Human Umbilical Cord Mesenchymal Stem Cells in Combination with Hyaluronic Acid Ameliorate the Progression of Knee Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. hUC-MSC Isolation and Identification
2.2. Hyaluronic Acid (HA) Preparation
2.3. Cell Viability Analysis of Human Umbilical Cord Mesenchymal Stem Cells (hUC-MSCs) with HA Treatment
2.4. Cytokine Array Analysis
2.5. ELISA Analysis
2.6. Mesenchymal Stem Cell Conditioned Medium (MSC-CM) Preparation and Treatment on H2O2-Damaged Human Chondrocyte Cells
2.7. Mono-Iodoacetate (MIA)-Induced KOA Model
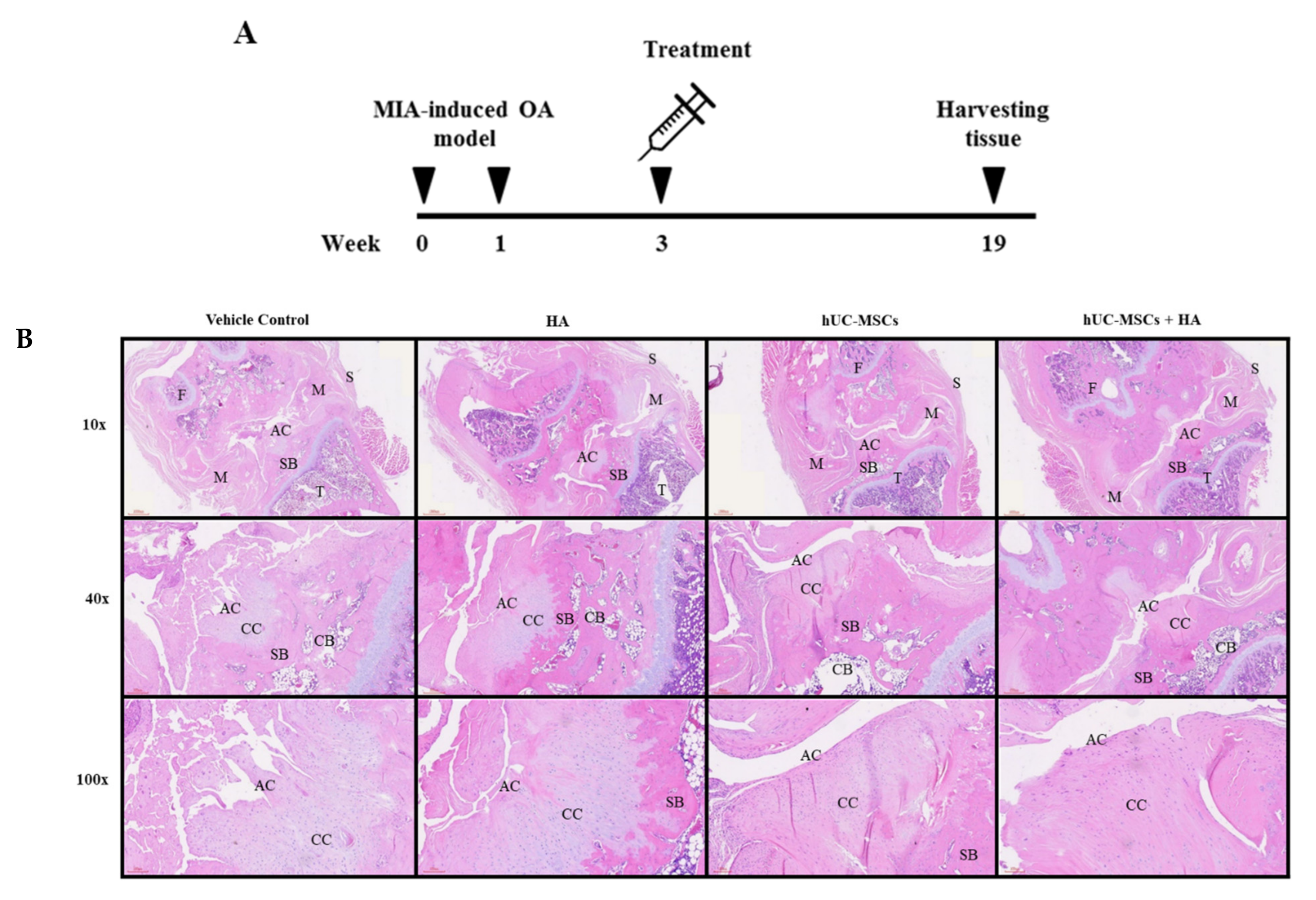
2.8. Histopathological Analysis
2.9. Cytokine Profiling of hUC-MSCs and HA Co-Culture with Osteoarthritis Synovial Fluid (OA SF)
2.10. Statistical Analysis
3. Results
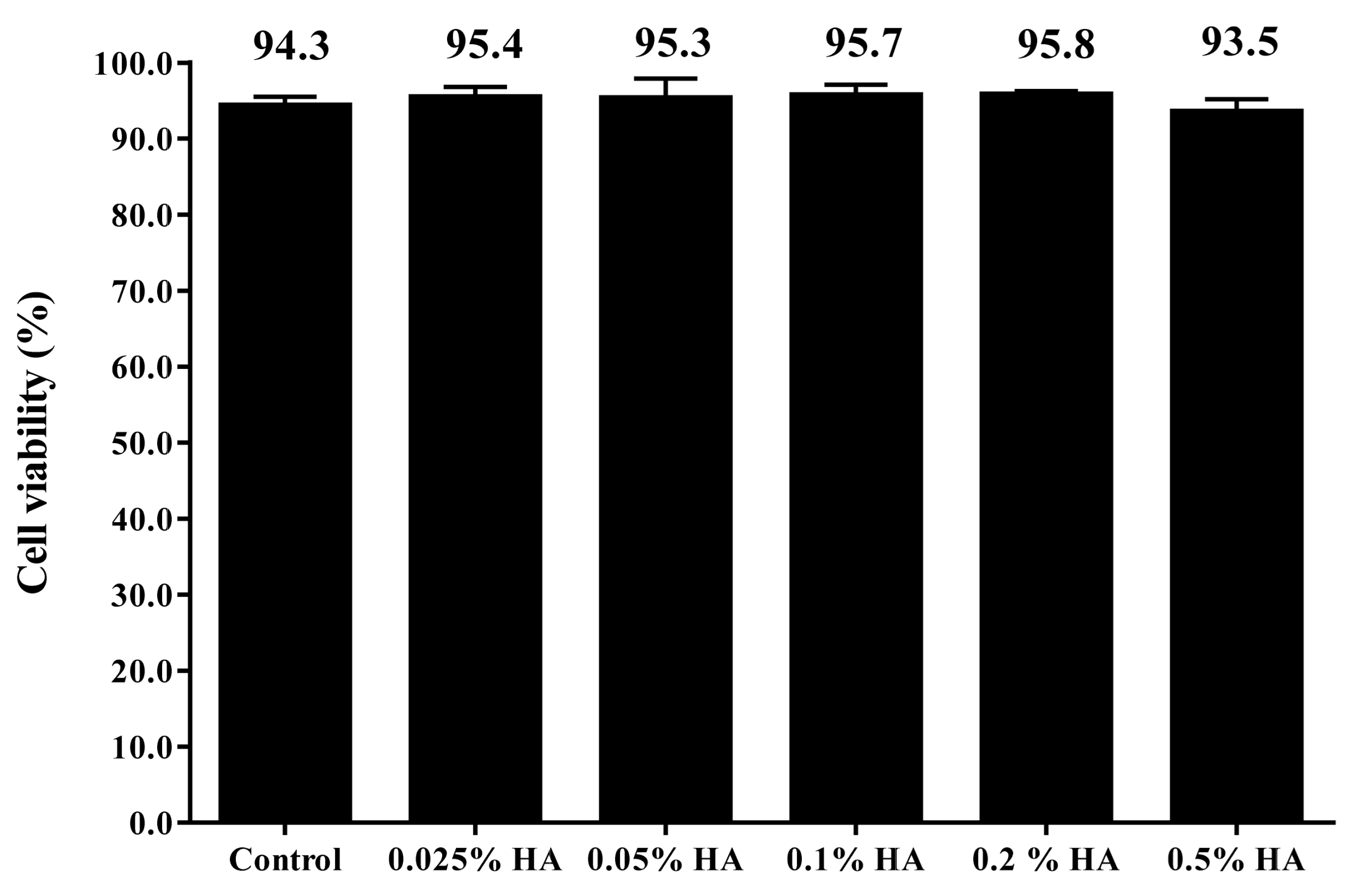
3.1. Different Concentrations of HA Had no Adverse Effects on the Viability of hUC-MSCs
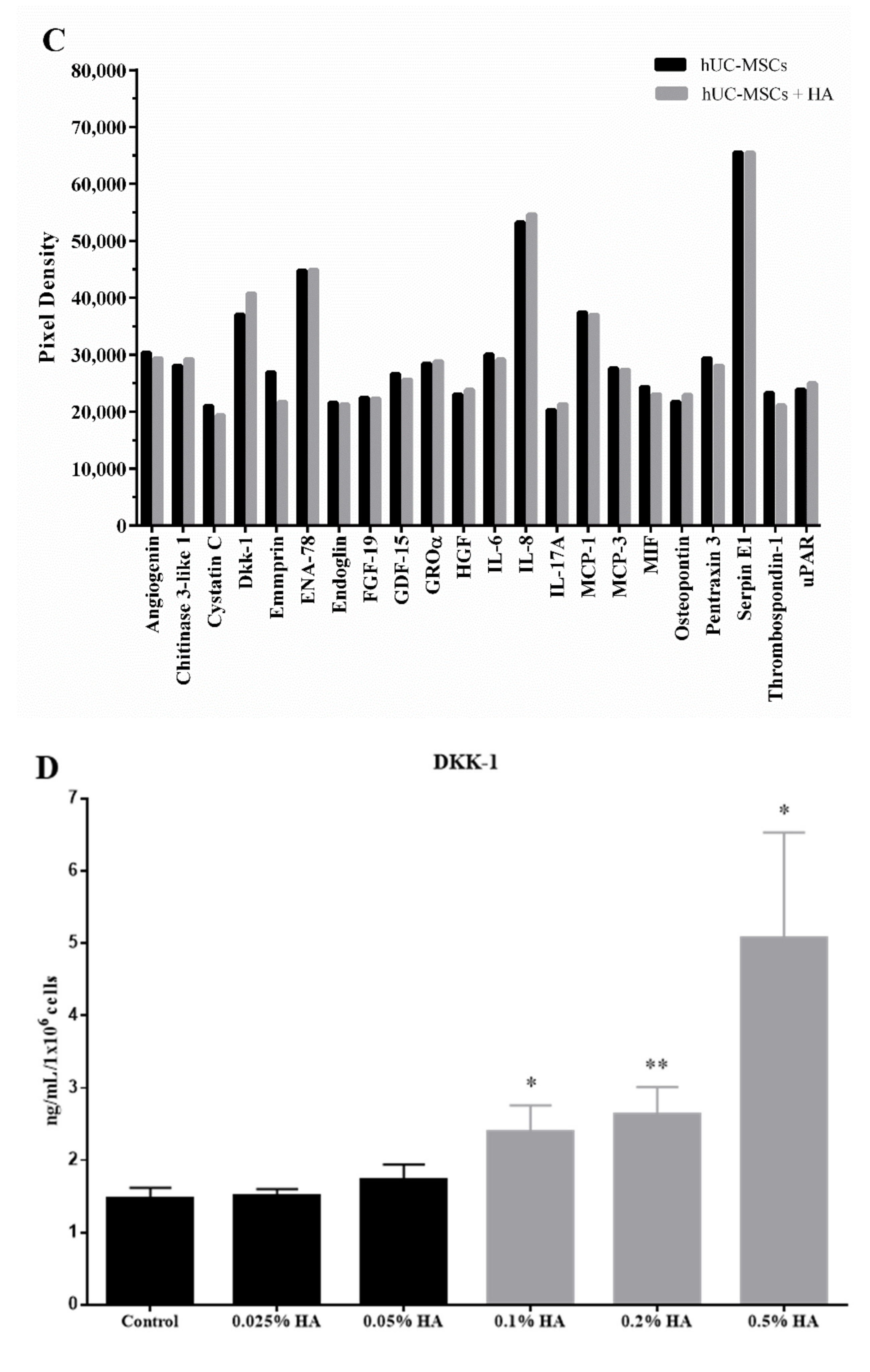
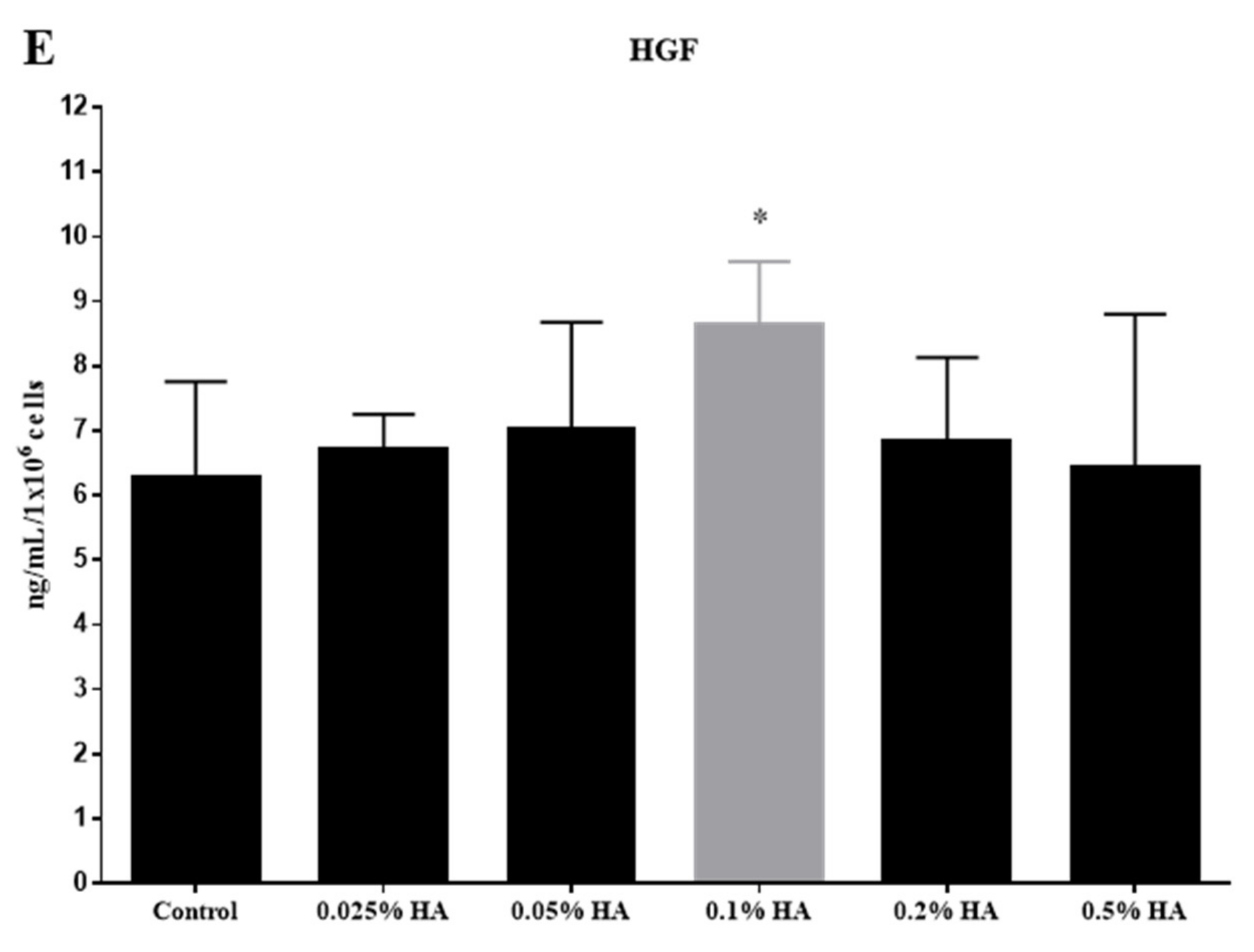
3.2. Cytokine Profiling of hUC-MSCs Treated with Various Concentrations of HA
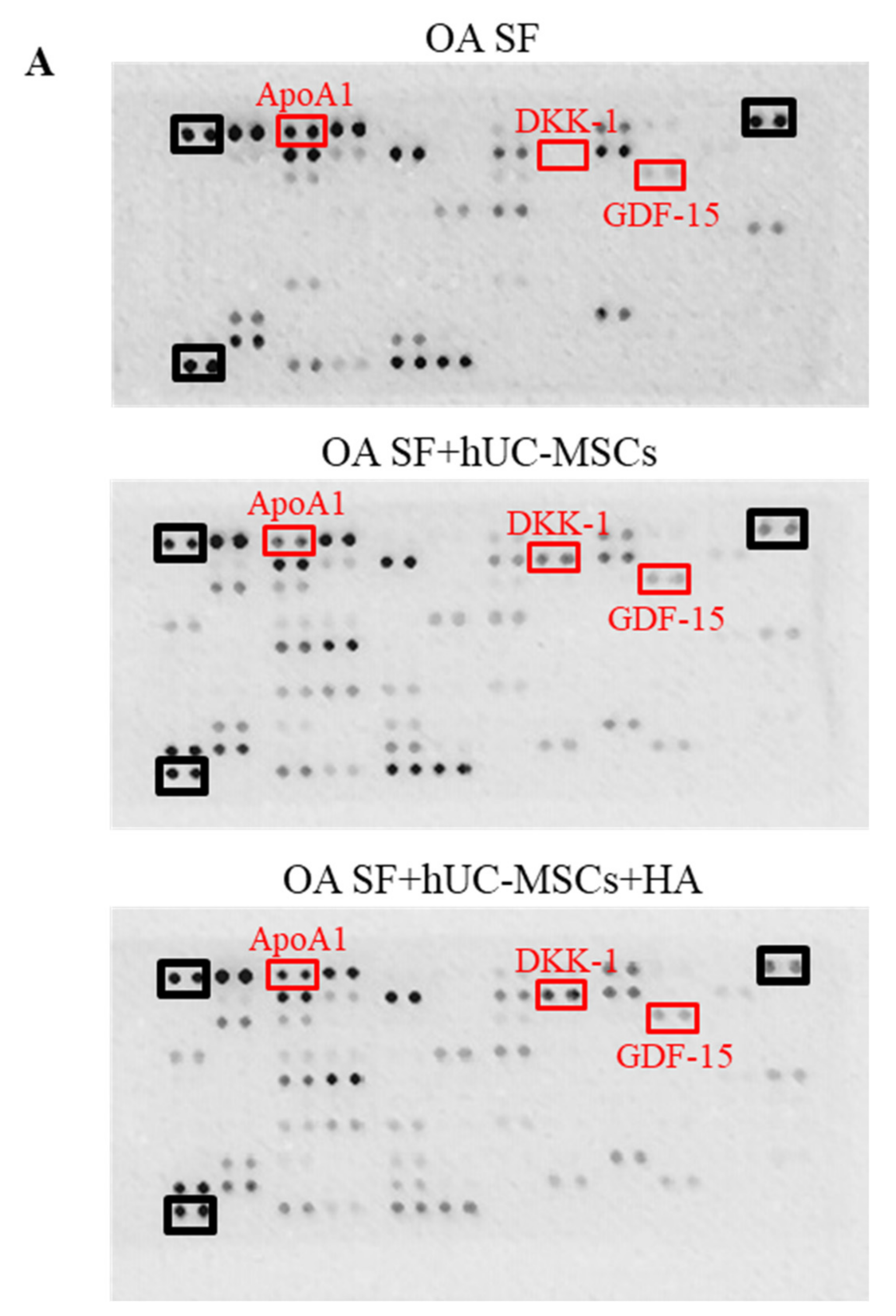
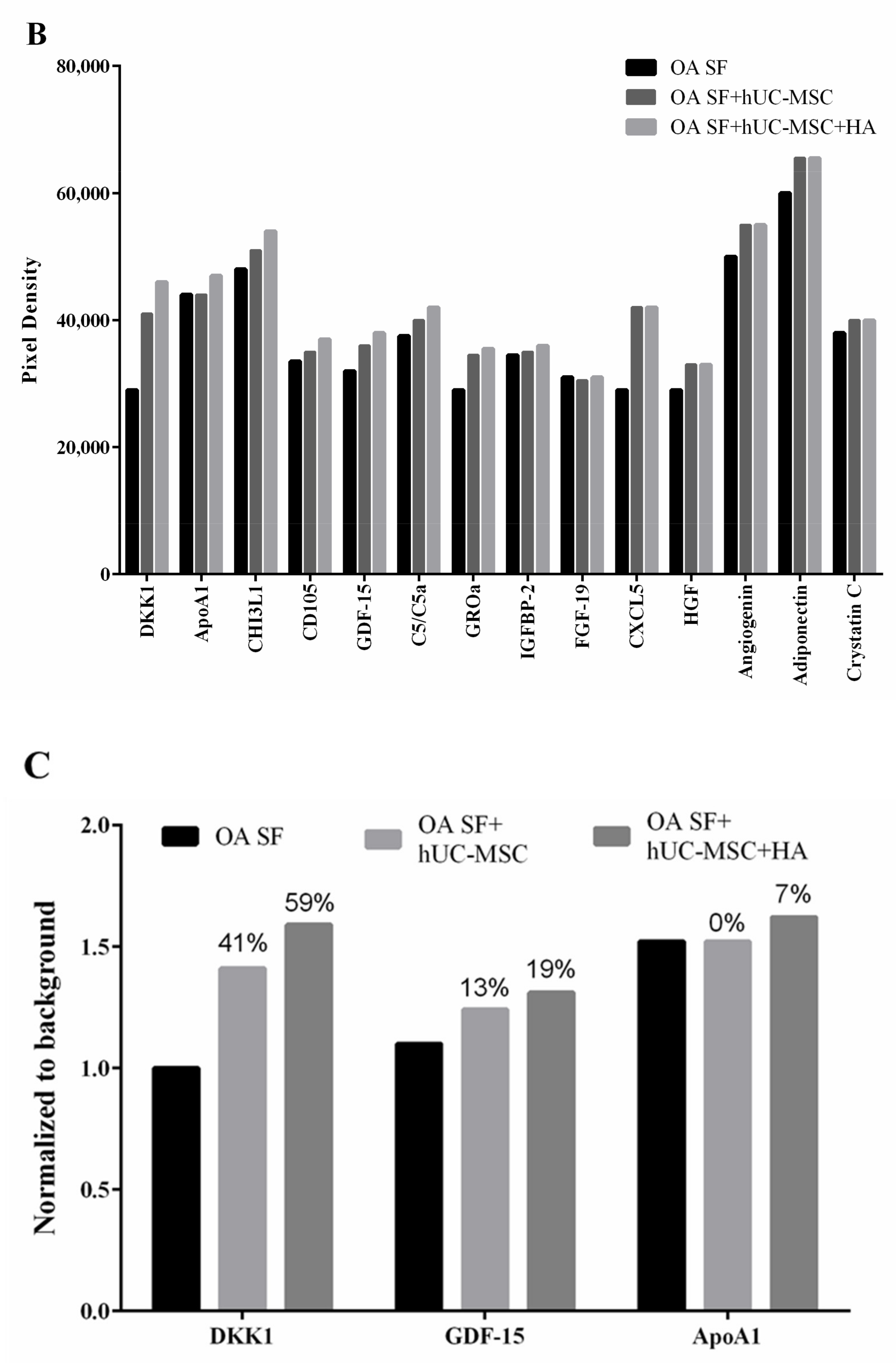
3.3. Cytokine Profiling of hUC-MSCs Cocultured with Osteoarthritis Synovial Fluid and Hyaluronic Acid
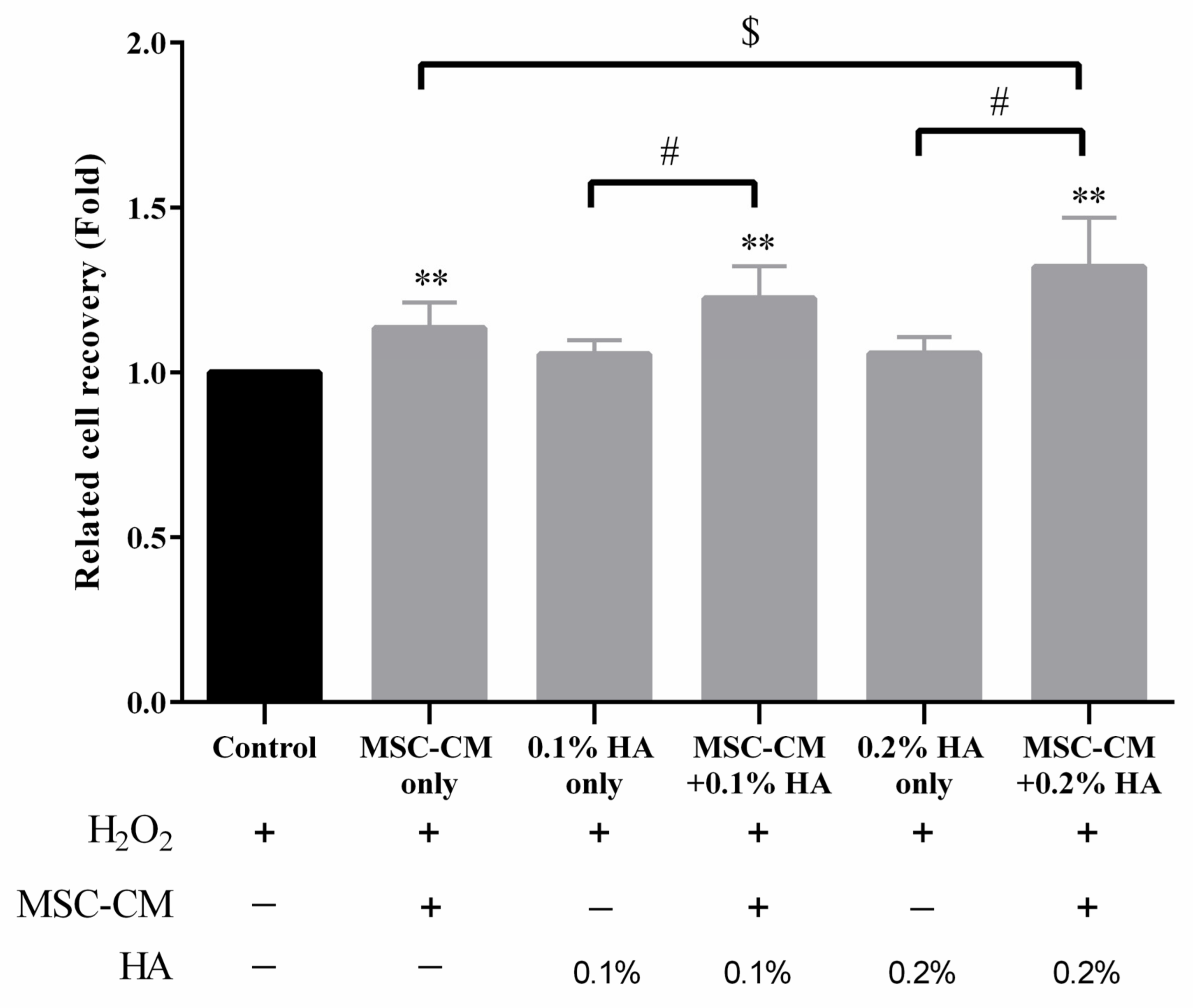
3.4. HA Enhanced Antioxidation Capacity of MSC-CM on Human Chondrocyte Cells
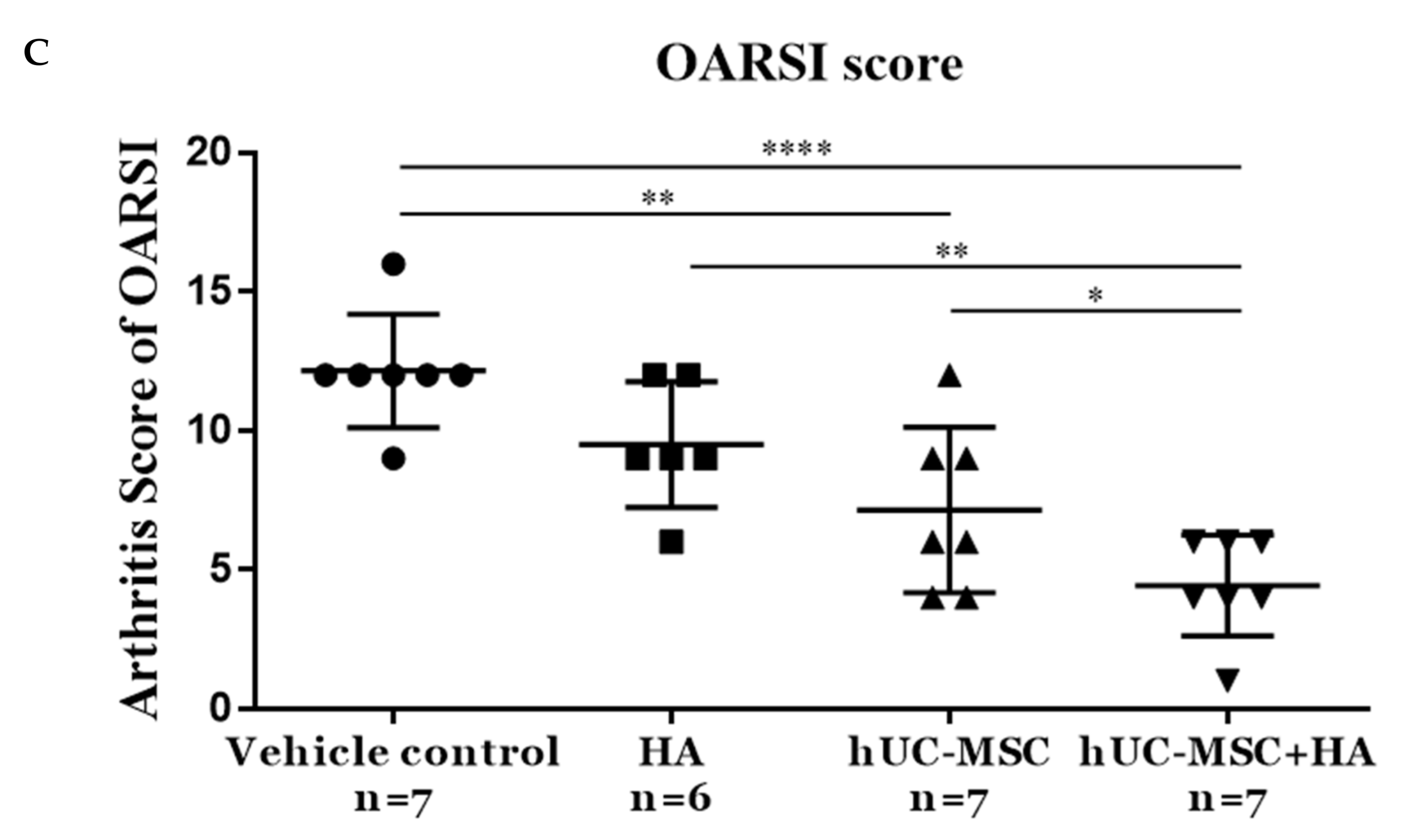
3.5. hUC-MSCs-HA Combinatorial Treatment Reduced Cartilage Damage in KOA Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magnusson, K.; Turkiewicz, A.; Englund, M. Nature vs nurture in knee osteoarthritis—the importance of age, sex and body mass index. Osteoarthr. Cartil. 2019, 27, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Inflammation and intracellular metabolism: New targets in OA. Osteoarthr. Cartil. 2015, 23, 1835–1842. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Spitaels, D.; Mamouris, P.; Vaes, B.; Smeets, M.; Luyten, F.; Hermens, R.; Vankrunkelsven, P. Epidemiology of knee osteoarthritis in general practice: A registry-based study. BMJ Open 2020, 10, e031734. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Siwiec, R.M. Knee Osteoarthritis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyere, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. (Hoboken) 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Wang, A.T.; Feng, Y.; Jia, H.H.; Zhao, M.; Yu, H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J. Stem Cells 2019, 11, 222–235. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Meirelles Lda, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Fei, C.M.; Guo, J.; Zhao, Y.S.; Zhao, S.D.; Zhen, Q.Q.; Shi, L.; Li, X.; Chang, C.K. Clinical significance of hyaluronan levels and its pro-osteogenic effect on mesenchymal stromal cells in myelodysplastic syndromes. J. Transl. Med. 2018, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, B.; Taraballi, F.; Martinez, J.O.; Minardi, S.; Basu, N.; Bauza, G.; Evangelopoulos, M.; Powell, S.; Corbo, C.; Tasciotti, E. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci. Rep. 2017, 7, 7991. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Ha, C.W.; Kim, J.A.; Rhim, J.H.; Park, Y.G.; Chung, J.Y.; Lee, H.J. Effect of Transplanting Various Concentrations of a Composite of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronic Acid Hydrogel on Articular Cartilage Repair in a Rabbit Model. PLoS ONE 2016, 11, e0165446. [Google Scholar] [CrossRef]
- Wen, Y.C.; Du, M.K.; Li, M.W.; Hsuan, Y.C.; Su, Y.C.; Lin, W. EphA2-positive human umbilical cord-derived mesenchymal stem cells exert anti-fibrosis and immunomodulatory activities via secretion of prostaglandin E2. Taiwan. J. Obs. Gynecol. 2018, 57, 722–725. [Google Scholar] [CrossRef]
- Chou, H.C.; Li, Y.T.; Chen, C.M. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am. J. Transl. Res. 2016, 8, 342–353. [Google Scholar]
- Enochson, L.; Stenberg, J.; Brittberg, M.; Lindahl, A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthr. Cartil. 2014, 22, 566–577. [Google Scholar] [CrossRef]
- Honsawek, S.; Tanavalee, A.; Yuktanandana, P.; Ngarmukos, S.; Saetan, N.; Tantavisut, S. Dickkopf-1 (Dkk-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskelet. Disord. 2010, 11, 257. [Google Scholar] [CrossRef]
- Tonomura, H.; Nagae, M.; Takatori, R.; Ishibashi, H.; Itsuji, T.; Takahashi, K. The Potential Role of Hepatocyte Growth Factor in Degenerative Disorders of the Synovial Joint and Spine. Int. J. Mol. Sci. 2020, 21, 8717. [Google Scholar] [CrossRef] [PubMed]
- Pfander, D.; Cramer, T.; Weseloh, G.; Pullig, O.; Schuppan, D.; Bauer, M.; Swoboda, B. Hepatocyte growth factor in human osteoarthritic cartilage. Osteoarthr. Cartil. 1999, 7, 548–559. [Google Scholar] [CrossRef][Green Version]
- Kavalkovich, K.W.; Boynton, R.E.; Murphy, J.M.; Barry, F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 457–466. [Google Scholar] [CrossRef]
- Wong, T.Y.; Chang, C.H.; Yu, C.H.; Huang, L.L.H. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell 2017, 16, 451–460. [Google Scholar] [CrossRef]
- Sirin, D.Y.; Kaplan, N.; Yilmaz, I.; Karaarslan, N.; Ozbek, H.; Akyuva, Y.; Kaya, Y.E.; Oznam, K.; Akkaya, N.; Guler, O.; et al. The association between different molecular weights of hyaluronic acid and CHAD, HIF-1alpha, COL2A1 expression in chondrocyte cultures. Exp. Med. 2018, 15, 4205–4212. [Google Scholar] [CrossRef]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Min. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghanbarvand, F.; Reza Behvarz, M.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Zhang, H.; Wang, Z.; Nan, G.; Li, Y.; Zhang, F.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: Implications in targeted cancer therapies. Lab. Invest. 2016, 96, 116–136. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, Y.S.; Kim, H.J.; Kim, C.H.; Lim, S.W.; Huh, J.W.; Lee, J.H.; Kim, H.R. CD44 enhances the epithelial-mesenchymal transition in association with colon cancer invasion. Int. J. Oncol. 2012, 41, 211–218. [Google Scholar] [CrossRef]
- Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G.; Wu, K. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets 2015, 8, 3783–3792. [Google Scholar] [CrossRef]
- Doumpas, N.; Lampart, F.; Robinson, M.D.; Lentini, A.; Nestor, C.E.; Cantu, C.; Basler, K. TCF/LEF dependent and independent transcriptional regulation of Wnt/beta-catenin target genes. Embo J. 2019, 38. [Google Scholar] [CrossRef]
- de Seny, D.; Cobraiville, G.; Charlier, E.; Neuville, S.; Lutteri, L.; Le Goff, C.; Malaise, D.; Malaise, O.; Chapelle, J.P.; Relic, B.; et al. Apolipoprotein-A1 as a damage-associated molecular patterns protein in osteoarthritis: Ex vivo and in vitro pro-inflammatory properties. PLoS ONE 2015, 10, e0122904. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, L.; van Helvoort, E.; Lafeber, F.; Mastbergen, S.; Hendriks, J.; Post, J.N.; Karperien, M. The Expressions of Dickkopf-Related Protein 1 and Frizzled-Related Protein Are Negatively Correlated to Local Inflammation and Osteoarthritis Severity. Cartilage 2019. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Malizos, K.N.; Oikonomou, P.; Tsezou, A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS ONE 2008, 3, e3740. [Google Scholar] [CrossRef]
- Tsezou, A.; Iliopoulos, D.; Malizos, K.N.; Simopoulou, T. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J. Orthop. Res. 2010, 28, 1033–1039. [Google Scholar] [CrossRef]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef]
- Perez-Garcia, S.; Carrion, M.; Villanueva-Romero, R.; Hermida-Gomez, T.; Fernandez-Moreno, M.; Mellado, M.; Blanco, F.J.; Juarranz, Y.; Gomariz, R.P. Wnt and RUNX2 mediate cartilage breakdown by osteoarthritis synovial fibroblast-derived ADAMTS-7 and -12. J. Cell. Mol. Med. 2019, 23, 3974–3983. [Google Scholar] [CrossRef]
- Xue, J.J.; Zhang, L.Y.; Hou, H.J.; Li, Y.; Liang, W.S.; Yang, K.H. Protective effect of propofol on hydrogen peroxide-induced human esophageal carcinoma via blocking the Wnt/beta-catenin signaling pathway. Iran. J. Basic Med. Sci. 2018, 21, 1297–1304. [Google Scholar] [CrossRef]
- Attie, A.D.; Kastelein, J.P.; Hayden, M.R. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001, 42, 1717–1726. [Google Scholar] [CrossRef]
- Sato, M.; Uchida, K.; Nakajima, H.; Miyazaki, T.; Guerrero, A.R.; Watanabe, S.; Roberts, S.; Baba, H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res. 2012, 14, R31. [Google Scholar] [CrossRef]
- Xing, D.; Wu, J.; Wang, B.; Liu, W.; Liu, W.; Zhao, Y.; Wang, L.; Li, J.J.; Liu, A.; Zhou, Q.; et al. Intra-articular delivery of umbilical cord-derived mesenchymal stem cells temporarily retard the progression of osteoarthritis in a rat model. Int. J. Rheum. Dis. 2020, 23, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Hui, J.H.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem. Cells 2007, 25, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Petrella, R.J.; Cogliano, A.; Decaria, J. Combining two hyaluronic acids in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled trial. Clin. Rheumatol. 2008, 27, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Salini, V. Efficacy and safety study on a new compound associating low and high molecular weight hyaluronic acid in the treatment of hip osteoarthritis. Int. J. Immunopathol. Pharm. 2017, 30, 89–93. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-L.; Wong, P.-C.; Ho, C.-W.; Chen, C.-H.; Liao, K.-Y.; Lovel, R.; Wu, T.B.-C.; Chang, W.-Y.; Lee, Y.-Z.; Lin, W. Human Umbilical Cord Mesenchymal Stem Cells in Combination with Hyaluronic Acid Ameliorate the Progression of Knee Osteoarthritis. Appl. Sci. 2021, 11, 6650. https://doi.org/10.3390/app11146650
Wu J-L, Wong P-C, Ho C-W, Chen C-H, Liao K-Y, Lovel R, Wu TB-C, Chang W-Y, Lee Y-Z, Lin W. Human Umbilical Cord Mesenchymal Stem Cells in Combination with Hyaluronic Acid Ameliorate the Progression of Knee Osteoarthritis. Applied Sciences. 2021; 11(14):6650. https://doi.org/10.3390/app11146650
Chicago/Turabian StyleWu, Jia-Lin, Pei-Chun Wong, Chung-Wei Ho, Chien-Han Chen, Kuan-Ya Liao, Ronald Lovel, Tang Bo-Chung Wu, Wen-Ying Chang, Yan-Zhang Lee, and Willie Lin. 2021. "Human Umbilical Cord Mesenchymal Stem Cells in Combination with Hyaluronic Acid Ameliorate the Progression of Knee Osteoarthritis" Applied Sciences 11, no. 14: 6650. https://doi.org/10.3390/app11146650
APA StyleWu, J.-L., Wong, P.-C., Ho, C.-W., Chen, C.-H., Liao, K.-Y., Lovel, R., Wu, T. B.-C., Chang, W.-Y., Lee, Y.-Z., & Lin, W. (2021). Human Umbilical Cord Mesenchymal Stem Cells in Combination with Hyaluronic Acid Ameliorate the Progression of Knee Osteoarthritis. Applied Sciences, 11(14), 6650. https://doi.org/10.3390/app11146650






