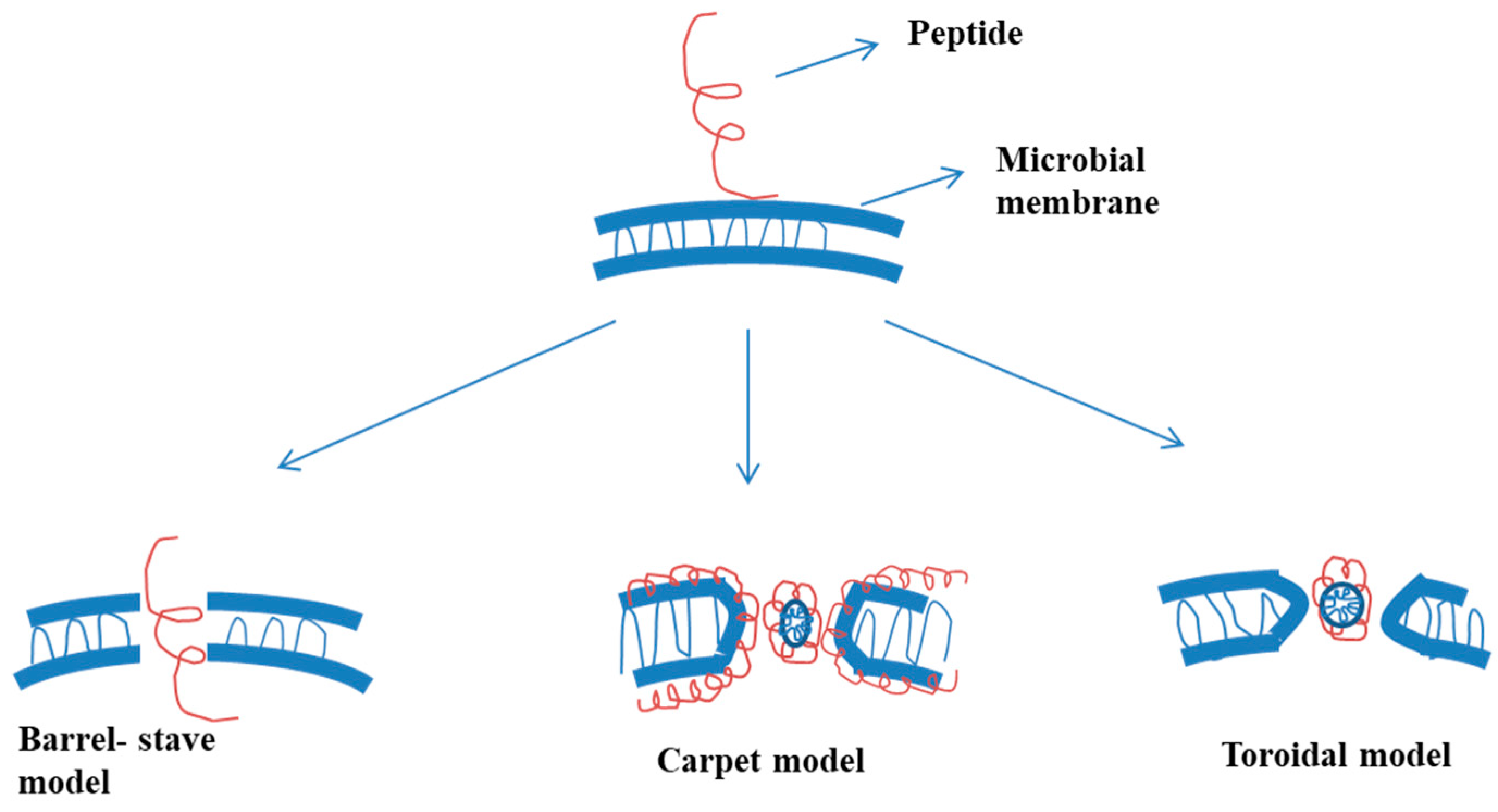
2.1. Mode of Activity against Bacteria
2.1.1. Barrel-Stave Model
2.1.2. Carpet Model
2.1.3. Toroidal Model
2.1.4. Non-Membranolytic Disruption of Bacterial Cell
2.2. Mode of Activity against Fungi
2.3. Mode of Activity against Virus
3. Categorisation of Antimicrobial Peptides
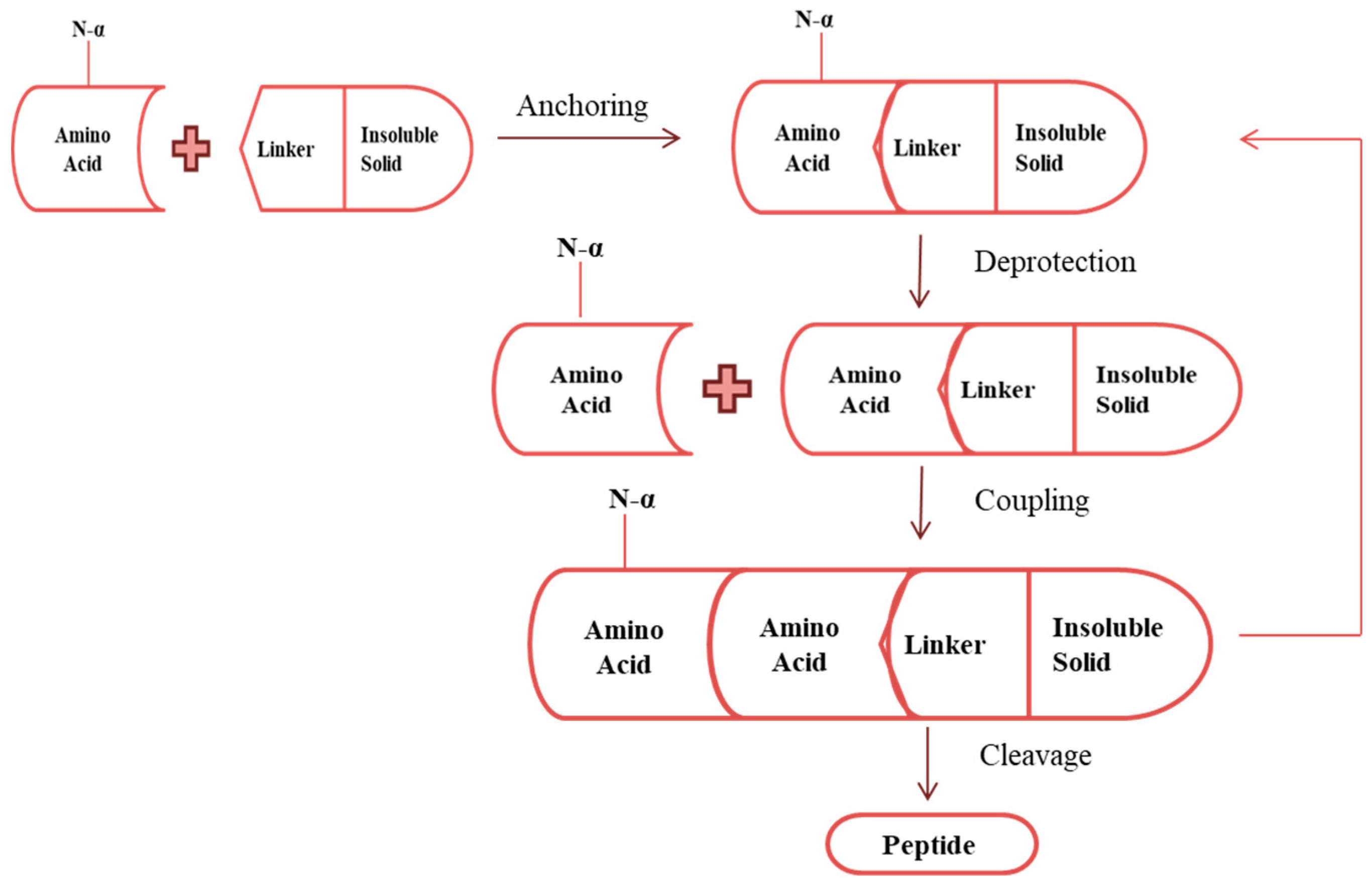
4. Synthesis of Antimicrobial Peptide
4.1. Chemical Synthesis Mechanism
4.2. Enzymatic Synthesis Mechanism
4.3. Synthesis Using Recombinant DNA Technology
5. Harvesting of Antimicrobial Peptides from Hermetia illucens
- Larva collection and rearing:
- 2.
- Incorporation of microorganism into larvae:
- 3.
- Storage conditions:
- 4.
- Sample collection:
- 5.
- Purification of isolated samples from collected sample:
- 6.
- Storage:
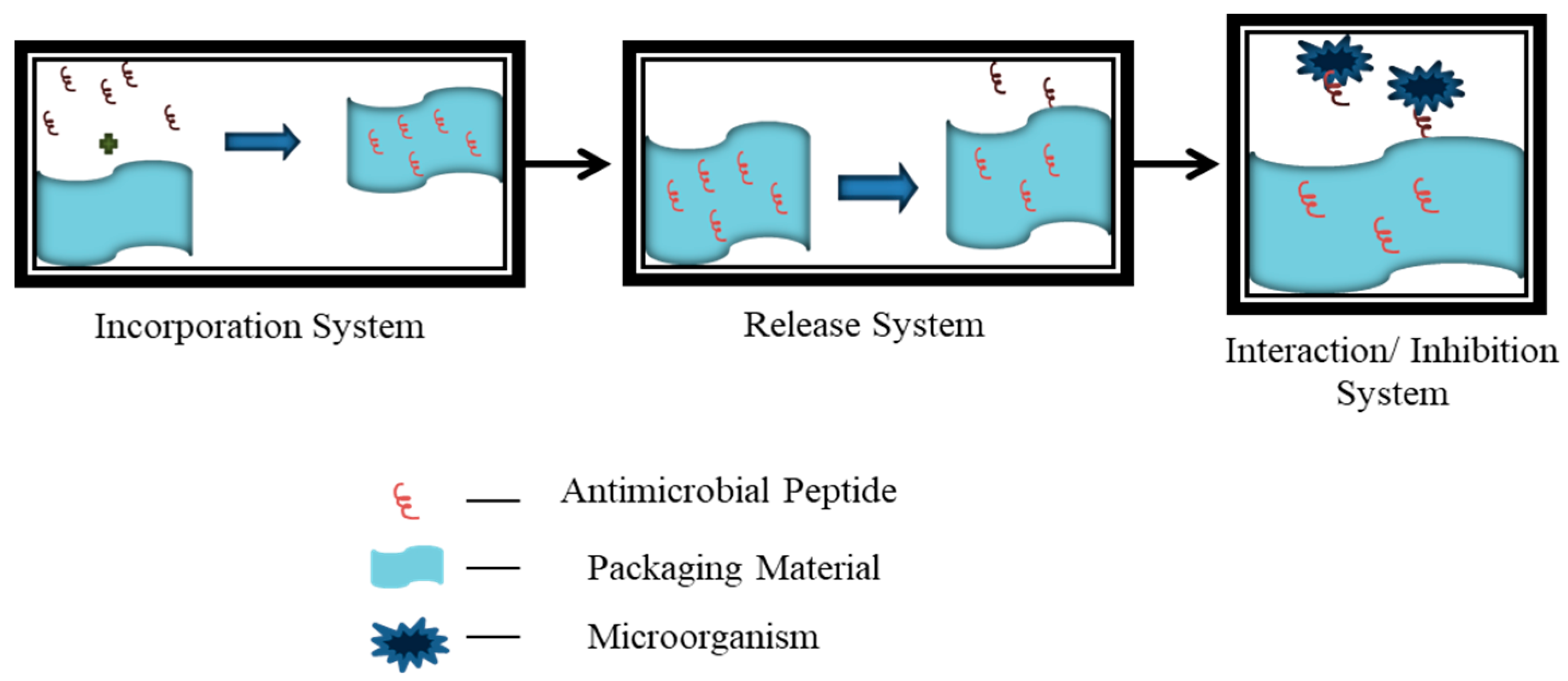
6. Applications of Antimicrobial Peptides in Active Packaging
6.1. Future Scope of AMP Derived from Insects in Food Packaging
6.2. Challenges in Incorporation of AMP into Food Packaging Material
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Martin, S.F. Adaptation in the innate immune system and heterologous innate immunity. Cell. Mol. life Sci. 2014, 71, 4115–4130. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, L.R.; Motta, A.D.; Brandelli, A. Mode of action of antimicrobial peptide P45 on Listeria monocytogenes. J. Basic Microbiol. 2008, 48, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Tonk, M.; Vilcinskas, A.; Rahnamaeian, M. Insect antimicrobial peptides: Potential tools for the prevention of skin cancer. Appl. Microbiol. Biotechnol. 2016, 100, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Nepovimova, E.; Klimova, B.; Wu, Q.; Kuca, K. Antimicrobial peptides: Amphibian host defense peptides. Curr. Med. Chem. 2019, 26, 5924–5946. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Antimicrobial activity of an extract of Hermetia illucens larvae immunized with Lactobacillus casei against Salmonella species. Insects 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Haine, E.R.; Moret, Y.; Siva-Jothy, M.T.; Rolff, J. Antimicrobial defense and persistent infection in insects. Science 2008, 322, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G.; Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Karnik, S.; Barai, R.S.; Jayaraman, V.K.; Idicula-Thomas, S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010, 38 (Suppl. 1), D774–D780. [Google Scholar] [CrossRef]
- Santos, J.C.; Sousa, R.C.; Otoni, C.G.; Moraes, A.R.; Souza, V.G.; Medeiros, E.A.; Espitia, P.J.; Pires, A.C.; Coimbra, J.S.; Soares, N.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef]
- Lu, H.L.; Leger, R.S. Insect immunity to entomopathogenic fungi. Adv. Genet. 2016, 94, 251–285. [Google Scholar]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Walsh, T.J. Antifungal peptides: Novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 1999, 43, 1–11. [Google Scholar] [CrossRef]
- Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- Chia, T.J.; Wu, Y.C.; Chen, J.Y.; Chi, S.C. Antimicrobial peptides (AMP) with antiviral activity against fish nodavirus. Fish Shellfish. Immunol. 2010, 28, 434–439. [Google Scholar] [CrossRef]
- Mohan, K.V.; Rao, S.S.; Atreya, C.D. Antiviral activity of selected antimicrobial peptides against vaccinia virus. Antivir. Res. 2010, 86, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr. Antibacterial peptides isolated from insects. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2000, 6, 497–511. [Google Scholar]
- Yaakobi, K.; Liebes-Peer, Y.; Kushmaro, A.; Rapaport, H. Designed amphiphilic β-sheet peptides as templates for paraoxon adsorption and detection. Langmuir 2013, 29, 6840–6848. [Google Scholar] [CrossRef]
- Freudenthal, O.; Quilès, F.; Francius, G. Discrepancies between cyclic and linear antimicrobial peptide actions on the spectrochemical and nanomechanical fingerprints of a young biofilm. ACS Omega 2017, 2, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Rufián-Henares, J.Á.; Martínez, R.G.; Miralles, B.; Bergillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: Activity and physicochemical property relationship of the peptide components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Podda, E.; Benincasa, M.; Pacor, S.; Micali, F.; Mattiuzzo, M.; Gennaro, R.; Scocchi, M. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 1732–1740. [Google Scholar] [CrossRef]
- Makarova, O.; Johnston, P.; Rodriguez-Rojas, A.; El Shazely, B.; Morales, J.M.; Rolff, J. Genomics of experimental adaptation of Staphylococcus aureus to a natural combination of insect antimicrobial peptides. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.I. Novel attacin from Hermetia illucens: cDNA cloning, characterization, and antibacterial properties. Prep. Biochem. Biotechnol. 2019, 49, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Improved methods for classification, prediction, and design of antimicrobial peptides. In Computational Peptidology; Humana Press: New York, NY, USA, 2015; pp. 43–66. [Google Scholar]
- Lee, J.H.; Kim, I.W.; Kim, M.; Ahn, M.Y.; Yun, E.Y.; Hwang, J.S. Antimicrobial activity of the scolopendrasin V peptide identified from the centipede Scolopendra subspinipes mutilans. J. Microbiol. Biotechnol. 2017, 27, 43–48. [Google Scholar] [CrossRef]
- Vizioli, J.; Salzet, M. Antimicrobial peptides from animals: Focus on invertebrates. Trends Pharmacol. Sci. 2002, 23, 494–496. [Google Scholar] [CrossRef]
- Li, F.F.; Brimble, M.A. Using chemical synthesis to optimise antimicrobial peptides in the fight against antimicrobial resistance. Pure Appl. Chem. 2019, 91, 181–198. [Google Scholar] [CrossRef]
- Guzmán, F.; Barberis, S.; Illanes, A. Peptide synthesis: Chemical or enzymatic. Electron. J. Biotechnol. 2007, 10, 279–314. [Google Scholar] [CrossRef]
- Perez Espitia, P.J.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Souza Cruz, R.; Alves Medeiros, E.A. Bioactive peptides: Synthesis, properties, and applications in the packaging and preservation of food. Compr. Rev. Food Sci. Food Saf. 2012, 11, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Burrill, G.S. The biotechnology industry: An engine of innovation. In Biotechnology Entrepreneurship; Academic Press: Cambridge, MA, USA, 2014; pp. 21–44. [Google Scholar]
- Cavaco, M.; Castanho, M.A.; Neves, V. Peptibodies: An elegant solution for a long-standing problem. Pept. Sci. 2018, 110, e23095. [Google Scholar] [CrossRef]
- Kent, S.B. Chemical synthesis of peptides and proteins. Annu. Rev. Biochem. 1988, 57, 957–989. [Google Scholar] [CrossRef] [PubMed]
- Borgia, J.A.; Fields, G.B. Chemical synthesis of proteins. Trends Biotechnol. 2000, 18, 243–251. [Google Scholar] [CrossRef]
- So, J.E.; Kang, S.H.; Kim, B.G. Lipase-catalyzed synthesis of peptides containing D-amino acid. Enzym. Microb. Technol. 1998, 23, 211–215. [Google Scholar] [CrossRef]
- Machado, A.; Liria, C.W.; Proti, P.B.; Remuzgo, C.; Miranda, M.T. Sínteses química e enzimática de peptídeos: Princípios básicos e aplicações. Química Nova 2004, 27, 781–789. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Frissen, A.E.; Boer, E.; van Kekem, K.; van Zoelen, D.J.; Eggen, I.F. Optimized enzymatic synthesis of C-terminal peptide amides using subtilisin A from Bacillus licheniformis. J. Mol. Catal. B Enzym. 2010, 66, 33–42. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, F.; Xin, Y.; Liu, J.; Luo, L.; Yin, Z. Expression and purification of antimicrobial peptide buforin IIb in Escherichia coli. Biotechnol. Lett. 2011, 33, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Recombinant production of antimicrobial peptides in Escherichia coli: A review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, K.H.; Nam, S.H.; Jang, B.G.; Kim, J.H.; Kim, D.W.; Yu, D.J. The effect on growth performance of chicken meat in broiler chicks by dietary supplementation of black soldier fly larvae, Hermetia illucens (Diptera: Stratmyidae). J. Sericultural Entomol. Sci. 2013, 51, 30–35. [Google Scholar] [CrossRef][Green Version]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Ren, M.; Ma, S.; Liu, X.; Chen, K.; Xia, H. Peptidoglycan recognition protein-S1 acts as a receptor to activate AMP expression through the IMD pathway in the silkworm Bombyx mori. Dev. Comp. Immunol. 2021, 115, 103903. [Google Scholar] [CrossRef]
- Nijhout, H.F. Physiological control of molting in insects. Am. Zool. 1981, 21, 631–640. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed–a review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Evaluation of the Antimicrobial Activity of an Extract of Lactobacillus casei-Infected Hermetia illucens Larvae Produced Using an Automatic Injection System. Animals 2020, 10, 2121. [Google Scholar] [CrossRef]
- Tegtmeier, D.; Hurka, S.; Klüber, P.; Brinkrolf, K.; Heise, P.; Vilcinskas, A. Cottonseed press cake as a potential diet for industrially farmed black soldier fly larvae triggers adaptations of their bacterial and fungal gut microbiota. Front. Microbiol. 2021, 12, 563. [Google Scholar] [CrossRef] [PubMed]
- Zdybicka-Barabas, A.; Bulak, P.; Polakowski, C.; Bieganowski, A.; Waśko, A.; Cytryńska, M. Immune response in the larvae of the black soldier fly Hermetia illucens. Invertebr. Surviv. J. 2017, 14, 9–17. [Google Scholar]
- Alvarez, D.; Wilkinson, K.A.; Treilhou, M.; Téné, N.; Castillo, D.; Sauvain, M. Prospecting peptides isolated from black soldier fly (Diptera: Stratiomyidae) with antimicrobial activity against Helicobacter pylori (Campylobacterales: Helicobacteraceae). J. Insect Sci. 2019, 19, 17. [Google Scholar] [CrossRef]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, expression, purification and functional characterization of novel antimicrobial peptide genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed. Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- Park, S.I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, H ermetia illucens (D iptera: S tratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Park, S.I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Harlystiarini, H.; Mutia, R.; Wibawan, I.W.; Astuti, D.A. In vitro antibacterial activity of black soldier fly (hermetia illucens) larva extracts against gram-negative bacteria. Bul. Peternak. 2019, 43. [Google Scholar] [CrossRef]
- Xu, J.; Luo, X.; Fang, G.; Zhan, S.; Wu, J.; Wang, D.; Huang, Y. Transgenic expression of antimicrobial peptides from black soldier fly enhance resistance against entomopathogenic bacteria in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2020, 127, 103487. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Calabrese, E.J. (Eds.) Hormesis: A Revolution in Biology, Toxicology and Medicine; Springer Science & Business Media: Totowa, NJ, USA, 1 December 2009. [Google Scholar]
- Wojda, I. Temperature stress and insect immunity. J. Therm. Biol. 2017, 68, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hetru, C.; Bulet, P. Strategies for the isolation and characterization of antimicrobial peptides of invertebrates. In Antibacterial Peptide Protocols; Humana Press: Totowa, NJ, USA, 1997; pp. 35–49. [Google Scholar]
- Tabunoki, H.; Dittmer, N.T.; Gorman, M.J.; Kanost, M.R. Development of a new method for collecting hemolymph and measuring phenoloxidase activity in Tribolium castaneum. BMC Res. Notes 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stawikowski, M.; Fields, G.B. Introduction to peptide synthesis. Curr. Protoc. Protein Sci. 2012, 69, 18.1.1–18.1.13. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Abreu, D.A.; Cruz, J.M.; Paseiro Losada, P. Active and intelligent packaging for the food industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Kour, H.; Wani, N.A.; Malik, A.; Kaul, R.; Chauhan, H.; Gupta, P.; Bhat, A.; Singh, J. Advances in food packaging—A review. Stewart Postharvest Rev. 2013, 9, 1–7. [Google Scholar]
- Nagarajarao, R.C. Recent advances in processing and packaging of fishery products: A review. Aquat. Procedia 2016, 7, 201–213. [Google Scholar] [CrossRef]
- Corrales, M.; Fernández, A.; Han, J.H. Antimicrobial packaging systems. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2014; pp. 133–170. [Google Scholar]
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Marvdashti, L.M.; Yavarmanesh, M.; Koocheki, A. Controlled release of nisin from polyvinyl alcohol-Alyssum homolocarpum seed gum composite films: Nisin kinetics. Food Biosci. 2019, 28, 133–139. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioğlu, A.; Altınkaya, S.A. Development of cellulose acetate based antimicrobial food packaging materials for controlled release of lysozyme. J. Food Eng. 2009, 90, 453–462. [Google Scholar] [CrossRef]
- Seo, H.S.; Bang, J.; Kim, H.; Beuchat, L.R.; Cho, S.Y.; Ryu, J.H. Development of an antimicrobial sachet containing encapsulated allyl isothiocyanate to inactivate Escherichia coli O157: H7 on spinach leaves. Int. J. Food Microbiol. 2012, 159, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef]
- Lee, T.H.; NHall, K.; Aguilar, M.I. Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef]
- Agrillo, B.; Balestrieri, M.; Gogliettino, M.; Palmieri, G.; Moretta, R.; Proroga, Y.T.; Rea, I.; Cornacchia, A.; Capuano, F.; Smaldone, G.; et al. Functionalized polymeric materials with bio-derived antimicrobial peptides for “active” packaging. Int. J. Mol. Sci. 2019, 20, 601. [Google Scholar] [CrossRef]
- Blanco Massani, M.; Fernandez, M.R.; Ariosti, A.; Eisenberg, P.; Vignolo, G. Development and characterization of an active polyethylene film containing Lactobacillus curvatus CRL705 bacteriocins. Food Addit. Contam. 2008, 25, 1424–1430. [Google Scholar] [CrossRef]
- Gogliettino, M.; Balestrieri, M.; Ambrosio, R.L.; Anastasio, A.; Smaldone, G.; Proroga, Y.T.; Moretta, R.; Rea, I.; De Stefano, L.; Agrillo, B.; et al. Extending the shelf-life of meat and dairy products via PET-modified packaging activated with the antimicrobial peptide MTP1. Front. Microbiol. 2020, 10, 2963. [Google Scholar] [CrossRef]
- Gomes, P.D.; Fernandes, M.H. Defensins in the oral cavity: Distribution and biological role. J. Oral Pathol. Med. 2010, 39, 1–9. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Viticchi, C.; Mocchegiani, F.; Riva, A.; Saba, V.; Scalise, G. Effect of mono-dose intraperitoneal cecropins in experimental septic shock. Crit. Care Med. 2001, 29, 1666–1669. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Liu, Z.; Liu, G.; Souffrant, W.B.; Yin, Y. Effect of lactoferrincin B and cecropin P1 against enterotoxigenic Escherichia coli in vitro. J. Food Agric. Environ. 2011, 9, 271–274. [Google Scholar]
- Carlsson, A.; Nyström, T.; de Cock, H.; Bennich, H. Attacin-an insect immune protein-binds LPS and triggers the specific inhibition of bacterial outer-membrane protein synthesis. Microbiology 1998, 144, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Bulet, P.; Dimarcq, J.L.; Hetru, C.; Lagueux, M.; Charlet, M.; Hegy, G.; Van Dorsselaer, A.; Hoffmann, J.A. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J. Biol. Chem. 1993, 268, 14893–14897. [Google Scholar] [CrossRef]
- Keppi, E.; Pugsley, A.P.; Lambert, J.; Wicker, C.; Dimarcq, J.L.; Hoffmann, J.A.; Hoffmann, D. Mode of action of diptericin A, a bactericidal peptide induced in the hemolymph of Phormia terranovae larvae. Arch. Insect Biochem. Physiol. 1989, 10, 229–239. [Google Scholar] [CrossRef]
- Blancard, D.; Laterrot, H.; Marchoux, G.; Candresse, T. 2-Diagnosis of parasitic and nonparasitic diseases. Tomato Dis. 2012, 35–411. [Google Scholar] [CrossRef]
- Levashina, E.A.; Ohresser, S.; Bulet, P.; Reichhart, J.M.; Hetru, C.; Hoffmann, J.A. Metchnikowin, a novel immune-inducible proline-rich peptide from Drosophila with antibacterial and antifungal properties. Eur. J. Biochem. 1995, 233, 694–700. [Google Scholar] [CrossRef]
- Shi, W.; Li, C.; Li, M.; Zong, X.; Han, D.; Chen, Y. Antimicrobial peptide melittin against Xanthomonas oryzae pv. oryzae, the bacterial leaf blight pathogen in rice. Appl. Microbiol. Biotechnol. 2016, 100, 5059–5067. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Saito, T.; Ohmura, T.; Kuroda, K.; Suita, K.; Ihara, K.; Isogai, E. Functional structure and antimicrobial activity of persulcatusin, an antimicrobial peptide from the hard tick Ixodes persulcatus. Parasites Vectors 2016, 9, 1–11. [Google Scholar] [CrossRef]
- da Silva, A.C.; Rodrigues, M.X.; Silva, N.C. Methicillin-resistant Staphylococcus aureus in food and the prevalence in Brazil: A review. Braz. J. Microbiol. 2020, 51, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Karanis, P. An overview of methods/techniques for the detection of Cryptosporidium in food samples. Parasitol. Res. 2018, 117, 629–653. [Google Scholar] [CrossRef]
- Boxell, A.; Lee, S.H.; Jefferies, R.; Watt, P.; Hopkins, R.; Reid, S.; Armson, A.; Ryan, U. Pyrrhocoricin as a potential drug delivery vehicle for Cryptosporidium parvum. Exp. Parasitol. 2008, 119, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Hashemi, S.M.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Xue, Y.; Ding, T.; Zhu, S.; Mao, M.; Zhang, L.; Han, Y. Polymer brush grafted antimicrobial peptide on hydroxyapatite nanorods for highly effective antibacterial performance. Chem. Eng. J. 2021, 423, 130133. [Google Scholar] [CrossRef]
- Ma, Y.N.; Ma, L.; Jiang, Y. Interpretation of the guideline of compatibility study of pharmaceutical products and packaging materials the assessment of experimental data. Chin. J. New Drugs 2014, 28, 940–943. [Google Scholar]
- Qian, Q.J.; Zhao, X.J.; Ma, Q.H.; Jiang, J.M.; Wei, R.; Chen, G.; Fu, L.F. Study on compatibility in between packaging materials and Haemophilus inflfluenzae type b conjugate vaccine. Prog. Microbiol. Immunol. 2012, 5, 12. [Google Scholar]
- Jones, A. Killer plastics: Antimicrobial additives for polymers. Plast. Eng. 2008, 64, 34–40. [Google Scholar] [CrossRef]
- Sierra, J.M.; Viñas, M. Future prospects for Antimicrobial peptide development: Peptidomimetics and antimicrobial combinations. Expert Opin. Drug Discov. 2021, 601–604. [Google Scholar] [CrossRef] [PubMed]




| S.No. | Criteria for Classification | Peptides | Description | Examples | References |
|---|---|---|---|---|---|
| 1. | Structure | α helical peptide | Intramolecular disulphide bridge is formed by the cysteine | Cecropins | [27] |
| Cysteine rich peptide | Peptides with cysteine residues | Defensis | [10] | ||
| Glycine rich peptide | Consists of 14% to 22% glycine residues | Attacins | [34] | ||
| Proline rich peptide | Composed of 14–39 amino acids and contains proline residues | Drosocins | [10] | ||
| β sheet peptide | Consist of a disulphide bond, which helps in stabilizing the conformation | Defensis | [35] | ||
| 2. | Mode of action | Membranolytic | These peptides enter the microbial cell wall by disruption | Scolopendin 2 | [36] |
| Non- membranolytic | Peptide that enters the cell by endocytosis | Scolopendin 1 | [36] | ||
| 3. | Electrostatic charge | Cationic | Peptide with positive charge | Cecropins | [37] |
| Non- cationic | Peptides with negative charge and isolated from mammalian epithelia | Enkelytin | [37] |
| S.No. | Type of Synthesis | Advantages | Challenges | References |
|---|---|---|---|---|
| 1. | Chemical | Easy separation from side products and impure compounds. | Toxic byproducts and low yields. | [38,39] |
| 2. | Enzymatic | Helpful in synthesis of short chain peptides. It also has good stereo selectivity. | It becomes challenging while synthesis of long chain peptides. Low productivity and high cost of catalyst. | [39,40] |
| 3. | Recombinant DNA Technology | Convenient for large scale production. | Takes more time due to lengthy process. | [41,42] |
| S.No. | Source | Peptide | Harvesting Technique | Microorganisms Inhibited | References |
|---|---|---|---|---|---|
| 1. | Haemolymph | Solid phase extraction | Helicobacter pylori | [58] | |
| 2. | Crushed larva | stomoxynZH1 | RNA extraction using Trizol | S. aureus, E. coli, Rhizoctonia solani and Sclerotinia sclerotiorum | [59] |
| 3. | Grounded larvae | Maceration | E. coli, P. fluorescens, M. luteus and B. subtilis | [32] | |
| 4. | Larvae | Directly used as feed for piglets | Lactobacilli, D-streptococci | [60] | |
| 5. | Lyophilized larvae | Homogenised and extracted with acidic methanol | Methicillin resistant Staphylococcus aureus | [61] | |
| 6. | Haemolymph | cecropin-like peptide 1 | Solid-phase extraction and reverse-phase chromatography | E. coli, Enterobacter aerogens and Pseudomonas areuginosa | [62] |
| 7. | Grounded larva | Maceration using methanol | Salmonella and E. coli | [63] | |
| 8. | Larvae whose digestive tract was removed. Followed by treating with liquid nitrogen | Hidefensin-1, Hidiptericin-1 and HiCG13551 | TRIeasy- RNA isolation kit | Streptococcus pneumonia, E. coli and Staphylococcus aureus | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, A.; Luo, H.; Ramakrishna, S. Harvesting of Antimicrobial Peptides from Insect (Hermetia illucens) and Its Applications in the Food Packaging. Appl. Sci. 2021, 11, 6991. https://doi.org/10.3390/app11156991
Sultana A, Luo H, Ramakrishna S. Harvesting of Antimicrobial Peptides from Insect (Hermetia illucens) and Its Applications in the Food Packaging. Applied Sciences. 2021; 11(15):6991. https://doi.org/10.3390/app11156991
Chicago/Turabian StyleSultana, Afreen, Hongrong Luo, and Seeram Ramakrishna. 2021. "Harvesting of Antimicrobial Peptides from Insect (Hermetia illucens) and Its Applications in the Food Packaging" Applied Sciences 11, no. 15: 6991. https://doi.org/10.3390/app11156991
APA StyleSultana, A., Luo, H., & Ramakrishna, S. (2021). Harvesting of Antimicrobial Peptides from Insect (Hermetia illucens) and Its Applications in the Food Packaging. Applied Sciences, 11(15), 6991. https://doi.org/10.3390/app11156991







