Identification of Novel Inhibitors of Type-I Mycobacterium Tuberculosis Fatty Acid Synthase Using Docking-Based Virtual Screening and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ligand Library Preparation
2.2. Docking-Based Screening
2.3. Molecular Dynamics Simulation
2.4. MM/PBSA-Based Protein–Ligand Interaction Energy Calculation
3. Results
3.1. Structural Model of NADPH-Bound Mtb FAS-I
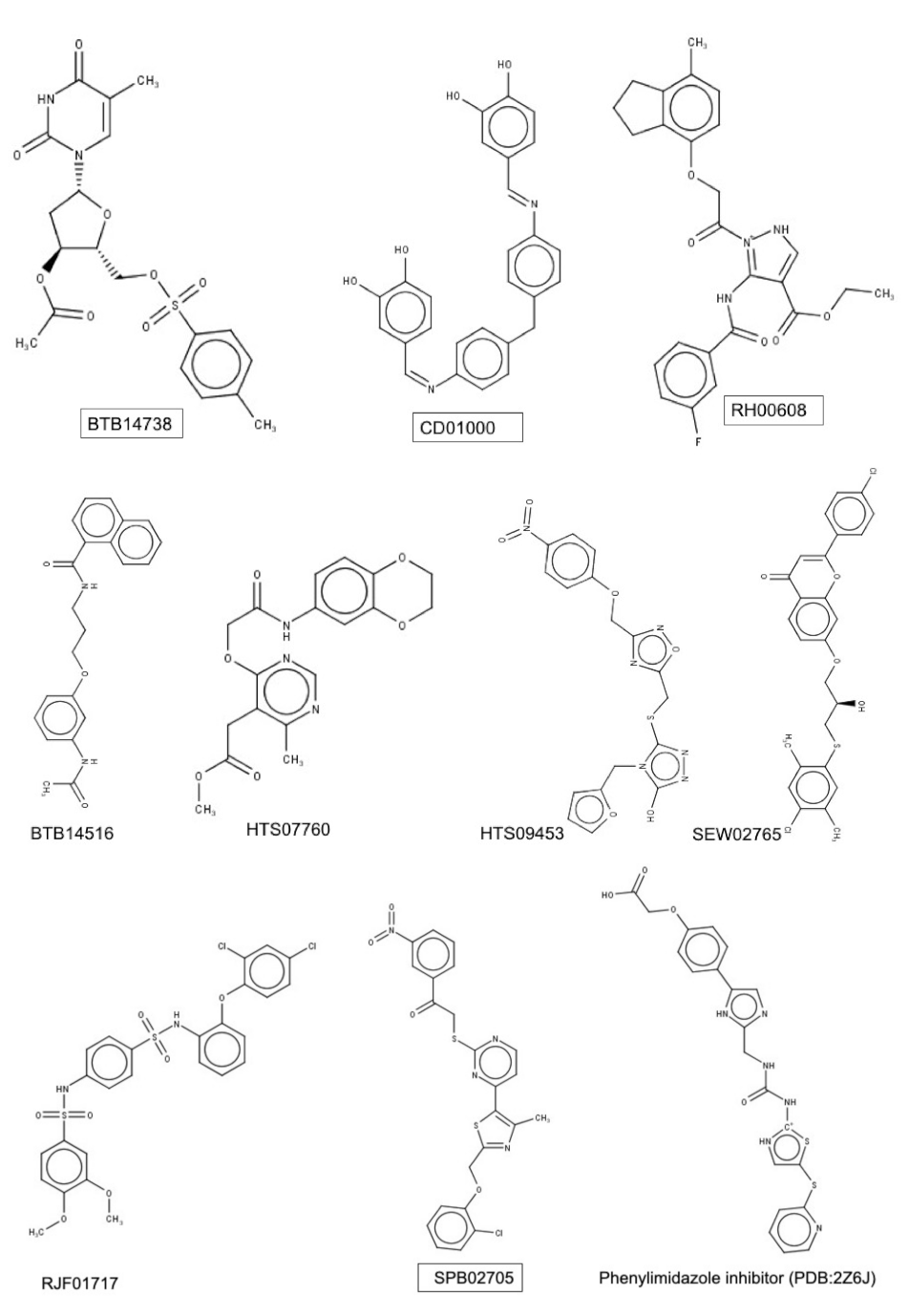
3.2. Docking-Based Screening
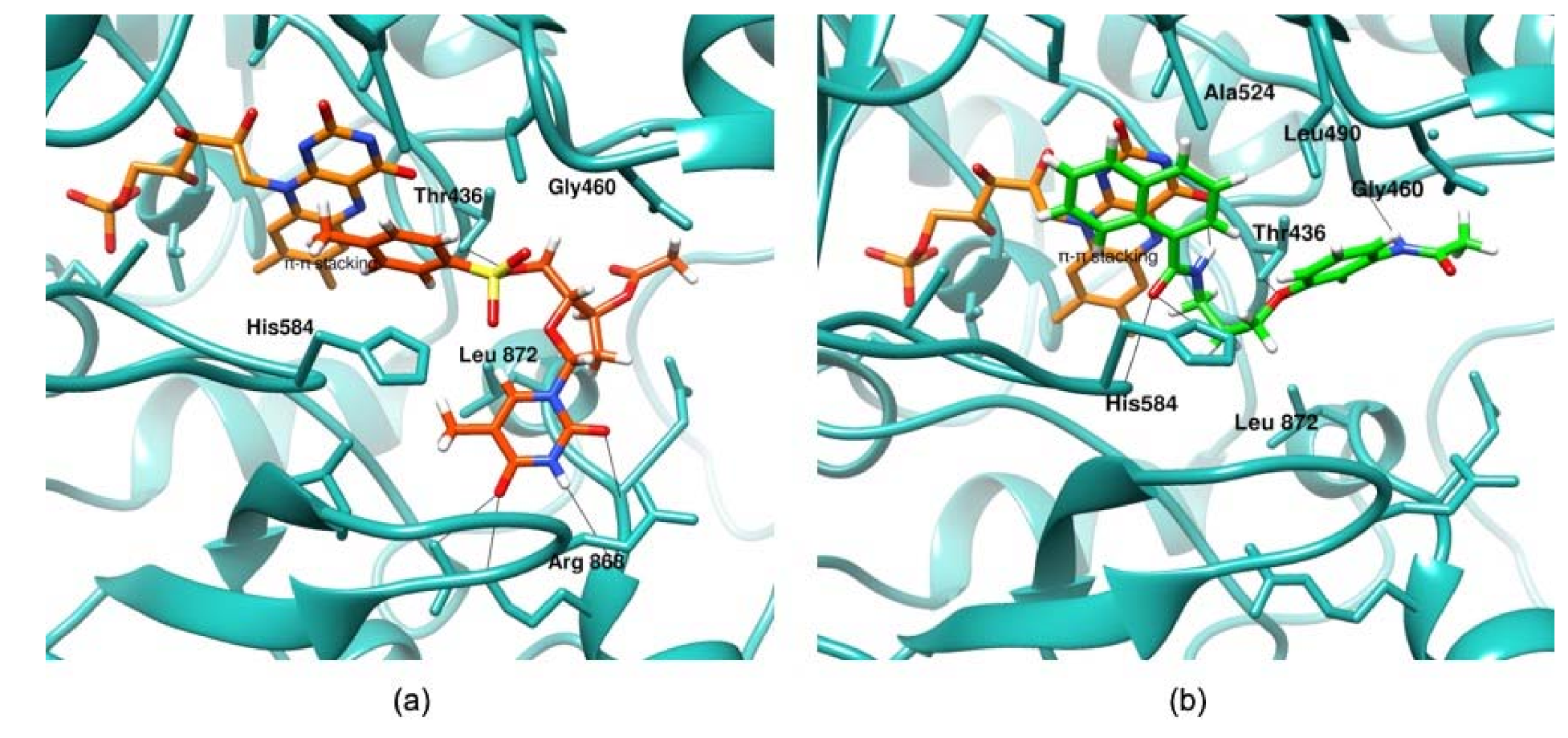
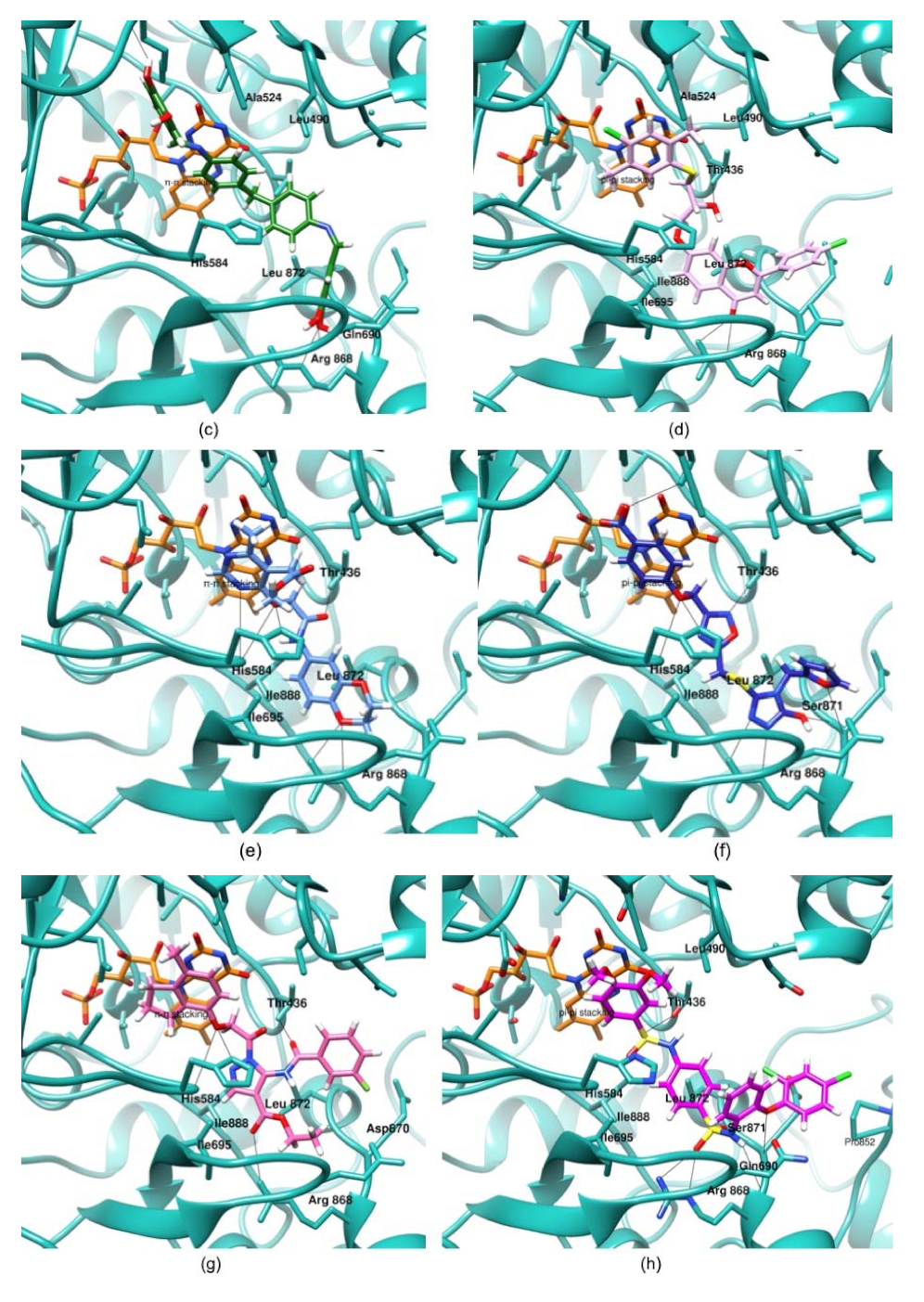
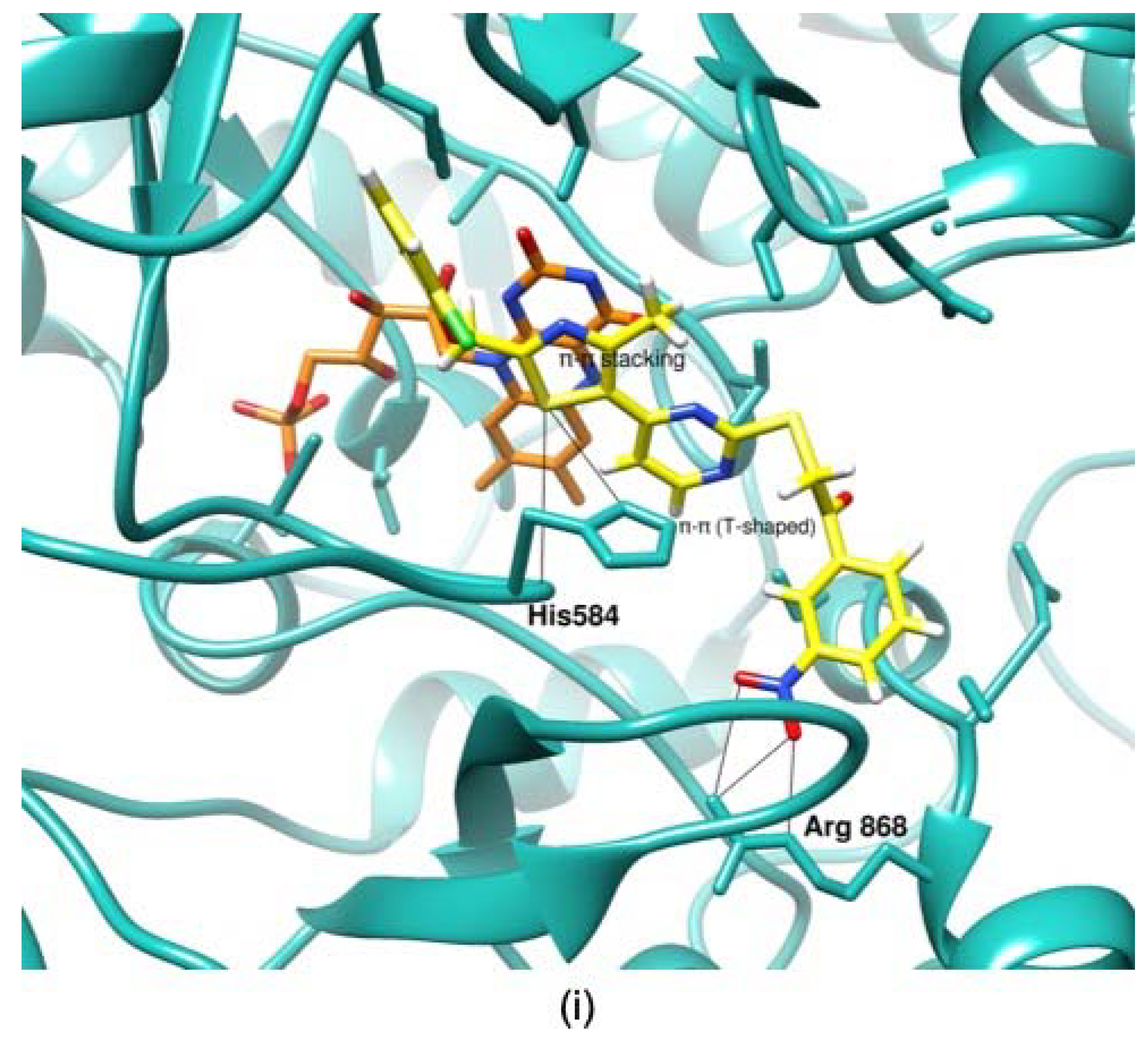
3.3. Molecular Docking and Proposed Mode of Binding of Putative Hits
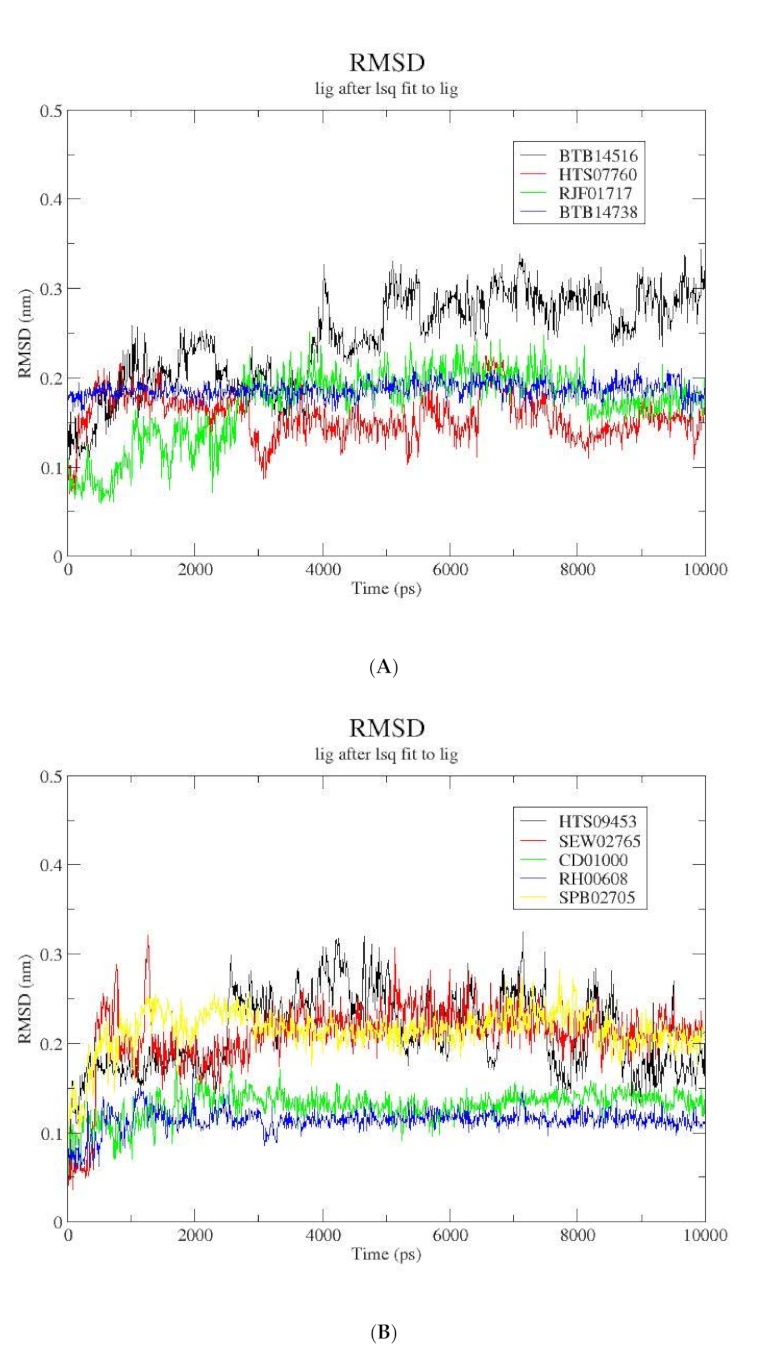
3.4. Molecular Dynamics Simulation
3.5. MM/PBSA-Based Interaction Energy Calculation
3.6. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Factsheet. 2019. Available online: https://www.who.int/tb/publications/factsheet_global.pdf?ua=1 (accessed on 22 October 2020).
- WHO. News. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 20 October 2020).
- Glickman, M.S.; Cox, J.S.; Jacobs, W.R. A Novel Mycolic Acid Cyclopropane Synthetase Is Required for Cording, Persistence, and Virulence of Mycobacterium tuberculosis. Mol. Cell 2000, 5, 717–727. [Google Scholar] [CrossRef]
- Barkan, D.; Liu, Z.; Sacchettini, J.C.; Glickman, M.S. Mycolic Acid Cyclopropanation is Essential for Viability, Drug Resistance, and Cell Wall Integrity of Mycobacterium tuberculosis. Chem. Biol. 2009, 16, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, V.; Varela, C.; Javid, A.; Singh, A.; Besra, G.S.; Bhatt, A. Mycolic acids: Deciphering and targeting the Achilles’ heel of the tubercle bacillus. Mol. Microbiol. 2015, 98, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Wang, C.; Besra, G.S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculo-sis. Clin. Microbiol. Rev. 2005, 18, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Molle, V.; Besra, G.S.; Jacobs, W.R., Jr.; Kremer, L. The Mycobacterium tuberculosis FAS-II condensing enzymes: Their role in mycolic acid biosynthesis, ac-id-fastness, pathogenesis and in future drug development. Mol. Microbiol. 2007, 64, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.P.; Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, E.; Hofmann, J. Microbial type I fatty acid synthases (FAS): Major players in a network of cellular FAS sys-tems. Microbiol. Mol. Biol. Rev. 2004, 68, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Zignol, M.; Blades, N.J.; Geiman, D.E.; Dougherty, A.; Grosset, J.; Broman, K.W.; Bishai, W.R. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: Applica-tion to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2003, 100, 7213–7218. [Google Scholar] [CrossRef]
- Sassetti, C.M.; Boyd, D.H.; Rubin, E.J. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl. Acad. Sci. USA 2001, 98, 12712–12717. [Google Scholar] [CrossRef]
- Ma, Z.; Lienhardt, C.; McIlleron, H.; Nunn, A.; Wang, X. Global tuberculosis drug development pipeline: The need and the reality. Lancet 2010, 375, 2100–2109. [Google Scholar] [CrossRef]
- Steele, M.A.; Prez, R.M.D. The Role of Pyrazinamide in Tuberculosis Chemotherapy. Chest 1988, 94, 845–850. [Google Scholar] [CrossRef]
- Sayahi, H.; Pugliese, K.M.; Zimhony, O.; Jacobs, W.R.; Shekhtman, A.; Welch, J.T. Analogs of the Antituberculous Agent Pyrazinamide Are Competitive Inhibitors of NADPH Binding to M. tuberculosis Fatty Acid Synthase I. Chem. Biodivers. 2012, 9, 2582–2596. [Google Scholar] [CrossRef]
- Zimhony, O.; Cox, J.S.; Welch, J.T.; Vilchèze, C.; Jacobs, W.R., Jr. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 2000, 6, 1043–1047. [Google Scholar] [CrossRef]
- Elad, N.; Baron, S.; Peleg, Y.; Albeck, S.; Grunwald, J.; Raviv, G.; Shakked, Z.; Zimhony, O.; Diskin, R. Structure of Type-I Mycobacterium tuberculosis fatty acid synthase at 3.3 Å resolution. Nat. Commun. 2018, 9, 3886. [Google Scholar] [CrossRef]
- Maia, E.H.B.; Assis, L.C.; De Oliveira, T.A.; Da Silva, A.M.; Taranto, A.G. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front. Chem. 2020, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Tiwari, S.; Srivastava, K.K.; Siddiqi, M.I. Identification of Novel Inhibitors of Mycobacterium tuberculosis PknG Using Pharmacophore Based Virtual Screening, Docking, Molecular Dynamics Simulation, and Their Biological Evaluation. J. Chem. Inf. Model. 2015, 55, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Siddiqi, M.I.; Miertus, S. New molecular scaffolds for the design of Mycobacterium tuberculosis type II dehy-droquinase inhibitors identified using ligand and receptor based virtual screening. J. Mol. Model. 2010, 16, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Sobarzo-Sanchez, E.; Santana, L.; Uriarte, E. Molecular Docking and Drug Discovery in β-Adrenergic Receptors. Curr. Med. Chem. 2017, 24, 4340–4359. [Google Scholar] [CrossRef] [PubMed]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-Based Virtual Screening for Drug Discovery: Principles, Applications and Recent Advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, M.-B.; Chen, Z.-J.; Chen, H.; Lin, J.-P.; Yang, L.-R. Fragment-based drug discovery and molecular docking in drug design. Curr. Pharm. Biotechnol. 2015, 16, 11–25. [Google Scholar] [CrossRef]
- Moro, W.B.; Yang, Z.; Kane, T.A.; Brouillette, C.G.; Brouillette, W.J. Virtual screening to identify lead inhibitors for bacterial NAD synthetase (NADs). Bioorg. Med. Chem. Lett. 2009, 19, 2001–2005. [Google Scholar] [CrossRef][Green Version]
- Mishra, A.K.; Singh, N.; Agnihotri, P.; Mishra, S.; Singh, S.P.; Kolli, B.K.; Chang, K.P.; Sahasrabuddhe, A.A.; Siddiqi, M.I.; Pratap, J.V. Discovery of novel inhibitors for Leishmania nucleoside diphosphatase kinase (NDK) based on its struc-tural and functional characterization. J. Comput. Aided. Mol. Des. 2017, 31, 547–562. [Google Scholar] [CrossRef]
- Lee, Y.-V.; Choi, S.B.; Wahab, H.A.; Lim, T.S.; Choong, Y.S. Applications of Ensemble Docking in Potential Inhibitor Screening forMycobacterium tuberculosisIsocitrate Lyase Using a Local Plant Database. J. Chem. Inf. Model. 2019, 59, 2487–2495. [Google Scholar] [CrossRef]
- Kwofie, S.K.; Adobor, C.; Quansah, E.; Bentil, J.; Ampadu, M.; Miller, W.A.; Wilson, M.D. Molecular docking and dynamics simulations studies of OmpATb identifies four potential novel natural product-derived anti-Mycobacterium tuberculosis compounds. Comput. Biol. Med. 2020, 122, 103811. [Google Scholar] [CrossRef]
- Zhao, W.; Xiong, M.; Yuan, X.; Li, M.; Sun, H.; Xu, Y. In Silico Screening-Based Discovery of Novel Inhibitors of Human Cyclic GMP–AMP Synthase: A Cross-Validation Study of Molecular Docking and Experimental Testing. J. Chem. Inf. Model. 2020, 60, 3265–3276. [Google Scholar] [CrossRef]
- Newton, A.S.; Faver, J.C.; Micevic, G.; Muthusamy, V.; Kudalkar, S.N.; Bertoletti, N.; Anderson, K.S.; Bosenberg, M.W.; Jorgensen, W.L. Structure-Guided Identification of DNMT3B Inhibitors. ACS Med. Chem. Lett. 2020, 11, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Jiménez, L.K.; Paz-González, A.D.; Juárez-Saldivar, A.; Uhrig, M.L.; Agusti, R.; Reyes-Arellano, A.; Nogueda-Torres, B.; Rivera, G. Structure-Based Virtual Screening of New Benzoic Acid Derivatives as Trypanosoma cruzi Trans-sialidase Inhibitors. Med. Chem. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, A.; Somvanshi, P.; Singh, A.; Khan, A.U. Structure-Based Screening of Non-β-Lactam Inhibitors against Class D β-Lactamases: An Approach of Docking and Molecular Dynamics. ACS Omega 2020, 5, 9356–9365. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Granchi, C.; Rizzolio, F.; Tuccinardi, T. Application of MM-PBSA Methods in Virtual Screening. Molecules 2020, 25, 1971. [Google Scholar] [CrossRef] [PubMed]
- Maybridge Library. Available online: http://www.maybridge.com/ (accessed on 25 December 2020).
- Jain, A.N. Surflex: Fully Automatic Flexible Molecular Docking Using a Molecular Similarity-Based Search Engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS—A message-passing parallel molecu-lar-dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.E.M.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2009, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D. Automation of the CHARMM General Force Field (CGenFF) II: As-signment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Available online: http://plasma-gate.weizmann.ac.il/Grace/ (accessed on 12 April 2020).
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the molecular mechanics/Poisson Boltzmann surface area and molecular me-chanics/generalized Born surface area methods. II. The accuracy of ranking poses generated from docking. J. Comput. Chem. 2011, 32, 866–877. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. g_mmpbsa—A GROMACS tool for high-throughput MM/PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Hall, B.A.; Kenway, O.A.; Jha, S.; Coveney, P.V. Computing Clinically Relevant Binding Free Energies of HIV-1 Protease Inhibitors. J. Chem. Theory Comput. 2014, 10, 1228–1241. [Google Scholar] [CrossRef]
- Xu, L.; Sun, H.; Li, Y.; Wang, J.; Hou, T. Assessing the Performance of MM/PBSA and MM/GBSA Methods. The Impact of Force Fields and Ligand Charge Models. J. Phys. Chem. B 2013, 117, 8408–8421. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson−Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Levy, R.M.; Zhang, L.Y.; Gallicchio, E.; Felts, A.K. On the nonpolar hydration free energy of proteins: Surface area and continuum solvent models for the so-lute-solvent interaction energy. J. Am. Chem. Soc. 2003, 125, 9523–9530. [Google Scholar] [CrossRef]
- Tan, C.; Tan, Y.-H.; Luo, R. Implicit Nonpolar Solvent Models. J. Phys. Chem. B 2007, 111, 12263–12274. [Google Scholar] [CrossRef]
- Jenni, S.; Leibundgut, M.; Boehringer, D.; Frick, C.; Mikolásek, B.; Ban, N. Structure of Fungal Fatty Acid Synthase and Implications for Iterative Substrate Shuttling. Science 2007, 316, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Botelho, F.D.; Gonçalves, A.S.; França, T.C.; LaPlante, S.R.; de Almeida, J.S. Identification of novel potential ricin inhibitors by virtual screening, molecular docking, molecular dy-namics and MM/PBSA calculations: A drug repurposing approach. J. Biomol. Struct. Dyn. 2021. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Laurini, E.; Col, V.D.; Mamolo, M.G.; Zampieri, D.; Posocco, P.; Fermeglia, M.; Vio, L.; Pricl, S. Homology Model and Docking-Based Virtual Screening for Ligands of the σ1 Receptor. ACS Med. Chem. Lett. 2011, 2, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Venken, T.; Krnavek, D.; Münch, J.; Kirchhoff, F.; Henklein, P.; De Maeyer, M.; Voet, A. An optimized MM/PBSA virtual screening approach applied to an HIV-1 gp41 fusion peptide inhibitor. Proteins Struct. Funct. Bioinform. 2011, 79, 3221–3235. [Google Scholar] [CrossRef]
- Saito, J.; Yamada, M.; Watanabe, T.; Iida, M.; Kitagawa, H.; Takahata, S.; Ozawa, T.; Takeuchi, Y.; Ohsawa, F. Crystal structure of enoyl-acyl carrier protein reductase (FabK) from Streptococcus pneumoniae reveals the binding mode of an inhibitor. Protein. Sci. 2008, 17, 691–699. [Google Scholar] [CrossRef]
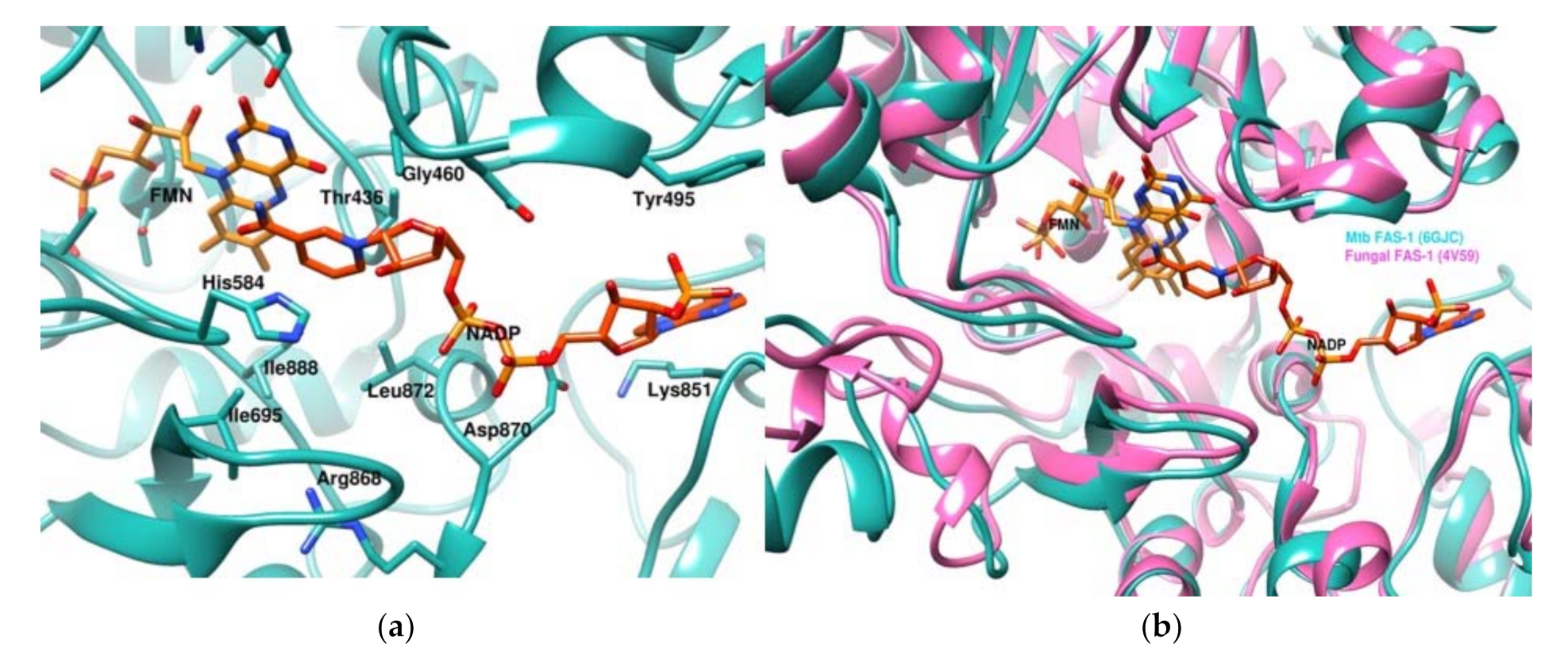

| NADPH Binding Site Residues from Thermomyces Lanuginosus (PDB Code: 4V59) | NADPH Binding Site Residues from Mtb (PDB Code: 6GJC) Obtained by Docking |
|---|---|
| Tyr636 | Tyr495 |
| Asp668 | - |
| Asp952 | - |
| Lys1026 | Lys851 |
| Pro1027 | Pro852 |
| Asp1045 | Asp870 |
| Ser1046 | Ser871 |
| Lys1044 | Ser869 |
| Leu1047 | Leu872 |
| Thr609 | Thr436 |
| Ile663 | Leu490 |
| Gly749 | Gly582 |
| Gly750 | Gly583 |
| His751 | His584 |
| Glu863 | - |
| Serial Number | Compound ID | Predicted Binding Free Energy (KJ/mol) | Molecular Docking Score |
|---|---|---|---|
| 1. | BTB14738 | −72.27 ± 12.63 | 9.55 |
| 2. | RH00608 | −68.06 ± 11.80 | 10.80 |
| 3. | SPB02705 | −63.57 ± 12.22 | 9.30 |
| 4. | HTS07760 | −53.17 ± 12.68 | 9.28 |
| 5. | CD01000 | −51.28 ± 13.74 | 10.41 |
| 6. | BTB14516 | −48.91 ± 11.37 | 10.01 |
| 7. | RJF01717 | −44.82 ± 15.77 | 10.18 |
| 8. | HTS09453 | −43.30 ± 14.27 | 10.46 |
| 9. | SEW02765 | −16.44 ± 13.22 | 9.75 |
| Compound Code | Van der Waals Energy | Electrostatic Energy | Polar Solvation Energy | SASA Energy | Binding Free Energy |
|---|---|---|---|---|---|
| BTB14738 | −165.92 ± 11.998 | −33.77 ± 6.45 | 146.82 ± 13.80 | −19.39 ± 0.97 | −72.27 ± 12.63 |
| RH00608 | −219.005 ± 10.53 | −14.61 ± 6.551 | 190.10 ± 10.54 | −24.55 ± 1.22 | −68.06 ± 11.80 |
| SPB02705 | −183.88 ± 16.99 | −2.59 ± 10.16 | 145.40 ± 21.44 | −22.50 ± 1.59 | −63.57 ± 12.22 |
| CD01000 | −201.98 ± 15.30 | −35.31 ± 9.28 | 209.04 ± 20.93 | −23.04 ± 1.60 | −51.28 ± 13.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Mao, S.-Q.; Li, W. Identification of Novel Inhibitors of Type-I Mycobacterium Tuberculosis Fatty Acid Synthase Using Docking-Based Virtual Screening and Molecular Dynamics Simulation. Appl. Sci. 2021, 11, 6977. https://doi.org/10.3390/app11156977
Singh N, Mao S-Q, Li W. Identification of Novel Inhibitors of Type-I Mycobacterium Tuberculosis Fatty Acid Synthase Using Docking-Based Virtual Screening and Molecular Dynamics Simulation. Applied Sciences. 2021; 11(15):6977. https://doi.org/10.3390/app11156977
Chicago/Turabian StyleSingh, Nidhi, Shi-Qing Mao, and Wenjin Li. 2021. "Identification of Novel Inhibitors of Type-I Mycobacterium Tuberculosis Fatty Acid Synthase Using Docking-Based Virtual Screening and Molecular Dynamics Simulation" Applied Sciences 11, no. 15: 6977. https://doi.org/10.3390/app11156977
APA StyleSingh, N., Mao, S.-Q., & Li, W. (2021). Identification of Novel Inhibitors of Type-I Mycobacterium Tuberculosis Fatty Acid Synthase Using Docking-Based Virtual Screening and Molecular Dynamics Simulation. Applied Sciences, 11(15), 6977. https://doi.org/10.3390/app11156977







