Optimization of Computed Tomography Angiography Protocols for Follow-Up Type B Aortic Dissection Patients by Using 3D Printed Model
Abstract
:1. Introduction
2. Materials and Methods
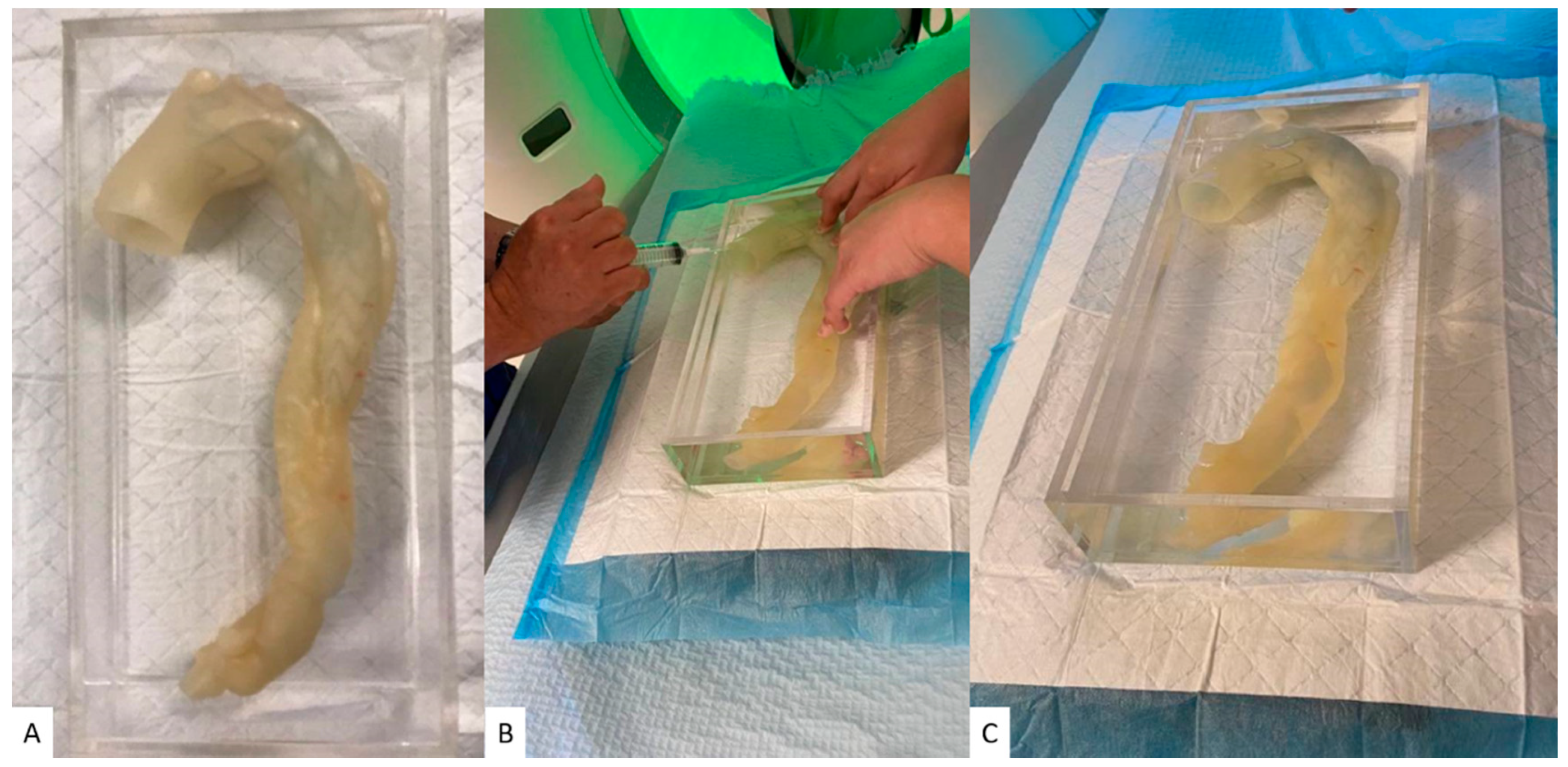
2.1. Selection of Sample Case and Segmentation
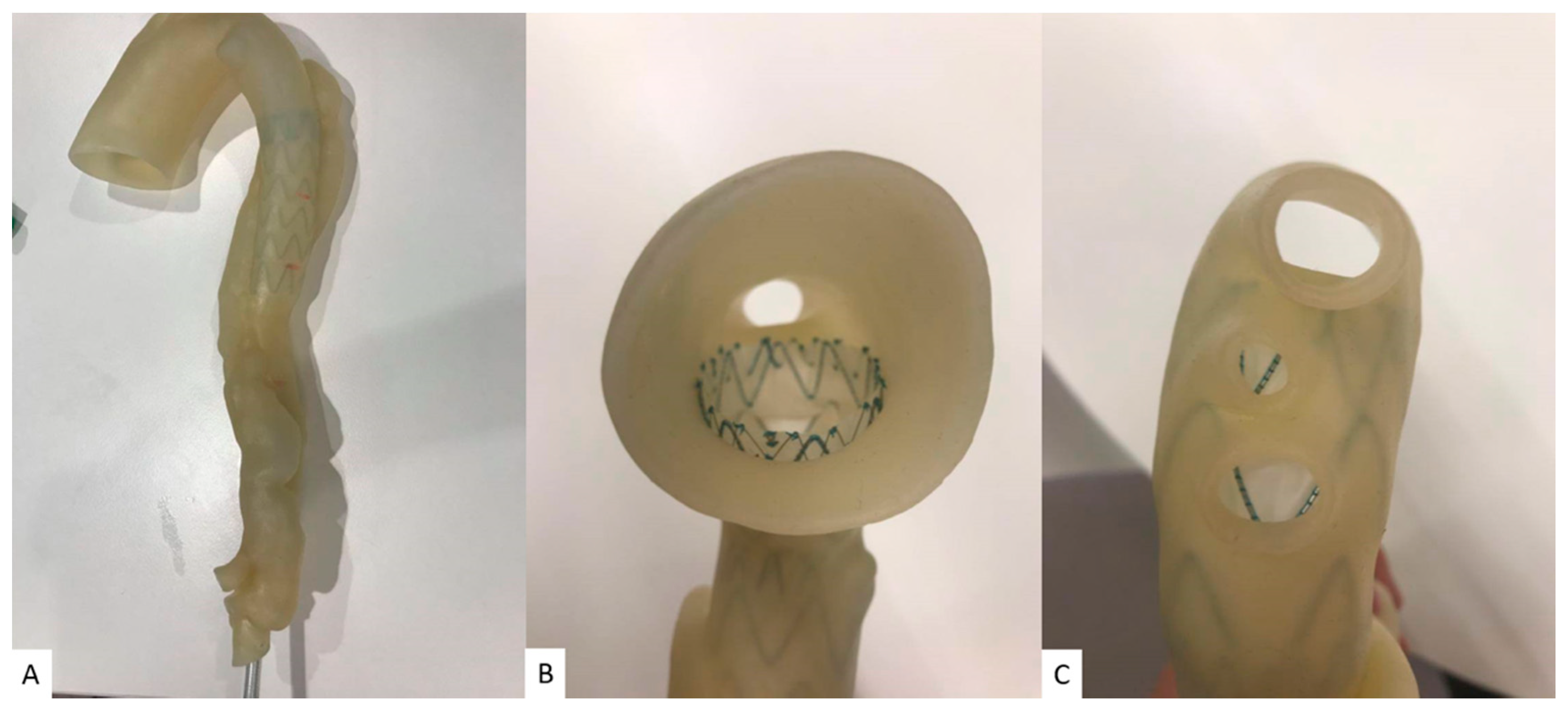
2.2. Selection of 3D Printing Materials
2.3. Deployment of Stent Graft
2.4. Aortic CTA Scanning Protocols
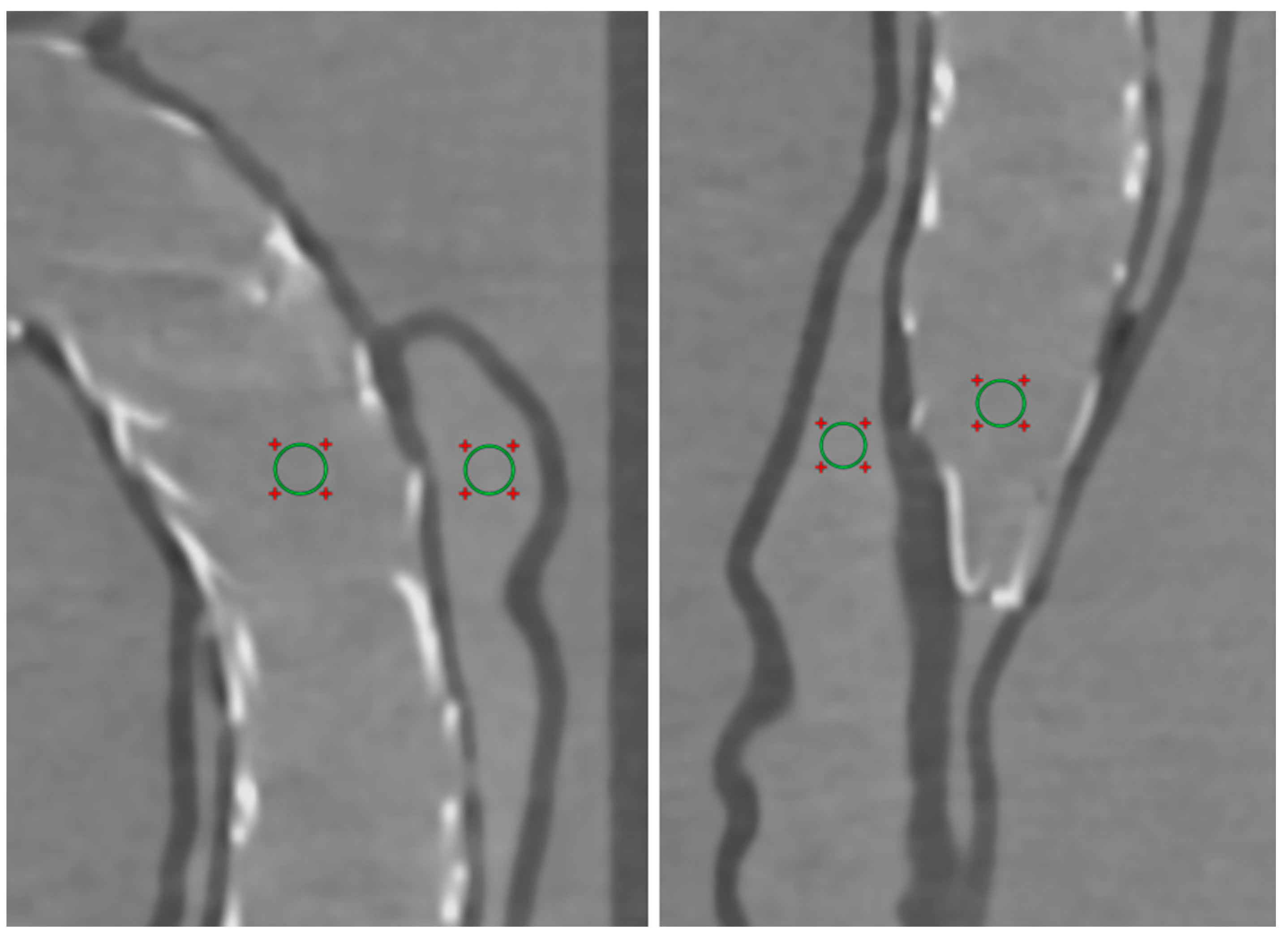
2.5. Quantitative Assessment of Image Quality
2.6. Radiation Dose
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szeto, W.Y.; McGarvey, M.; Pochettino, A.; Moser, W.; Hoboken, A.; Cornelius, K.; Woo, E.Y.; Carpenter, J.P.; Fairman, R.M.; Bavaria, J.E. Results of a new surgical paradigm: Endovascular repair for acute complicated type B aortic dissection. Ann. Thorac. Surg. 2008, 86, 87–94. [Google Scholar] [CrossRef]
- Thrumurthy, S.G.; Karthikesalingam, A.; Patterson, B.O.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 632–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavaria, J.E.; Brinkman, W.T.; Hughes, C.; Khoynezhad, A.; Szeto, W.Y.; Azizzadeh, A.; Lee, W.A.; White, R.A. Outcomes of thoracic endovascular aortic repair in acute type B aortic dissection: Results from the Valiant United States investigational device exemption study. Ann. Thorac. Surg. 2015, 100, 802–809. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Kische, S.; Rousseau, H.; Eggebrecht, H.; Rehders, T.C.; Kundt, G.; Glass, A.; Scheinert, D.; Czerny, M.; Kleinfeldt, T.; et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013, 6, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Hsu, H.-L.; Chen, P.-L.; Chen, I.-M.; Hsu, C.-P.; Shih, C.-C. The impact of distal stent graft–induced new entry on aortic remodeling of chronic type B dissection. Ann. Thorac. Surg. 2018, 105, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Hossack, M.; Patel, S.; Gambardella, I.; Neequaye, S.; Antoniou, G.A.; Torella, F. Endovascular vs. medical management for uncomplicated acute and sub-acute type B aortic dissection: A meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.R.; Bavaria, J.E. The role of thoracic endovascular repair in chronic type B aortic dissection. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 21–24. [Google Scholar] [CrossRef]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Fukui, T. Management of acute aortic dissection and thoracic aortic rupture. J. Intensive Care. 2018, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Apfaltrer, P.; Hanna, E.L.; Schoepf, U.J.; Spears, J.R.; Schoenberg, S.O.; Fink, C.; Vliegenthart, R. Radiation dose and image quality at high-pitch CT angiography of the aorta: Intraindividual and interindividual comparisons with conventional CT angiography. Am. J. Roentgenol. 2012, 199, 1402–1409. [Google Scholar] [CrossRef]
- Sun, Z.; Al Moudi, M.; Cao, Y. CT angiography in the diagnosis of cardiovascular disease: A transformation in cardiovascular CT practice. Quant. Imaging Med. Surg. 2014, 4, 376–396. [Google Scholar]
- Hoffmann, A.; Engelfriet, P.; Mulder, B. Radiation exposure during follow-up of adults with congenital heart disease. Int. J. Cardiol. 2007, 118, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, Z.; Xu, L.; Li, Y.; Zhang, N.; Yan, Z.; Fan, Z. High-pitch, low-voltage and low-iodine-concentration CT angiography of aorta: Assessment of image quality and radiation dose with iterative reconstruction. PLoS ONE 2015, 10, e0117469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felmly, L.M.; Cecco, C.N.; Schoepf, U.J.; Varga-Szemes, A.; Mangold, S.; McQuiston, A.D.; Litwin, S.E.; Bayer II, R.R.; Vogl, T.J.; Wichmann, J.L. Low contrast medium-volume third-generation dual-source computed tomography angiography for transcatheter aortic valve replacement planning. Eur. Radiol. 2017, 27, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Euler, A.; Taslimi, T.; Eberhard, M.; Kobe, A.; Reeve, K.; Zimmermann, A.; Krauss, A.; Gutjahr, R.; Schmidt, B.; Alkadhi, H. Computed tomography angiography of the aorta—Optimization of automatic tube voltage selection settings to reduce radiation dose or contrast medium in a prospective randomized rrial. Investig. Radiol. 2021, 56, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Insights into 3D printing in medical applications. Quant. Imaging Med. Surg. 2019, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Byrne, N.; Belasco, M.; Tandon, A.; Hussain, T. A systematic review of image segmentation methodology, used in the additive manufacture of patient-specific 3D printed models of the cardiovascular system. JRSM Cardiovasc. Dis. 2016, 5, 2048004016645467. [Google Scholar] [CrossRef] [Green Version]
- Foley, T.A.; El Sabbagh, A.; Anavekar, N.S.; Williamson, E.E.; Matsumoto, J.M. 3D-Printing: Applications in Cardiovascular Imaging. Curr. Radiol. Rep. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Giannopoulos, A.A.; Steigner, M.; George, E.; Barile, M.; Hunsaker, A.R.; Rybicki, F.J.; Mitsouras, D. Cardiothoracic applications of 3-D printing. J. Thorac. Imaging 2016, 31, 253–272. [Google Scholar] [CrossRef] [Green Version]
- Eshkalak, S.K.; Ghomi, E.R.; Dai, Y.; Choudhury, D.; Ramakrishna, S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020, 108940. [Google Scholar] [CrossRef]
- Sun, Z. Clinical applications of patient-specific 3D printed models in aardiovascular disease: Current status and future directions. Biomolecules 2020, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-A.; Squelch, A.; Sun, Z. Investigation of three-dimensional printing materials for printing aorta model replicating Type B aortic dissection. Curr. Med. Imaging 2021, 17, 843–849. [Google Scholar]
- Ratinam, R.; Quayle, M.; Crock, J.; Lazarus, M.; Fogg, Q.; McMenamin, P. Challenges in creating dissectible anatomical 3D prints for surgical teaching. J. Anat. 2019, 234, 419–437. [Google Scholar] [CrossRef] [Green Version]
- Riedle, H.; Mukai, B.; Molz, P.; Franke, J. Determination of the mechanical properties of aortic tissue for 3D printed surgical models. In Proceedings of the 2018 11th Biomedical Engineering International Conference (BMEiCON), Chiang Mai, Thailand, 21–24 November 2018; pp. 1–5. [Google Scholar] [CrossRef]
- McCollough, C.H.; Primak, A.N.; Braun, N.; Kofler, J.; Yu, L.; Christner, J. Strategies for reducing radiation dose in CT. Radiol. Clin. North Am. 2009, 47, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Kimura, N.; Yamaguchi, A.; Adachi, H. In-hospital and long-term results of surgery for acute type A aortic dissection: 243 consecutive patients. Ann. Thorac. Cardiovasc. Surg. 2012, 18, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Freyhardt, P.; Solowjowa, N.; Böning, G.; Kahn, J.; Aufmesser, B.; Haage, P.; Streitparth, F. CT-angiography of the aorta in patients with Marfan disease-High-pitch MDCT at different levels of tube voltage combined with Sinogram Affirmed Iterative Reconstruction. Clin. Imaging. 2018, 51, 123–132. [Google Scholar] [CrossRef]
- Flors, L.; Leiva-Salinas, C.; Norton, P.T.; Patrie, J.T.; Hagspiel, K.D. Imaging follow-up of endovascular repair of type B aortic dissection with dual-source, dual-energy CT and late delayed-phase scans. J. Vasc. Interv. Radiol. 2014, 25, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Karmonik, C.; Duran, C.; Shah, D.J.; Anaya-Ayala, J.E.; Davies, M.G.; Lumsden, A.B.; Bismuth, J. Preliminary findings in quantification of changes in septal motion during follow-up of type B aortic dissections. J. Vasc. Surg. 2012, 55, 1419–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Ng, C.K.; Squelch, A. Synchrotron radiation computed tomography assessment of calcified plaques and coronary stenosis with different slice thicknesses and beam energies on 3D printed coronary models. Quant. Imaging Med. Surg. 2019, 9, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jansen, S. Personalized 3D printed coronary models in coronary stenting. Quant. Imaging Med. Surg. 2019, 9, 1356–1367. [Google Scholar] [CrossRef]
- Aldosari, S.; Jansen, S.; Sun, Z. Patient-specific 3D printed pulmonary artery model with simulation of peripheral pulmonary embolism for developing optimal computed tomography pulmonary angiography protocols. Quant. Imaging Med. Surg. 2019, 9, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldosari, S.; Jansen, S.; Sun, Z. Optimization of computed tomography pulmonary angiography protocols using 3D printed model with simulation of pulmonary embolism. Quant. Imaging Med. Surg. 2019, 9, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Lee, S.; Kim, H.; Yang, D.H.; Kim, Y.-H.; Kyung, Y.S.; Kim, C.-S.; Choi, S.H.; Kim, B.J.; Ha, H.; et al. Three-dimensional printing: Basic principles and applications in medicine and radiology. Korean J. Radiol. 2016, 17, 182–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.-H.; Yu, T.; Zhou, M.-J.; Liu, C.; Zhou, M.; Jiang, Q.; Liu, C.-J.; Li, X.-Q.; Liu, Z. Use of 3D printing to guide creation of fenestrations in physician-modified stent-grafts for treatment of thoracoabdominal aortic disease. J. Endovasc. Ther. 2020, 27, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Tong, Y.H.; Li, X.Q.; Liu, C.J.; Liu, C.; Liu, Z. Total endovascular repair of an intraoperative stent-graft deployed in the false lumen of Stanford type A aortic dissection: A case report. World J. Clin. Cases 2020, 8, 954. [Google Scholar] [CrossRef]
- Zhang, M.; Tong, Y.H.; Liu, C.; Li, X.Q.; Liu, C.J.; Liu, Z. Treatment of Stanford type A aortic dissection with triple pre-fenestration, reduced diameter, and three-dimensional-printing techniques: A case report. World J. Clin. Cases 2021, 9, 183. [Google Scholar] [CrossRef]

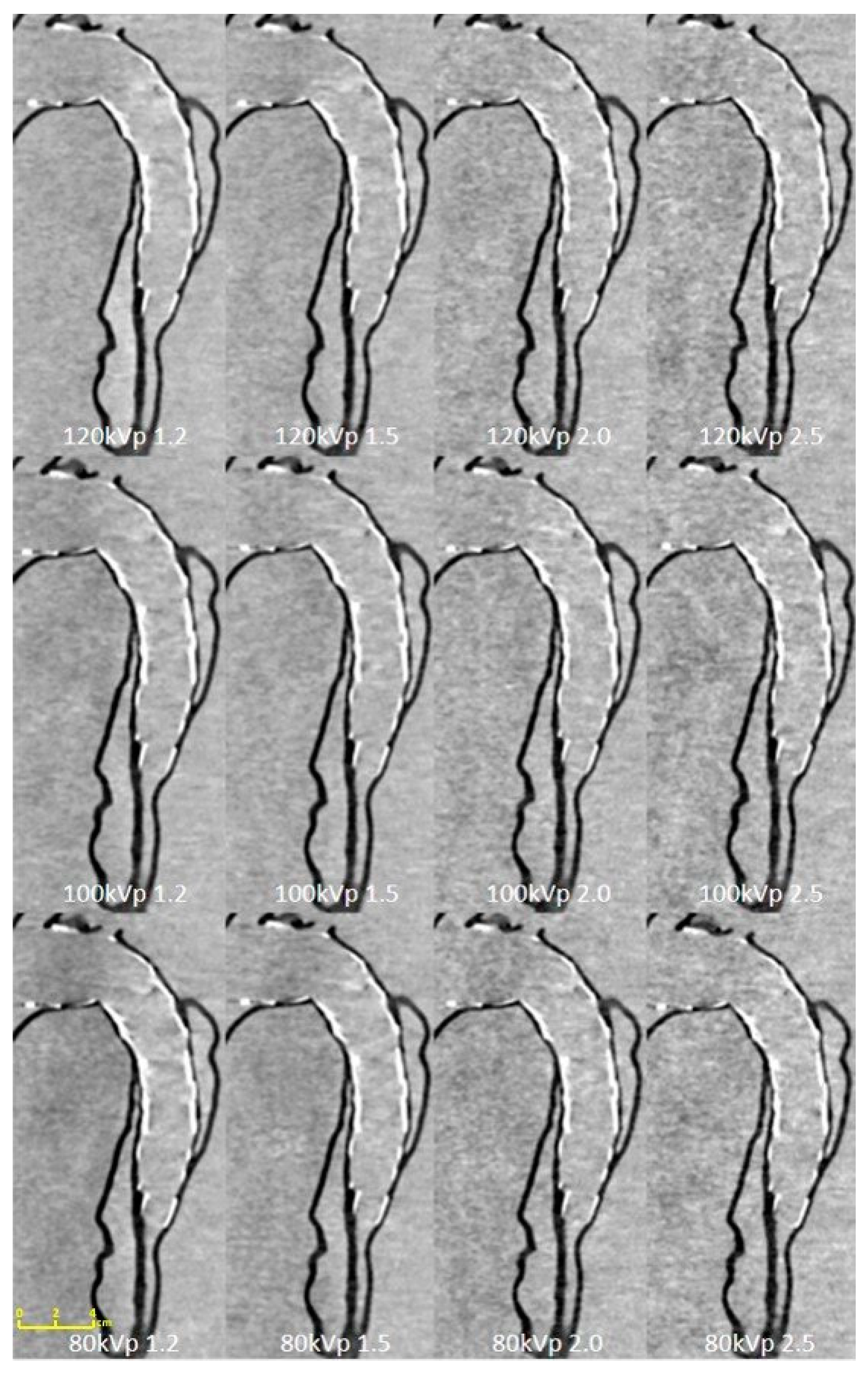
| Tube Voltage | 80 kVp | 100 kVp | 120 kVp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pitch Value | 1.2 | 1.5 | 2.0 | 2.5 | 1.2 | 1.5 | 2.0 | 2.5 | 1.2 | 1.5 | 2.0 | 2.5 |
| True lumen of proximal descending aorta | 23.93 ± 9.34 | 20.30 ± 1.69 | 19.64 ± 1.03 | 13.70 ± 1.42 | 27.60 ± 1.47 | 22.19 ± 2.14 | 19.14 ± 3.75 | 12.60 ± 0.38 | 25.64 ± 4.69 | 25.10 ± 2.74 | 19.93 ± 0.67 | 19.23 ± 3.74 |
| False lumen of proximal descending aorta | 24.70 ± 10.66 | 23.40 ± 0.90 | 20.86 ± 1.98 | 21.34 ± 2.08 | 24.18 ± 2.86 | 23.82 ± 0.64 | 16.63 ± 3.31 | 17.04 ± 1.24 | 26.18 ± 2.44 | 27.61 ± 3.93 | 22.06 ± 1.51 | 24.00 ± 2.94 |
| True lumen of middle descending aorta | 25.29 ± 7.43 | 25.10 ± 3.58 | 18.66 ± 1.19 | 16.62 ± 1.28 | 25.65 ± 2.16 | 25.91 ± 0.29 | 14.74 ± 0.58 | 18.40 ± 1.92 | 23.68 ± 2.33 | 26.21 ± 0.26 | 20.61 ± 1.24 | 19.81 ± 1.04 |
| False lumen of middle descending aorta | 18.12 ± 2.80 | 22.13 ± 1.54 | 10.11 ± 0.60 | 12.47 ± 0.54 | 20.86 ± 2.23 | 26.27 ± 3.77 | 15.62 ± 0.77 | 12.16 ± 1.16 | 20.15 ± 1.29 | 29.91 ± 4.84 | 14.03 ± 0.80 | 13.53 ± 0.51 |
| CTDIvol (mGy) | 0.07 | 0.08 | 0.06 | 0.06 | 0.1 | 0.1 | 0.08 | 0.07 | 0.11 | 0.1 | 0.08 | 0.07 |
| DLP (mGy/cm) | 2.8 | 2.9 | 2.2 | 2.1 | 3.9 | 3.8 | 2.7 | 2.4 | 4.1 | 3.8 | 2.8 | 2.5 |
| Effective dose (mSv) | 0.04 | 0.04 | 0.03 | 0.03 | 0.05 | 0.05 | 0.04 | 0.03 | 0.06 | 0.05 | 0.04 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-A.; Squelch, A.; Jansen, S.; Sun, Z. Optimization of Computed Tomography Angiography Protocols for Follow-Up Type B Aortic Dissection Patients by Using 3D Printed Model. Appl. Sci. 2021, 11, 6844. https://doi.org/10.3390/app11156844
Wu C-A, Squelch A, Jansen S, Sun Z. Optimization of Computed Tomography Angiography Protocols for Follow-Up Type B Aortic Dissection Patients by Using 3D Printed Model. Applied Sciences. 2021; 11(15):6844. https://doi.org/10.3390/app11156844
Chicago/Turabian StyleWu, Chia-An, Andrew Squelch, Shirley Jansen, and Zhonghua Sun. 2021. "Optimization of Computed Tomography Angiography Protocols for Follow-Up Type B Aortic Dissection Patients by Using 3D Printed Model" Applied Sciences 11, no. 15: 6844. https://doi.org/10.3390/app11156844
APA StyleWu, C.-A., Squelch, A., Jansen, S., & Sun, Z. (2021). Optimization of Computed Tomography Angiography Protocols for Follow-Up Type B Aortic Dissection Patients by Using 3D Printed Model. Applied Sciences, 11(15), 6844. https://doi.org/10.3390/app11156844







