Antioxidant and Anti-Inflammatory Potential and Consumer Acceptance of Wafers Enriched with Freeze-Dried Raspberry Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Raspberry Pomace
2.3. HPLC Analysis of Anthocyanins
2.4. Preparation of Wafers Enriched with Freeze-Dried Raspberry Pomace
2.5. Preparation of Extracts
2.5.1. Ethanolic Extracts (EtOH)
2.5.2. Buffer Extracts (PBS)
2.5.3. In Vitro Digestion (DIG)
2.6. Analysis of Phenolics
2.7. Antioxidant Properties of Enriched Wafers
2.7.1. Antiradical Activity
2.7.2. Chelating Ability and Reduction Power
2.8. Potential Anti-Inflammatory Activity
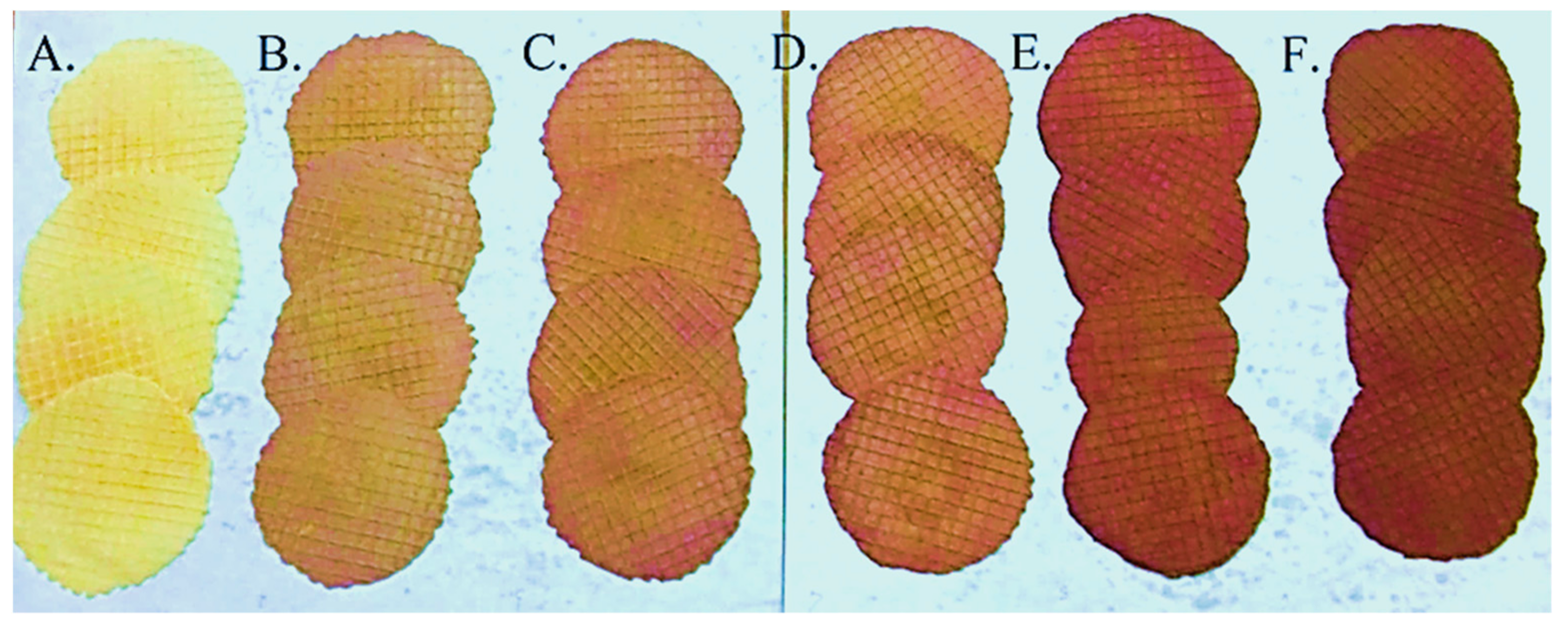
2.9. Color Measurement
2.10. Sensory Analysis
2.11. Statistical Analysis
3. Results
3.1. Anthocyanin Profile in Raspberry Pomace
3.2. Sensory and Color Analysis of Fortified Wafers
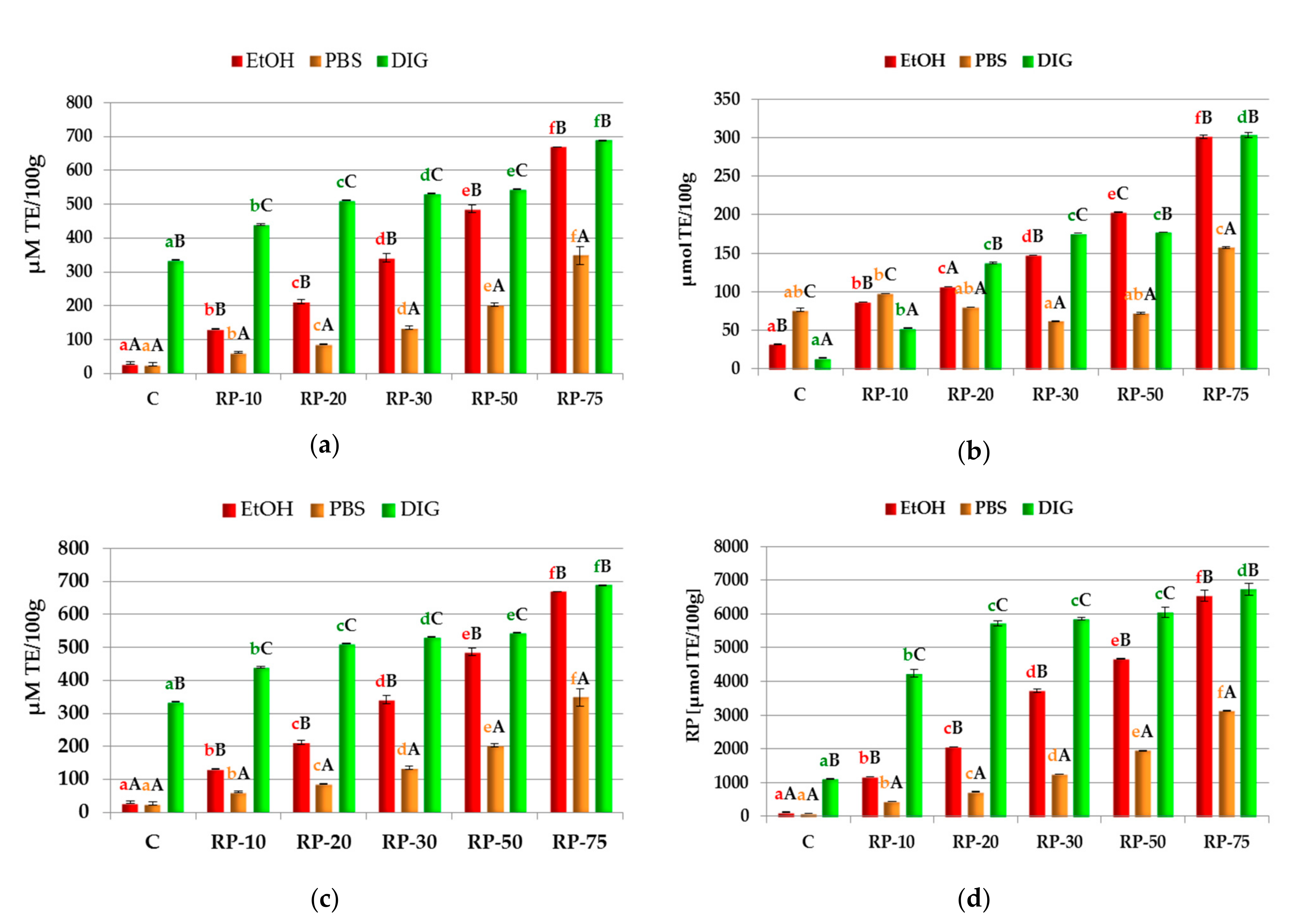
3.3. Phenolic Content
3.4. Antioxidant Activity of Raspberry Pomace-Enriched Wafers
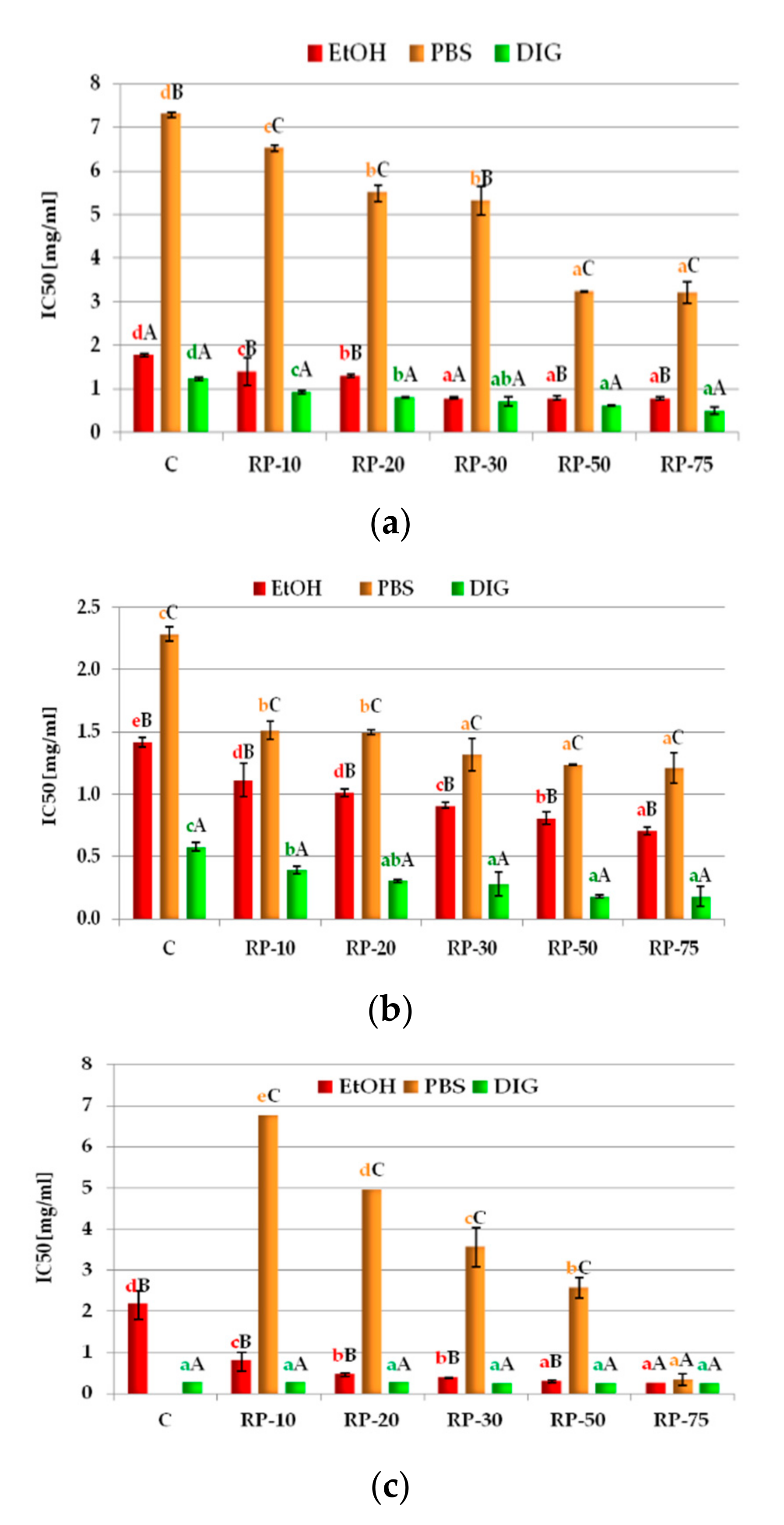
3.5. Potential Anti-Inflammatory Properties
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ARR Fruit Market in Poland. Poland is a Significant Fruit Producer Worldwide and in the EU. 2014. Available online: https://www.kowr.gov.pl/uploads/rynek-owocow2014-en.pdf (accessed on 1 June 2021).
- IRO2018-Poland_OK_4c01e.pdf. IRO Raspberry. Available online: https://www.internationalraspberry.net/ (accessed on 6 June 2021).
- Brodowska, A.J. Raspberry pomace—composition, properties and application. Eur. J. Biol. Res. 2017, 7, 86–96. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to im prove the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivokapić, S.; Vlaovićc, M.; Damjanović Vratnica, B.; Perović, A.; Perović, S. Biowaste as a Potential Source of Bioactive Compounds—A Case Study of Raspberry Fruit Pomace. Foods 2021, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Markowski, J. Grinding levels of raspberry pomace affect intestinal microbial activity, lipid and glucose metabolism in Wistar rats. Food Res. Int. 2019, 120, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kucharczyk, E.; Kaliszan, R.; Markuszewski, M.; Fotschki, B.; Juśkiewicz, J.; Borkowska-Sztachańska, M.; Ognik, K. The Characterization of Ground Raspberry Seeds and the Physiological Response to Supplementation in Hypertensive and Normotensive Rats. Nutrients 2020, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Viskelis, J.; Rubinskiene, M.; Bobinas, C.; Bobinaite, R. Enrichment of fruit leathers with berry press cake powder increase product functionality. In Proceedings of the FOODBALT 2017, Jelgava, Latvia, 27–28 April 2017; pp. 75–79. [Google Scholar] [CrossRef]
- Tańska, M.; Roszkowska, B.; Czaplicki, S.; Borowska, E.J.; Bojarska, J.; Dąbrowska, A. Effect of fruit pomace addition on shortbread cookies to improve their physical and nutritional values. Plant Foods Hum. Nutr. 2016, 71, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, S.; Simsek, S.; Eker, A.T.; Pineda-Vadillo, C.; Dupont, D.; Perez, B.; Viadel, B.; Sanz-Buenhombre, M.; Rodriguez, A.G.; Kertész, Z.; et al. Stability and bioaccessibility of anthocyanins in bakery products enriched with anthocyanins. Food Funct. 2016, 7, 3488–3496. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2016, 52, 291–305. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.-Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice—In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Szaufer-Hajdrych, M.; Goślińska, O. The quantitative determination of phenolic acids and antimicrobial activity of Symphoricarpos albus (L.) Blake. Acta Pol. Pharm.-Drug Res. 2004, 61, 69–74. [Google Scholar]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux flavonoides des fleurs et des feuilles de crataegus monogyna jacq. Et de crataegus laevigata (poiret) dc. En fonction de la periode de vegetation. Plantes Med. Phyther. 1991, 25, 12–16. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Guo, J.-T.; Lee, H.-L.; Chiang, S.-H.; Lin, F.-I.; Chang, C.-Y. Antioxidant Properties of the Extracts from Different Parts of Broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Szymanowska, U.; Karaś, M.; Złotek, U.; Jakubczyk, A. Effect of Fortification with Raspberry Juice on the Antioxidant and Potentially Anti-Inflammatory Activity of Wafers Subjected to In Vitro Digestion. Foods 2021, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-C.; Wang, H.-B.; Zhou, Q.; Hu, B.; Wen, J.-H.; Zhang, J.-L. Screening of effective xanthine oxidase inhibitors in dietary anthocyanins from purple sweet potato (Ipomoea batatas L. Cultivar Eshu No.8) and deciphering of the underlying mechanisms in vitro. J. Funct. Foods 2017, 36, 102–111. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, L.U.; Al-Said, F. Colour measurement and analysis in fresh and processed food: A review. Food Bioprocess Technol. 2014, 6, 36–60. [Google Scholar] [CrossRef]
- Bobinaite, R.; Viškelis, P.; Venskutonis, P.R. Chemical Composition of Raspberry (Rubus spp.) Cultivars. In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2016; pp. 713–731. ISBN 9780124081178. [Google Scholar]
- Bradish, C.M.; Perkins-Veazie, P.; Fernandez, G.E.; Xie, G.; Jia, W. Comparison of Flavonoid Composition of Red Raspberries (Rubus idaeus L.) Grown in the Southern United States. J. Agric. Food Chem. 2011, 60, 5779–5786. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Čechovičienė, I.; Jukniūtė, K.; Šlepetienė, A.; Paulauskienė, A. Qualitative properties of cookies enriched with berries pomace. Food Sci. Technol. 2020, 41, 474–481. [Google Scholar] [CrossRef]
- Scaronarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef] [Green Version]
- Seker, I.T.; Ertop, M.H.; Hayta, M. Physicochemical and bioactive properties of cakes incorporated with gilaburu fruit (Viburnum opulus) pomace. Qual. Assur. Saf. Crop. Foods 2016, 8, 261–266. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.L. Vitamin E, α-tocopherol. In Antioxidants in Higher Plants; CRC Press: Boca Raton, FL, USA, 2017; pp. 111–134. ISBN 9781351369152. [Google Scholar]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavaila-bility of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Calixto, F.D.S. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.; Tagliani, C.; Arcia, P.; Cozzano, S.; Curutchet, A. Blueberry by-product used as an ingredient in the development of functional cookies. Food Sci. Technol. Int. 2017, 24, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of Action of Flavonoids as Anti-inflammatory Agents: A Review. Inflamm. Allergy-Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Anti-inflammatory, Immunomodulatory, and Prebiotic Properties of Dietary Flavonoids. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 327–345. [Google Scholar]
- Nile, S.; Khobragade, C.N. In Vitro Anti-inflammatory and Xanthine Oxidase Inhibitory Activity of Tephrosia purpurea Shoot Extract. Nat. Prod. Commun. 2011, 6, 1437–1440. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.P. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
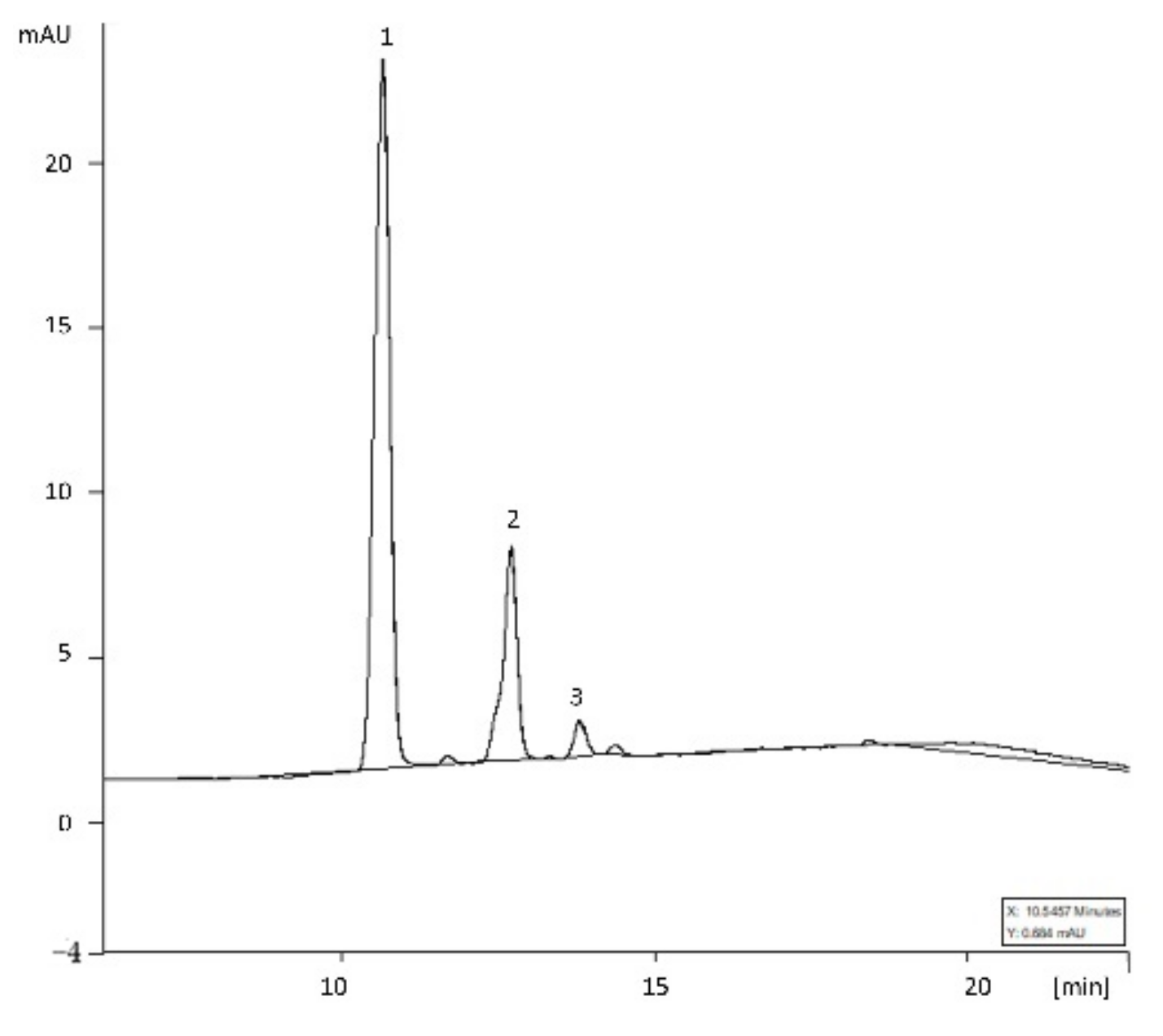
| Compound | Content [mg/100 g d.w.] |
|---|---|
| cyanidin-3-O-sophoroside | 105.44 ± 6.15 |
| cyanidin-3-O-glucoside | 35.64 ± 2.08 |
| cyanidin-3-O-rutinoside | 7.43 ± 0.43 |
| Sum [mg/100 g d.w.] * | 148.5 ± 8.66 |
| Quality | Wafer Variants | ||||||
| C | RP-10 | RP-20 | RP-30 | RP-50 | RP-75 |  | |
| Shape | 4.06 ± 0.20 a | 4.17 ± 0.19 ab | 4.11 ± 0.06 ab | 4 ± 0.15 c | 4.56 ± 0.23 a | 4.06 ± 0.10 a | |
| Color | 4.72 ± 0.07 d | 3.11 ± 0.16 a | 3.67 ± 0.08 b | 3.64 ± 0.13 b | 4.17 ± 0.11 c | 4.39 ± 0.09 c | |
| Surface | 4.44 ± 0.02 d | 4.22 ± 0.06 b | 4.39 ± 0.05 cd | 4.28 ± 0.01 bc | 4.22 ± 0.07 b | 4.06 ± 0.05 a | |
| Consistency | 4.17 ± 0.18 c | 4.17 ± 0.06 c | 4.56 ± 0.04 d | 3.44 ± 0.17 b | 2.78 ± 0.14 a | 2.89 ± 0.06 a | |
| Aroma | 4.17 ± 0.04 d | 3.89 ± 0.02 cd | 3.83 ± 0.20 bc | 3.56 ± 0.12 ab | 3.67 ± 0.09 abc | 3.5 ± 0.04 a | |
| Taste | 4.28 ± 0.05 c | 4.06 ± 0.05 bc | 4.00 ± 0.13 bc | 3.61 ± 0.09 a | 3.78 ± 0.17 ab | 3.56 ± 0.09 a | |
| Overall acceptance | 4.31 ± 0.06 b | 3.94 ± 0.20 ab | 4.09 ± 0.22 ab | 3.75 ± 0.13 a | 3.86 ± 0.09 a | 3.74 ± 0.15 a | |
| Sample | Color Characteristic | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | ΔE | |
| C | 80.69 ± 3.46 c | 5.5 ± 0.06 a | 31.11 ± 1.16 b | 31.59 ± 1.13 b | 1.39 ± 0.008 c | - |
| RP-10 | 57.14 ± 0.38 b | 13.08 ± 1.05 b | 14.11 ± 3.00 a | 19.24 ± 2.9 a | 0.82 ± 0.066 b | 30.05 ± 3.18 a |
| RP-20 | 51.55 ± 0.54 b | 17.68 ± 0.94 c | 12.4 ± 2.05 a | 21.59 ± 0.40 a | 0.61 ± 0.102 ab | 36.71 ± 3.07 a |
| RP-30 | 52.09 ± 1.36 b | 18.52 ± 0.12 c | 13.52 ± 2.10 a | 22.93 ± 1.33 a | 0.63 ± 0.071 ab | 36.11 ± 0.10 a |
| RP-50 | 39.33 ± 0.03 a | 21.87 ± 0.53 d | 10.17 ± 0.03 a | 24.12 ± 0.47 a | 0.43 ± 0.010 a | 49.21 ± 2.23 b |
| RP-75 | 35.02 ± 0.36 a | 21.68 ± 0.87 d | 11.76 ± 0.67 a | 24.66 ± 1.08 a | 0.49 ± 0.007 a | 52.22 ± 2.92 b |
| Phenolic Compound Content | Sample | |||||
|---|---|---|---|---|---|---|
| C | RP-10 | RP-20 | RP-30 | RP-50 | RP-75 | |
| EtOH | ||||||
| TPC [mg/100 g d.w.] | 80.62 ± 8.23 aA | 183.68 ± 10.58 bA | 245.18 ± 1.18 cA | 392.29 ± 16.46 dA | 588.43 ± 32.91 eA | 752.99 ± 0.00 fA |
| TPA [µg/100 g d.w.] | 14.41 ± 3.40 aA | 50.45 ± 3.40 bA | 85.29 ± 5.10 cA | 121.32 ± 1.70 dA | 159.76 ± 5.10 eA | 249.86 ± 3.40 fA |
| TFd [mg/100 g d.w.] | 42.10 ± 2.29 aA | 84.20 ± 2.29 bA | 123.06 ± 6.87 cA | 187.82 ± 11.45 dA | 334.36 ± 3.43 eA | 387.79 ± 19.46 fA |
| TAc [mg/100 g d.w.] | Nd | 6.99 ± 1.03 aB | 15.66 ± 2.07 bB | 22.65 ± 1.33 cC | 37.89 ± 3.10 dB | 62.73 ± 2.80 eB |
| PBS | ||||||
| TPC | 64.83 ± 18.81 aA | 103.89 ± 3.53 bB | 122.17 ± 10.58 bB | 172.87 ± 4.70 cB | 216.92 ± 8.23 dB | 251.83 ± 3.53 eB |
| TPA | 10.81 ± 5.10 aA | 37.24 ± 1.70 bB | 58.86 ± 8.49 bB | 93.70 ± 13.59 cB | 133.34 ± 15.29 dB | 198.20 ± 22.08 eB |
| TFd | 36.43 ± 5.72 aA | 48.58 ± 0.00 abB | 80.96 ± 22.90 abcB | 101.20 ± 40.07 bcB | 125.49 ± 5.72 dB | 222.64 ± 51.52 eB |
| TAc | Nd | 3.13 ± 0.89 aA | 5.11 ± 1.03 bA | 11.2 ± 0.59 cA | 17.95 ± 0.30 dA | 28.60 ± 1.48 eA |
| DIG | ||||||
| TPC | 389.79 ± 19.98 aB | 561.84 ± 2.35 bC | 596.74 ± 2.35 cC | 646.61 ± 14.10 dC | 690.66 ± 3.53 eC | 802.03 ± 19.9 fC |
| TPA | 300.31 ± 16.99 aB | 338.75 ± 6.80 bC | 421.63 ± 5.10 cC | 464.88 ± 5.10 dC | 90.10 ± 20.39 eC | 559.77 ± 6.80 fC |
| TFd | 240.85 ± 20.04 aB | 350.15 ± 25.76 bC | 392.65 ± 22.90 bcC | 441.22 ± 5.72 cC | 522.18 ± 11.45 dC | 692.20 ± 57.25 eC |
| TAc | Nd | 6.54 ± 0.60 aB | 13.91 ± 3.38 bB | 17.95 ± 0.65 cB | 37.92 ± 1.67 dB | 57.40 ± 2.92 eB |
| EtOH | ||||
| TPC | TPA | TFd | TAc | |
| DPPH | 0.99 | 1.00 | 0.97 | 1.00 |
| ABTS | 1.00 | 0.99 | 0.99 | 0.99 |
| CHP | 0.98 | 0.99 | 0.96 | 0.97 |
| RP | 0.99 | 0.99 | 0.97 | 0.98 |
| XO | −0.88 | −0.86 | −0.87 | −0.82 |
| LOX | −0.93 | −0.93 | −0.91 | −0.90 |
| COX-2 | −0.72 | −0.73 | −0.70 | −0.68 |
| PBS | ||||
| TPC | TPA | TFd | TAc | |
| DPPH | 0.50 | 0.63 | 0.72 | 0.64 |
| ABTS | 0.96 | 0.99 | 0.99 | 0.99 |
| CHP | 0.92 | 0.87 | 0.79 | 0.83 |
| RP | 0.97 | 1.00 | 0.99 | 1.00 |
| XO | −0.97 | −0.95 | −0.88 | −0.93 |
| LOX | −0.83 | −0.77 | −0.69 | −0.72 |
| COX-2 | −0.97 | −0.98 | −0.96 | −0.98 |
| DIG | ||||
| TPC | TPA | TFd | TAc | |
| DPPH | 0.92 | 0.95 | 0.95 | 0.92 |
| ABTS | 0.98 | 0.97 | 0.98 | 0.92 |
| CHP | 0.97 | 0.97 | 0.96 | 0.95 |
| RP | 0.94 | 0.89 | 0.84 | 0.75 |
| XO | −0.99 | −0.96 | −0.94 | −0.89 |
| LOX | −0.96 | −0.93 | −0.89 | −0.85 |
| COX-2 | −0.97 | −0.96 | −0.99 | −0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanowska, U.; Karaś, M.; Bochnak-Niedźwiecka, J. Antioxidant and Anti-Inflammatory Potential and Consumer Acceptance of Wafers Enriched with Freeze-Dried Raspberry Pomace. Appl. Sci. 2021, 11, 6807. https://doi.org/10.3390/app11156807
Szymanowska U, Karaś M, Bochnak-Niedźwiecka J. Antioxidant and Anti-Inflammatory Potential and Consumer Acceptance of Wafers Enriched with Freeze-Dried Raspberry Pomace. Applied Sciences. 2021; 11(15):6807. https://doi.org/10.3390/app11156807
Chicago/Turabian StyleSzymanowska, Urszula, Monika Karaś, and Justyna Bochnak-Niedźwiecka. 2021. "Antioxidant and Anti-Inflammatory Potential and Consumer Acceptance of Wafers Enriched with Freeze-Dried Raspberry Pomace" Applied Sciences 11, no. 15: 6807. https://doi.org/10.3390/app11156807
APA StyleSzymanowska, U., Karaś, M., & Bochnak-Niedźwiecka, J. (2021). Antioxidant and Anti-Inflammatory Potential and Consumer Acceptance of Wafers Enriched with Freeze-Dried Raspberry Pomace. Applied Sciences, 11(15), 6807. https://doi.org/10.3390/app11156807









