1. Introduction
In the field of condensed-matter physics, ferroelectricity has been studied for decades because spontaneous electrical polarization can be obtained without an electrostatic potential, and the direction of polarization can be tuned by using an external electric field [
1,
2,
3]. Ferroelectric materials are widely applicable in piezoelectric devices, condensers, IR detectors, nonvolatile memory devices, and ferroelectric random-access memory [
4,
5,
6].
Perovskite BaTiO
3 (BTO), which is the best-known ferroelectric material, undergoes a distinct transition between the ferroelectric and paraelectric phases with high latent heat (~0.3 J/g) as the transition temperature, which is called the Curie temperature (T
C) [
7,
8]. The ferro-paraelectric transition in BTO originates from the spontaneous transition between tetragonal and cubic structures around the T
C of 120 °C [
9,
10]. When the room-temperature ferroelectric phase, tetragonal BTO, is heated above T
C, the lattice constants of a and c become identical, resulting in a cubic structure, which exhibits no spontaneous polarization.
BTO has the formula of perovskite (ABO
3), and the cations Ba
2+ and Ti
4+ occupy the A and B sites, respectively. The dopant atoms usually have preferred substitutional sites (A or B in the perovskite ABO
3 system) depending on their atomic radius, the valence states, or their coordination numbers [
11,
12,
13]. For example, Ce
3+ atoms are more likely to occupy the A sites, while Ce
4+ atoms are more likely to occur at the B sites [
14]. Many doped BTO systems have been reported so far, and it is known that proper doping at an A or B site can effectively influence the crystal structure of BTO, as well as its ferroelectricity [
15,
16,
17]. For example, A-site doping with divalent atoms, such as Sr
2+ and Ca
2+, or B site-doping with tetravalent atoms, such as Zr
4+, Sn
4+, or Hf
4+ atoms, were expected to have an effective influence on ferroelectric properties, such as T
C and latent heat [
18,
19,
20]. However, the effects of multivalent atoms, such as transition metal atoms, are hardly predictable for exact substitutional sites. Moreover, the ferroelectric properties can also be diverse depending on the synthetic conditions, such as temperature, ambient gas, or pressure [
7].
There have been many studies of BTO systems that were doped with Sr
2+ and that had high solubility [
21]. The T
C values of Sr-doped BTO decreased with an increase in the content of Sr atoms [
15]. In the case of transition metal dopants, such as tungsten (W), W dopants have possible valence states of 4+, 5+, or 6+ when tungsten atoms substitute a Ti
4+ atom into a BTO system. Devi et al. reported that substitutional tungsten atoms in the hexavalent state (W
6+) in BTO could effectively improve the ferroelectric properties by creating trivalent titanium (Ti
3+) atoms due to the charge neutrality of the system [
22]. Because dopants with hetero-valence states create vacancies or interstitial states, the ferroelectric properties and the phase stability of W-doped BTO are still under debate.
In this study, we showed the change in structural phase transition in polycrystalline BaTiO3 through the doping of the A or B sites with Sr or W atoms. The Ba atoms were replaced with Sr atoms at the A sites, whereas the Ti atoms were replaced with W atoms at the B sites. X-ray diffraction revealed that the Sr- and W-doped BTO has a tetragonal structure for all samples in this study. The ratio of the lattice constants in the tetragonal structure (c/a) decreased from 1.008 to 1.005 as the content of Sr and W dopants increased. The transition between the ferroelectric tetragonal (room-temperature phase) and paraelectric cubic (high-temperature phase) structures was checked by using differential scanning calorimetry (DSC) measurements. At the transition temperature (TC), the ferroelectricity disappeared because of the cubic structure above TC. The values of TC decreased while increasing the Sr content, but TC was almost invariant with respect to the W content. The values of the latent heat during the transition gradually decreased and approached zero with the increase in the content of the Sr and W dopants. The results of this study show that the room-temperature phases of Sr-and W-doped BTO have a cube-like structure with a reduced c/a ratio; thus, the ferroelectricity of tetragonal BTO can be suppressed by doping the A and B sites in BTO.
2. Materials and Methods
BTO and BTO doped with Sr and W were synthesized by using a solid-state reaction of raw BaCO3 (Samchun, 99.0%), TiO2 (Sigma Aldrich, 99.8%), SrCO3 (Sigma Aldrich, 99.9%), and WO3 (Sigma Aldrich, 99.9%). The stoichiometric mixture was calcinated at 800 °C for 24 h and reheated at 1000 °C for 24–30 h. Intermediate fine grinding was performed between heating processes.
The differential scanning calorimetry (DSC) curves and measurements were obtained by using TA DSC 250 on a powder sample. The powder sample was put into a pan, which was then covered using a lid. An empty pan that was sealed with a lid was also used as a reference sample. The powder sample and reference sample were placed in a DSC cell. The DSC experiment was conducted in a temperature range from 25 to 184 °C with a heating rate of 10 °C/min. Then, the DSC cell was cooled to 25 °C with a cooling rate of 10 °C/min. The thermodynamic data of the powder sample were recorded during the heating and cooling processes.
The crystal structures of the BTO powders were evaluated by using X-ray diffraction (XRD, Expert pro-MPD, PANalytical) with Cu Kα radiation (0.15418 nm) at the Western Seoul Center, Korea Basic Science Institute and the XRD data were analyzed by using the Rietveld refinement program of Fullprof.
The surface morphology of Sr- and W-doped BTO was studied by using a field emission scanning electron microscopy (FE-SEM) system (Hitachi SU8220 FE-SEM with a base pressure of ~1 × 10−8 Torr) at the Western Seoul Center, Korea Basic Science Institute.
3. Results and Discussion
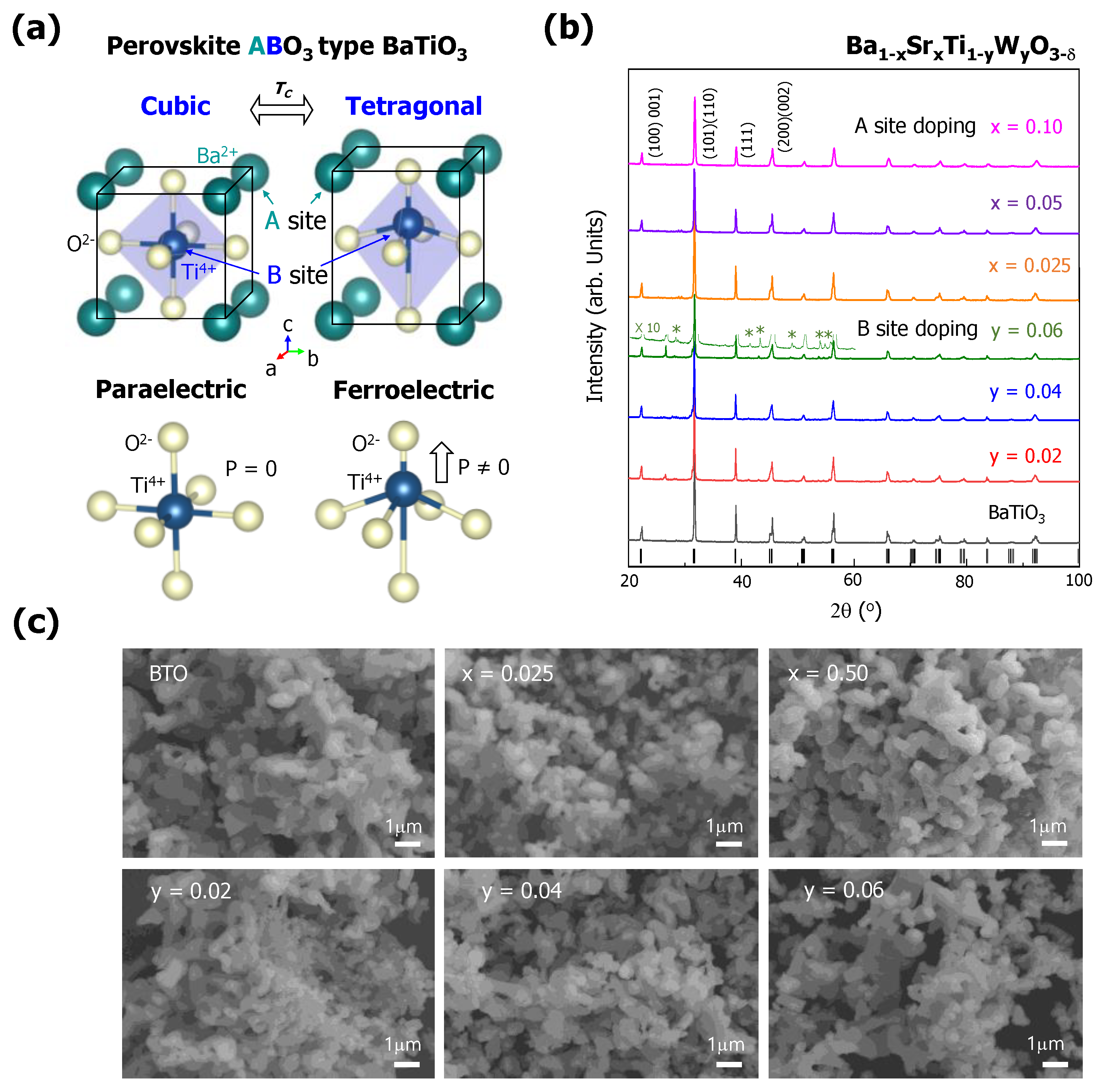
BTO has a perovskite structure with the chemical formula of ABO
3. In this case, the Sr
2+ atoms are located at the eight corners (A sites) of the cuboid, and the Ti
4+ atoms are located at the center (B sites) of the cuboid, as seen in
Figure 1a. BTO has two different phases, each of which exhibits a cubic (high-temperature phase, above the T
C) or tetragonal (room-temperature phase, below the T
C) structure. Both crystal structures are perovskite structures with different ratios between the lattice constants (c/a) (i.e., 1 for the cubic structure and >1 for the tetragonal structure). The Ti atoms are surrounded by six O
2− atoms, which form an octahedral shape, thereby occupying the B sites. In cubic BTO, all of the lattice constants are identical, leading to a paraelectric condition with no polarization. On the other hand, tetragonal BTO exhibits an elongation of the lattice along the c-axis. Thus, the Ti-O octahedrons have down-shifted O
2− atoms and up-shifted Ti
4+ atoms, resulting in permanent electrical polarization. Therefore, the polarization curves of tetragonal BTO can have hysteresis curves as a function of the applied electric field (E-field), and they can retain a certain amount of polarization after removing the E-field.
The X-ray diffraction patterns of the BTO doped with Sr and W atoms are shown in
Figure 1b. The BTO systems with Sr and W substitutions retained the tetragonal structure when the dopant content was x = 0.1 for Sr and y = 0.06 for W. The Sr-doped BTO had a single phase (tetragonal structure) for all values of the Sr content, but with high W content (y = 0.06). W-doped BTO has several small secondary peaks that correspond to TiO
2 and SrO [
23]. This indicates that Sr atoms have a greater solubility than W atoms in the BTO system. The SEM images revealed that the influence of Sr and W doping on the morphology and particle size was insignificant, as shown in
Figure 1c.
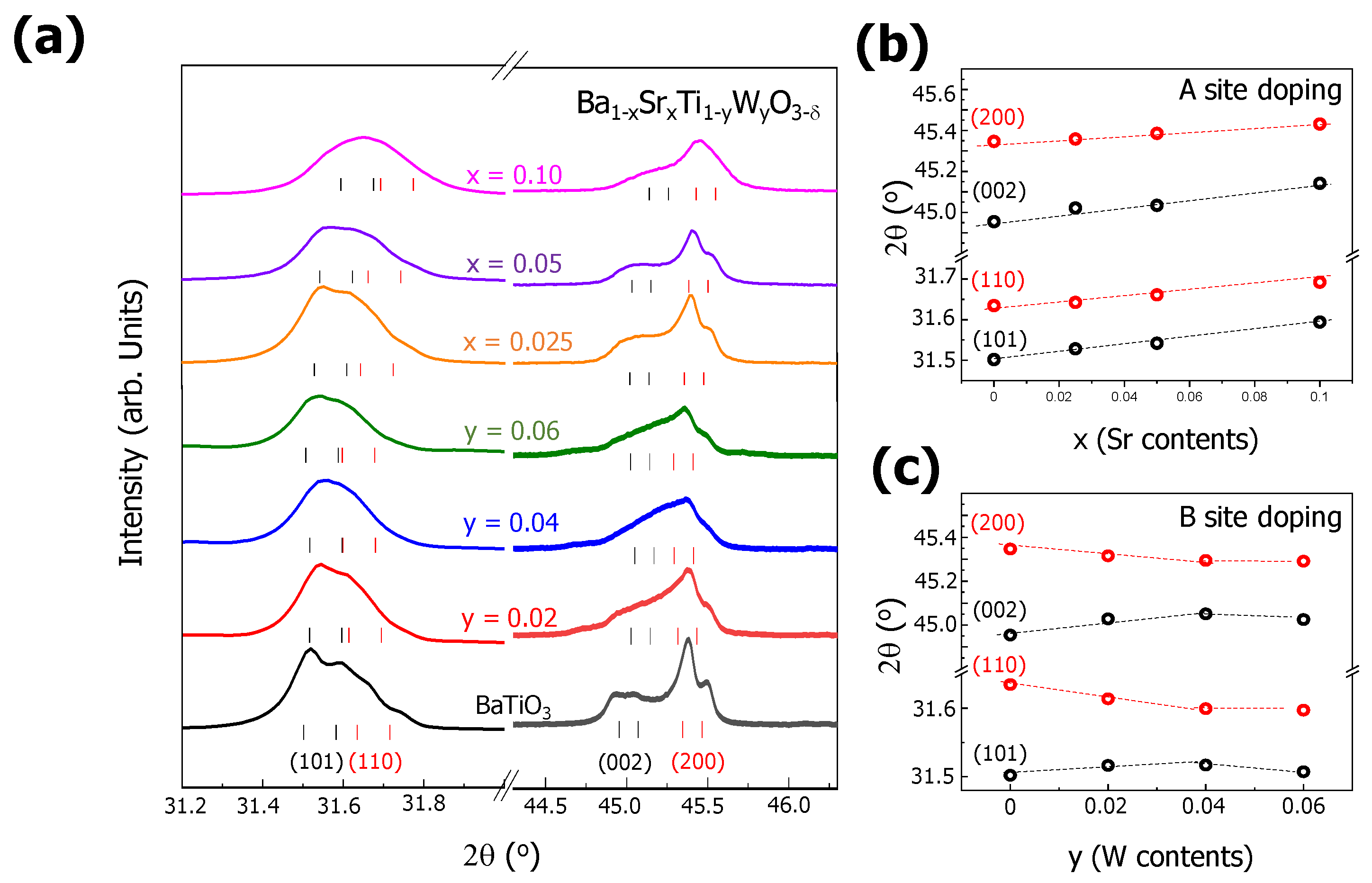
To see the changes in the BTO lattice, we analyzed the diffraction peaks located around 32.0° and 45.0°, which corresponded to the diffractions of the (101), (110), (002), and (200) planes in tetragonal BTO. The diffraction angles of these XRD peaks (as marked with black and red bars in
Figure 2a) were deduced from the Rietveld refinement results, which are summarized in
Table 1. As the Sr content x increased from 0.025 to 0.1, all of the diffraction peaks of (101), (110), (002), and (200) shifted to higher diffraction angles (see
Figure 2b). This indicated that the lattice constants of a and c in the Sr-doped BTO became smaller. However, with the increase in the W content y from 0.02 to 0.06, the diffraction peaks of (101), (110), (002), and (200) shifted, but the tendency of these shifts was different from that in the case of the Sr-doped BTO. With y = 0.02 and 0.04, the peaks of (101) and (002) were shifted to higher angles, while the peaks of (110) and (200) were shifted to lower angles, as seen in
Figure 2c. This indicated that the lattice constant of a became larger while that of c became smaller in the case of W-doped BTO. Above y = 0.04, the diffraction angles of (101), (110), (002), and (200) were saturated without significant changes, indicating that BTO has a solubility limit for W doping at around y = 0.04. Considering the ionic radii of Ti
4+ (60.5 pm) and W (66 pm, 62 pm, and 60 pm for W
4+, W
5+, and W
6+), Ti
4+ atoms might be replaced by hexavalent W
6+ atoms because they have similar effective ionic radii [
24]. However, as mentioned before, hexavalent W
6+ doping creates a significant charge imbalance in the system, leading to an unstable system with considerable numbers of vacancies and interstitials. Therefore, we could also consider doping a BTO system with W
4+ or W
5+. The oxygen vacancies could not be expected in the BTO system doped with W
4+ or W
5+ for the charge compensation. Considering that the amount of oxygen was larger than the stoichiometric values in the W- and Sr-doped BTO systems (the value of 3-δ was roughly 3.08 according to the EDS measurements), we could not exclude the possibility of excess oxygen in the W-doped BTO system.
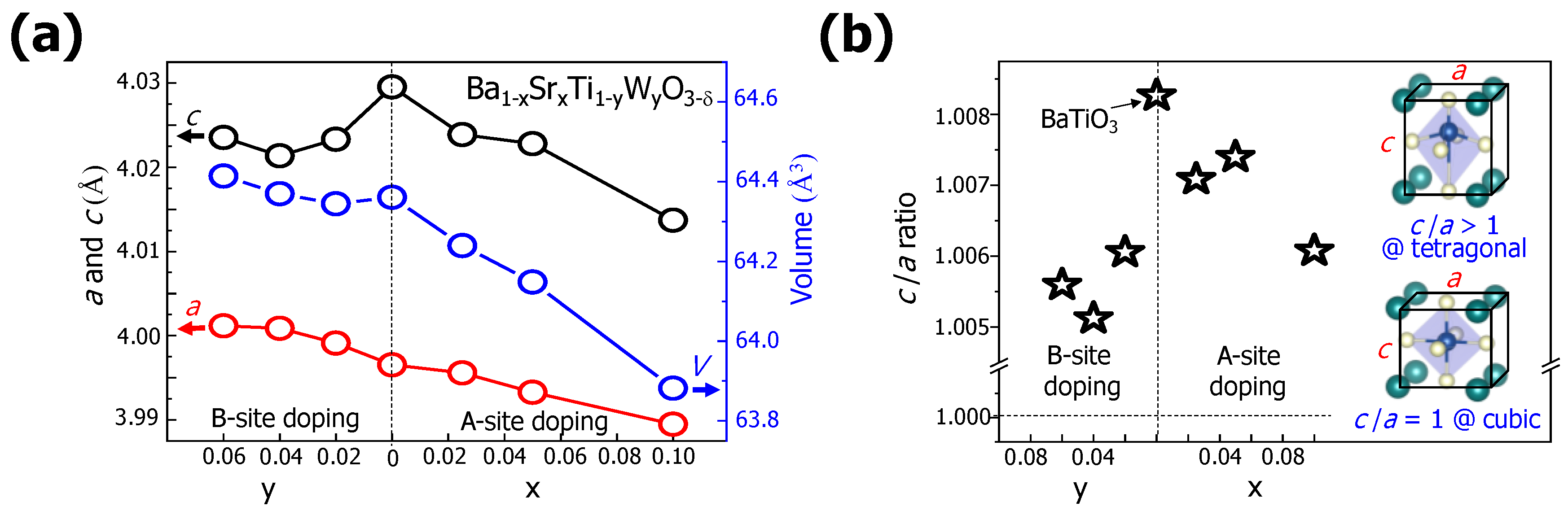
Figure 3a shows the changes in the BTO lattice constants a and c caused by substitution with Sr and W atoms at the A and B sites. With the increase in the Sr content x, the values of the lattice constants a and b simultaneously decreased. The volume of the tetragonal structure of Sr-doped BTO also decreased from 64.36(1) to 63.88(2) Å
3 (0.74%). Considering that the ionic radius of Sr
2+ (118 pm) is smaller than that of Ba
2+ (135 pm), the decreases in the lattice constants and the volume were reasonable. However, with the increase in the dopant content (W) in BTO, the lattice constant a increased while the lattice constant c decreased. With y = 0.06, the change in the lattice constants seemed to be saturated because a = 4.001(1) Å and c = 4.023(5) Å. The volume of W-doped BTO slightly increased with the increase in y from 64.36(1) to 64.41(4) Å
3, indicating that W
4+ or W
5+ atoms could also be candidates as substitutional atoms at the B sites in a BTO system.
In the tetragonal BTO, the lattice constants a and c had different values. We obtained the ratio of a and c to study the effects of doping at the A and B sites on the crystal structure of BTO. Initially, the BTO had a c/a ratio of 1.008, as seen in
Figure 3b. The B-site doping with W atoms resulted in a c/a ratio of about 1.005–1.006, regardless of the W dopant content, while the A-site doping with Sr atoms gradually decreased the c/a ratio from 1.008 (BTO) to 1.006 (x = 0.1) as a function of x. Because the differences between a and c in the tetragonal structure became smaller, the crystal structures of the BTO doped with Sr and with W became similar to the cubic structure. This could result in an indistinct transition between the tetragonal and cubic structures in a doped BTO system with large amounts of Sr and W atoms.
To study the effects of A- and B-site doping on the transition between the tetragonal and cubic BTO structures, the DSC curves of BTO, Ba
1-xSr
xTiO
3 (x = 0.025, 0.05, and 0.1), and BaTi
1-yW
yO
3 (y = 0.02, 0.04, and 0.06) were taken with increasing and decreasing temperatures (from room temperature to 200 °C), as seen in
Figure 4a,c. With the Sr and W substitutions, the endothermic and exothermic peaks in the DSC curves were flattened and the transition ranges were broadened. The transition temperature, T
C, was obtained from the positions of the peaks in the DSC curves, and the latent heat—the enthalpy at T
C—was calculated from the integrated area of the DSC curves. The T
C of BTO was found at 123.1 °C with heating and 114.7 °C with cooling. As the Sr content x increased, the intensity of the DSC peaks around T
C decreased, and the enthalpy (marked with colors) decreased to almost zero. With x = 0.1, the transition seemed to nearly disappear in the DSC curves. The T
C also decreased as the Sr content increased. Considering the significant decrease in the ratio of the lattice constants in the doped BTO, as mentioned above, we concluded that the substitutional Sr atoms at the A sites play a certain role in suppressing the distinct transition between the ferroelectric and paraelectric phases in BTO. This is because of the modified cube-like crystal structure, which has lattice constants (a and c) that are similar.
Figure 4c shows the DSC curves of BaTi
1-yW
yO
3 (y = 0.02, 0.04, and 0.06), which were obtained under the same experimental conditions as in the case of Ba
1-xSr
xTiO
3. Considering the strong dependency of the Sr content on the T
C and the enthalpy in Sr-doped BTO, the T
C of the W-doped BTO seemed to be invariant with all doping contents (i.e., 123.1 °C with heating and 114.7 °C with cooling). The enthalpy of W-doped BTO gradually decreased, providing a similar change to that in the Sr-doped BTO case.
The subsequent decreases in the lattice constants in the Sr-doped BTO indicated that smaller Sr2+ atoms occupied the spaces that were previously occupied by larger Ba2+ atoms. Therefore, a significant decrease in volume was observed, together with a decrease in TC and the latent heat in the Sr-doped BTO. On the other hand, in the W-doped BTO, the lattice constants changed, but the volume and TC seemed to be invariant, while the latent heat in the W-doped BTO decreased with the increase in the dopant content of W. The A-site doping with Sr atoms effectively contributed to the change in the volume of the system, which indirectly influenced the c/a ratio. However, the B-site doping with W atoms contributed to a change in the lattice constants in the tetragonal structure, which directly influenced the c/a ratio. Considering that the W atoms were located at the center of an oxygen octahedron in the W-doped BTO, the ferroelectricity originating from the off-center O2− could be degraded through B-site doping by using a precisely modulated crystal field for the oxygen octahedron without changing the volume. Therefore, we insist that the doping of both the A and B sites in BTO can prevent the transition between the tetragonal and cubic structures by reducing the c/a ratio. The amount of latent heat seemed to be influenced by the c/a ratio, while the values of TC seemed to be influenced by the volume.