Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reaction of PEDOT:PSS with Epoxyalkanes
2.3. Preparation of the Films
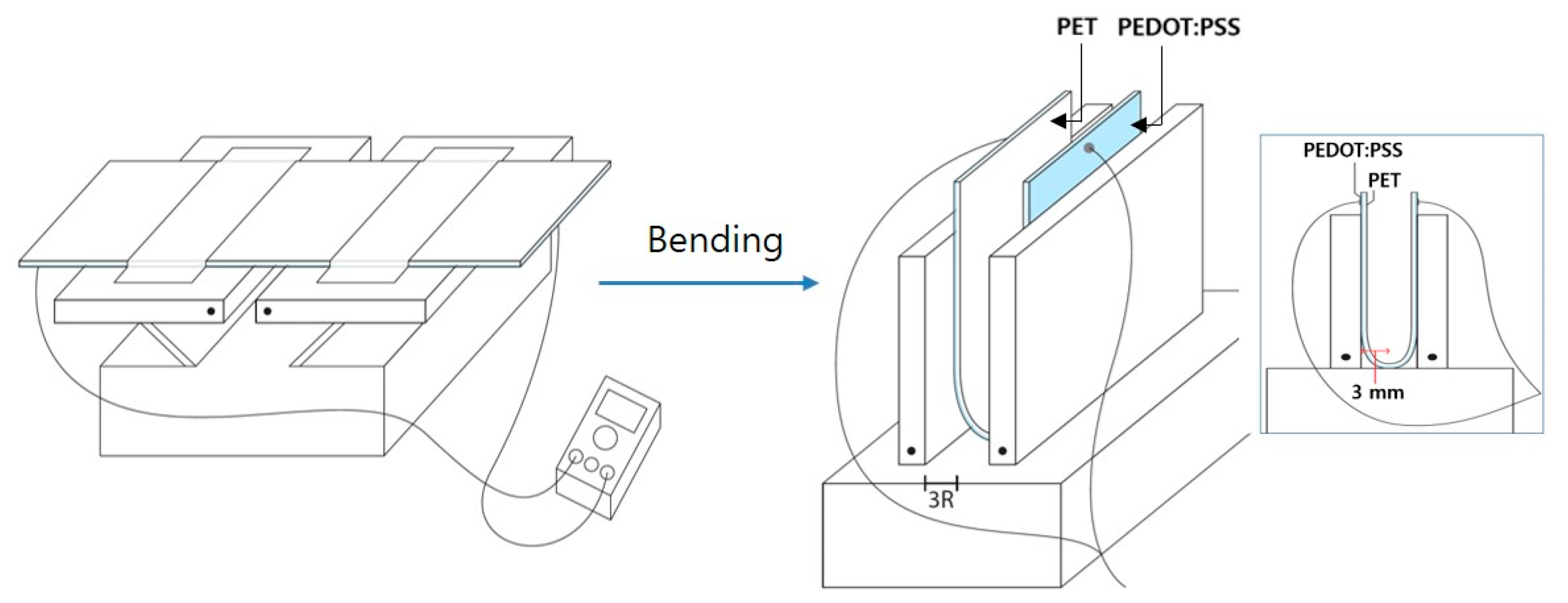
2.4. Bending Tests for the PEDOT:PSS-C6 Films
3. Results
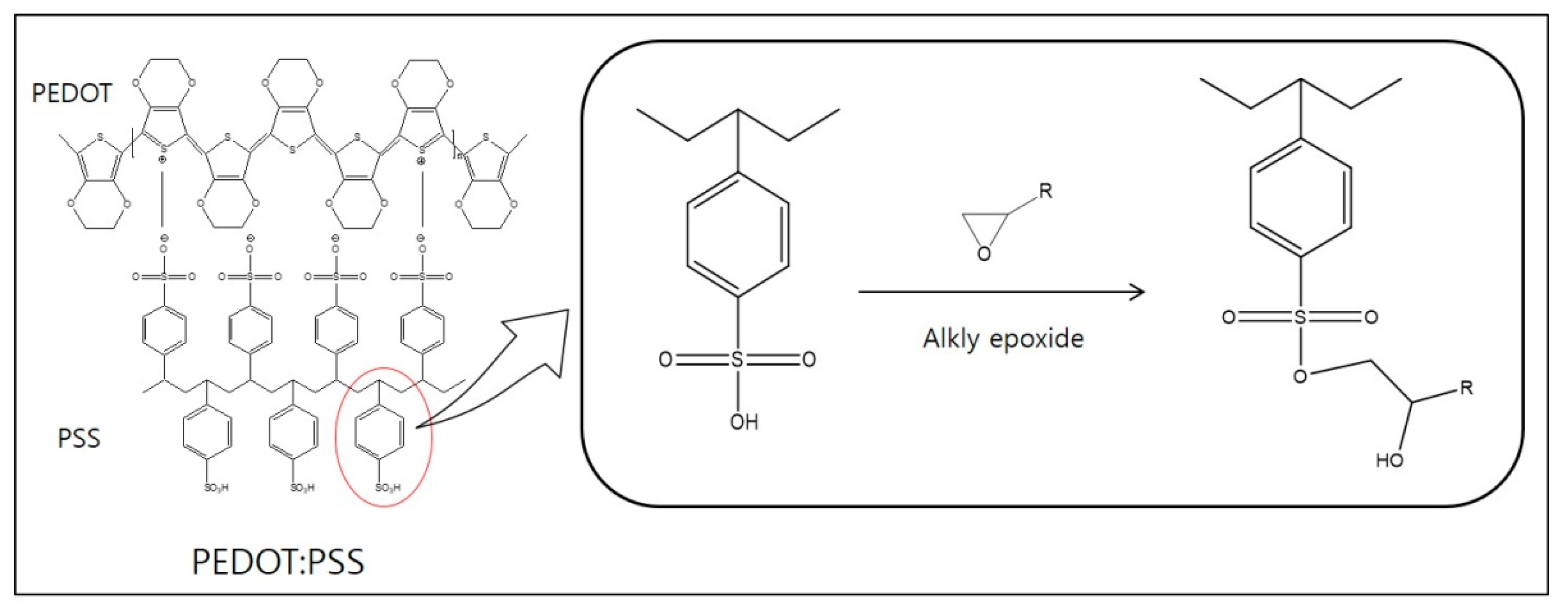
3.1. Reaction of PEDOT:PSS with Epoxyalkanes
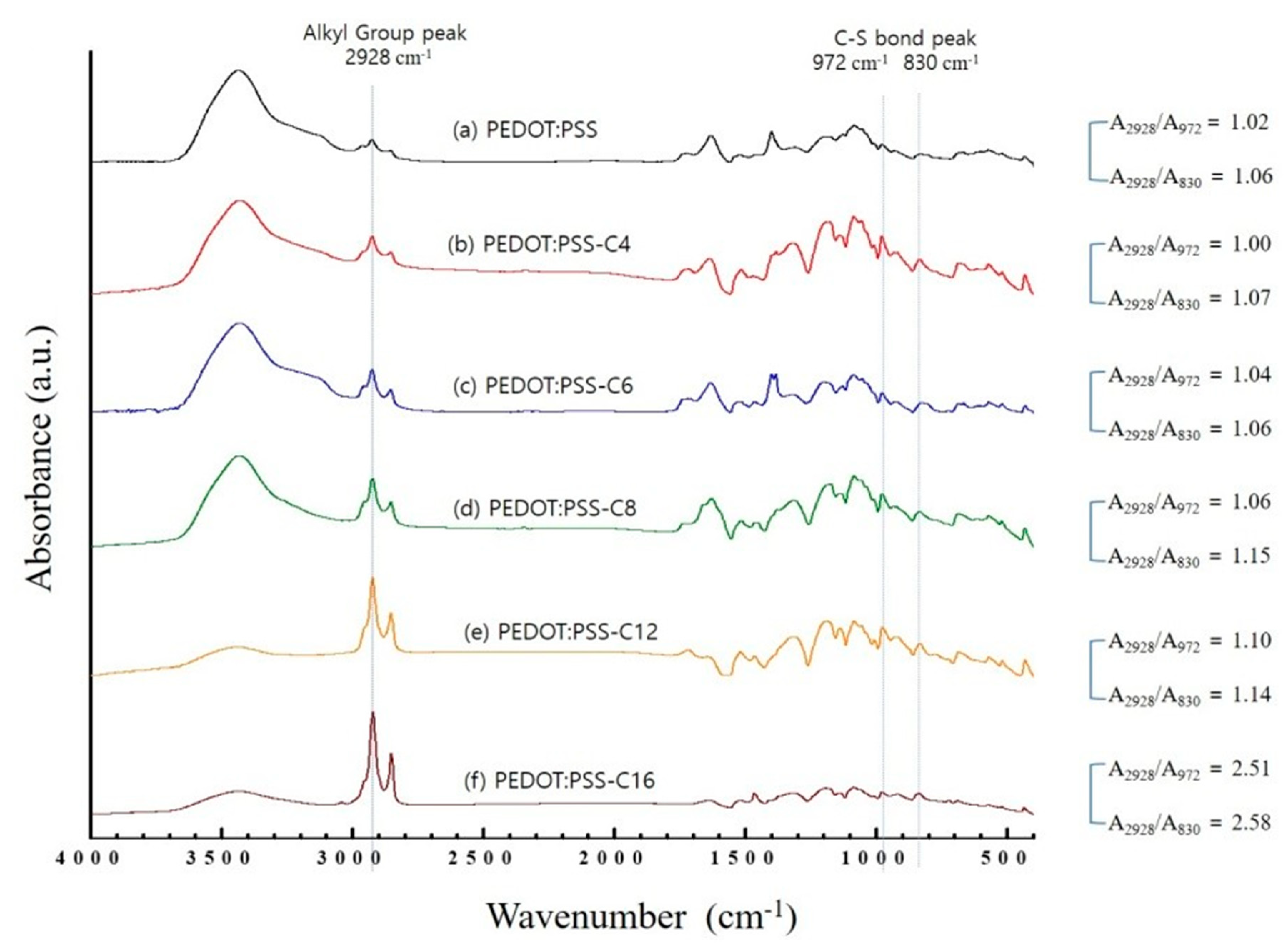
3.2. Analysis of Chemical Structures
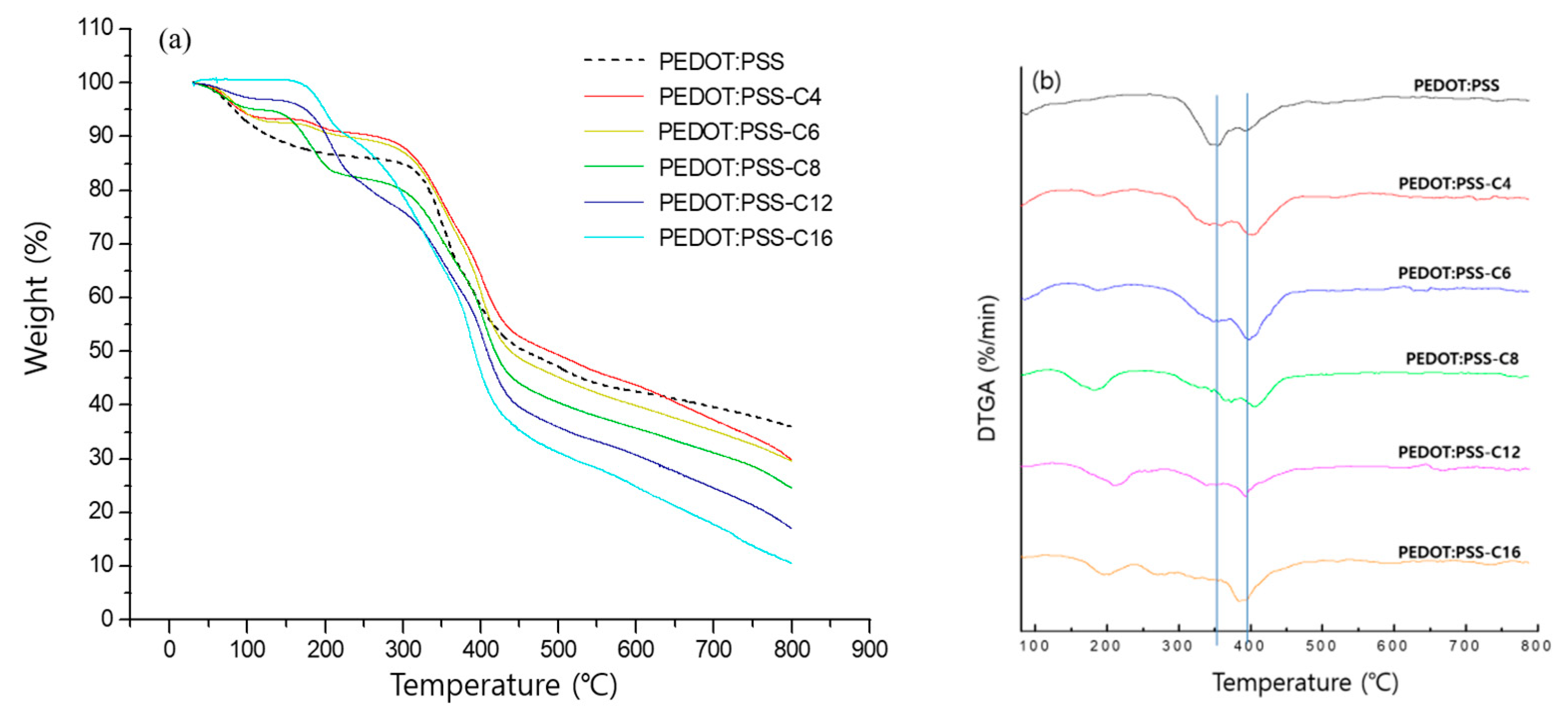
3.3. Properties of Epoxyalkane Derivatives
3.4. Properties of PEDOT:PSS-C6 Films
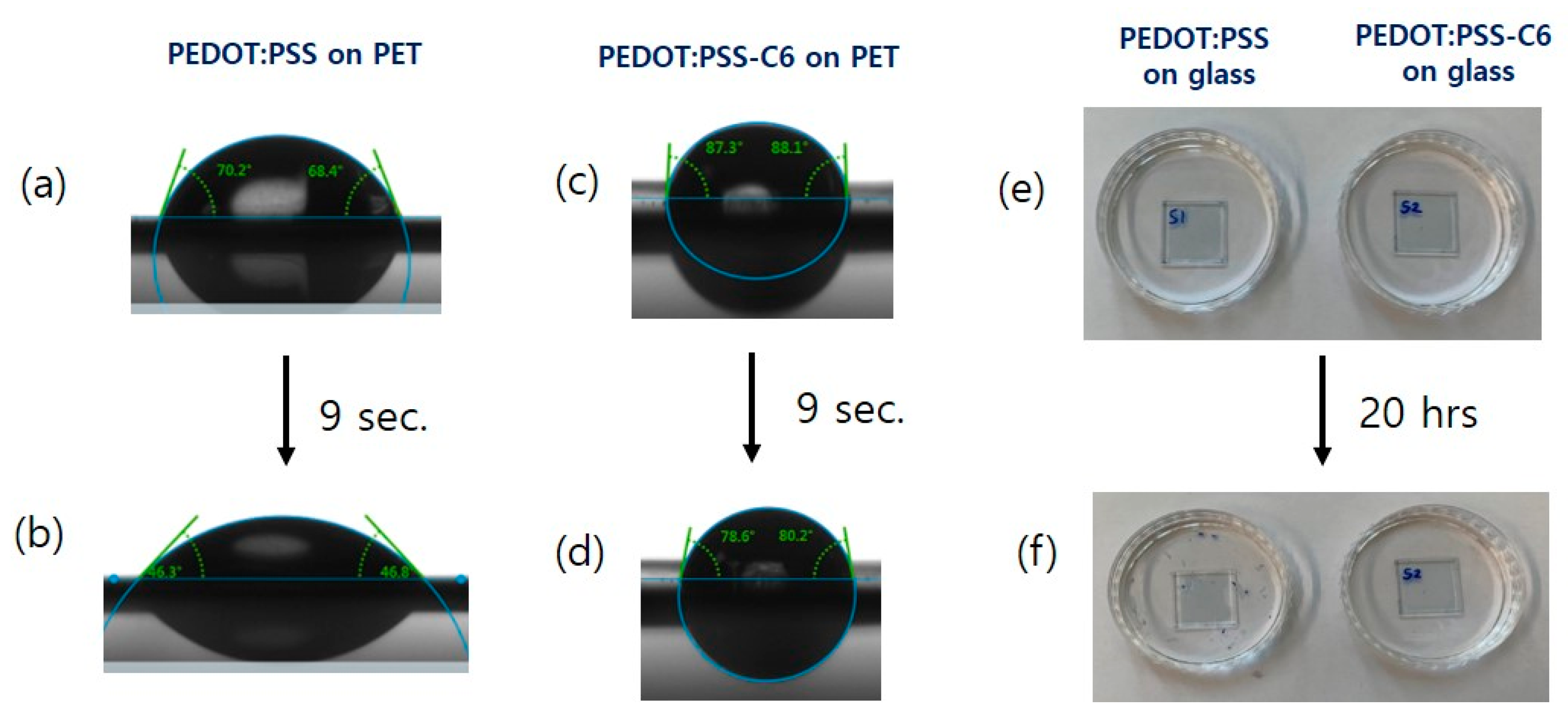
3.5. Flexible Properties of PEDOT:PSS-C6 Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Asplund, M.; Nyberg, T.; Inganäs, O. Electroactive polymers for neural interfaces. Polym. Chem. 2010, 1, 1374–1391. [Google Scholar] [CrossRef]
- Yoon, D.G.; Kang, M.G.; Kim, J.B.; Kang, K.T. Nozzle Printed-PEDOT:PSS for Organic Light Emitting Diodes with Various Dilution Rates of Ethanol. Appl. Sci. 2018, 8, 203. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X. Fiber-based wearable electronics: A review of materials, fabrication, devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Yan, W.; Dong, C.; Xiang, Y.; Xiang, Y.; Jiang, S.; Leber, A.; Loke, G.; Xu, W.; Hou, C.; Zhou, S.; et al. Thermally drawn advanced functional fibers: New frontier of flexible electronics. Mater. Today 2020, 35, 168–194. [Google Scholar] [CrossRef]
- Chen, S.; Song, L.; Tao, Z.; Shao, X.; Huang, Y.; Cui, Q.; Guo, X. Neutral-pH PEDOT:PSS as over-coating layer for stable silver nanowire flexible transparent conductive films. Org. Electron. 2014, 15, 3654–3659. [Google Scholar] [CrossRef]
- Greczynski, G.; Kugler, T.; Salaneck, W.R. Characterization of the PEDOT-PSS system by means of X-ray and ultraviolet photoelectron spectroscopy. Thin Solid Film. 1999, 354, 129–135. [Google Scholar] [CrossRef]
- De Jong, M.P.; van IJzendoorn, L.J.; De Voigt, M.J.A. Stability of the interface between indium-tin-oxide and poly (3,4-ethylenedioxythiophene)/poly (styrene sulfonate) in polymer light-emitting diodes. Appl. Phys. Lett. 2000, 77, 2255–2257. [Google Scholar] [CrossRef]
- Crispin, X.; Marciniak, S.; Osikowicz, W.; Zotti, G.; van der Gon, A.W.D.; Louwet, F.; Fahlman, M.; Groenendaal, L.; De Schryver, F.; Salaneck, W.R. Conductivity, Morphology, Interfacial Chemistry, and Stability of Poly(3,4-ethylene dioxythiophene)-Poly(styrene sulfonate): A Photoelectron Spectroscopy Study. Pol. Phys. 2003, 41, 2561–2583. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y. Chemical modification of Nafion membrane with 3,4-ethylenedioxythiophene for direct methanol fuel cell application. J. Power Sources 2007, 175, 256–260. [Google Scholar] [CrossRef]
- Kim, T.Y.; Suh, M.W.; Kwon, S.J.; Lee, T.H.; Kim, J.E.; Lee, Y.J.; Kim, J.H.; Hong, M.P. Poly(3,4-ethylenedioxythiophene) Derived from Poly(ionic liquid) for the Use as Hole-Injecting Material in Organic Light-Emitting Diodes. Macromol. Rapid Commun. 2009, 30, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, R.; Ochoteco, E.; Pozo-Gonzalo, C.; Grande, H.; Pomposo, J.A.; Mecerreyes, D. New Organic Dispersions of Conducting Polymers Using Polymeric Ionic Liquids as Stabilizers. Macromol. Rapid Commun. 2005, 26, 1122–1126. [Google Scholar] [CrossRef]
- Berezhetska, O.; Liberelle, B.; De Crescenzo, G.; Cicoira, F. A simple approach for protein covalent grafting on conducting polymer films. J. Mater. Chem. B 2015, 3, 5087–5094. [Google Scholar] [CrossRef]
- Hakansson, A.; Han, S.B.; Wang, S.H.; Lu, J.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X.; Fabiano, S. Effect of (3-glycidyloxypropyl) trimethoxysilane (GOPS) on the electrical properties of PEDOT:PSS films. J. Polym. Sci. Pol. Phys. 2017, 55, 814. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Herve, T.; Sanaur, S.; Bernard, C.; et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Doublet, T.; Gurfinkel, M.; Quilichini, P.; Ismailova, E.; Leleux, P.; Herve, T.; Sanaur, S.; Bernard, C.; Malliaras, G.G. Highly Conformable Conducting Polymer Electrodes for In Vivo Recordings. Adv. Healthc. Mater. 2011, 23, H268–H272. [Google Scholar] [CrossRef] [PubMed]
- Perloff, D.S. Four-point sheet Resistance Correction Factors for Thin Rectangular samples. Solid-State Electron. 1977, 20, 681–687. [Google Scholar] [CrossRef]
- Park, M.U.; Lee, M.L.; Chung, D.W. Model system of cross-linked PEDOT:PSS adaptable to an application for an electrode with enhanced water stability. Synth. Met. 2019, 258, 116195. [Google Scholar] [CrossRef]
- Jonsson, S.K.M.; Birgerson, J.; Crispin, X.; Greczynski, G.; Osikowicz, W.; Denier van der Gon, A.W.; Salaneck, W.R.; Fahlman, M. The effects of solvents on the morphology and sheet resistance in poly (3,4-ethylenedioxythiophene)-polystyrenesulfonic acid (PEDOT-PSS) films. Synth. Met. 2003, 139, 1–10. [Google Scholar] [CrossRef]
- Cho, W.; Wu, J.; Shim, B.S.; Kuan, W.F.; Mastroianni, S.E.; Young, W.S.; Kuo, C.C.; Epps, T.H.; Martin, D.C. Synthesis and characterization of bicontinuous cubic poly (3,4-ethylene dioxythiophene) gyroid (PEDOT GYR) gels. Chem. Phys. 2015, 17, 5115–5123. [Google Scholar] [CrossRef]
- Ahmad, I.; McCarthy, J.E.; Baranov, A.; Gun’ko, Y.K. Development of Graphene Nano-Platelet Based Counter Electrodes for Solar Cells. Materials 2015, 8, 5953–5973. [Google Scholar] [CrossRef]
- Friedel, B.; Keivanidis, P.E.; Brenner, T.J.K.; Abrusci, A.; McNeill, C.R.; Friend, R.H.; Greenham, N.C. Effects of Layer Thickness and Annealing of PEDOT:PSS Layers in Organic Photodetectors. Macromolecules 2009, 42, 6741–6747. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348. [Google Scholar] [CrossRef]
- Lee, C.J.; Tsai, I.S. Preparation and Properties of PEDOT/PSS Conductive Polymer Blended with Graphene/PVDF. Adv. Mater. Res. 2013, 608, 1318–1326. [Google Scholar] [CrossRef]
- Park, N.I.; Lee, S.B.; Lee, S.M.; Chung, D. Preparation and Characterization of PEDOT/PSS Hybrid with Graphene Derivative Wrapped by Water-soluble Polymer. Appl. Chem. Eng. 2014, 25, 581–585. [Google Scholar] [CrossRef][Green Version]
- Greczynski, G.; Kugler, T.; Keil, M.; Osikowicz, W.; Fahlman, M.; Salaneck, W.R. Photoelectron spectroscopy of thin films of PEDOT-PSS conjugated polymer blend: A mini-review and some new results. J. Electron Spectrosc. Relat. Phenom. 2001, 121, 1–17. [Google Scholar] [CrossRef]
- Ahn, M.H.; Cho, E.S.; Kwon, S.J. Process Optimization of ITO Film on PC Substrate Deposited by In-line Sputtering Method for a Resistive-type Touch Panel. J. Korean Vac. Soc. 2009, 18, 440–446. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly (3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly (3,4-ethylenedioxythiophene)/poly (4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Badre, C.; Marquant, M.; Alsayed, A.M.; Hough, L.A. Highly Conductive Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) Films Using 1-Ethyl-3-methylimidazolium Tetracyanoborate Ionic Liquid. Adv. Funct. Mater. 2012, 22, 2723–2727. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Lee, J.A.; Vosgueritchian, M.; Tee, B.C.K.; Bolander, J.A.; Bao, Z. Electronic properties of transparent conductive films of PEDOT:PSS on stretchable substrates. Chem. Mater. 2012, 24, 373. [Google Scholar] [CrossRef]
- Yeo, J.S.; Yun, J.M.; Kim, D.Y.; Park, S.; Kim, S.S.; Yoon, M.H.; Kim, T.W.; Na, S.I. Significant Vertical Phase Separation in Solvent-Vapor-Annealed Poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate) Composite Films Leading to Better Conductivity and Work Function for High-Performance Indium Tin Oxide-Free Optoelectronics. ACS Appl. Mater. Interface 2012, 4, 2551. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. Significant Conductivity Enhancement of Conductive Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) Films through a Treatment with Organic Carboxylic Acids and Inorganic Acids. ACS Appl. Mater. Interface 2010, 2, 474. [Google Scholar] [CrossRef]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT:PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Donoval, M.; Micjan, M.; Novota, M.; Nevrela, J.; Kovacova, S.; Pavuk, M.; Juhasz, P.; Jagelka, M.; Kovac Jr, J.; Jakabovic, J.; et al. Relation between secondary doping and phase separation in PEDOT:PSS films. Appl. Sur. Sci. 2017, 395, 86–91. [Google Scholar] [CrossRef]
- Lang, U.; Muller, E.; Naujoks, N.; Dual, J. Microscopical Investigations of PEDOT:PSS Thin Films. Adv. Func. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.Y.; Ho, K.C.; Chu, C.W. Highly conductive PEDOT:PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Park, M.U.; Shin, C.Y.; Kim, H.J.; Kim, S.Y.; Choi, Y.J.; Chung, D. Improved Coating of PEDOT:PSS onto CVD Graphene by the Addition of PVA. Appl. Chem. Eng. 2018, 29, 734–739. [Google Scholar]
- Hwang, B.; Lim, S. PEDOT:PSS Overcoating Layer for Mechanically and Chemically Stable Ag Nanowire Flexible Transparent Electrode. J. Nanomater. 2017, 1489186. [Google Scholar] [CrossRef]
- Park, M.U.; Song, M.; Lee, S.M.; Ryu, S.; Chung, D. Fabrication Process of Bilayer RGO/PEDOT:PSS Film for Flexible Transparent Conductive Electrode. J. Nanosci. Nanotechnol. 2018, 18, 6147–6151. [Google Scholar] [CrossRef] [PubMed]
- Jeadd, A.; Halik, M. Toward strain resistant flexible organic thin film transistors. Appl. Phys. Lett. 2009, 95, 103309. [Google Scholar]
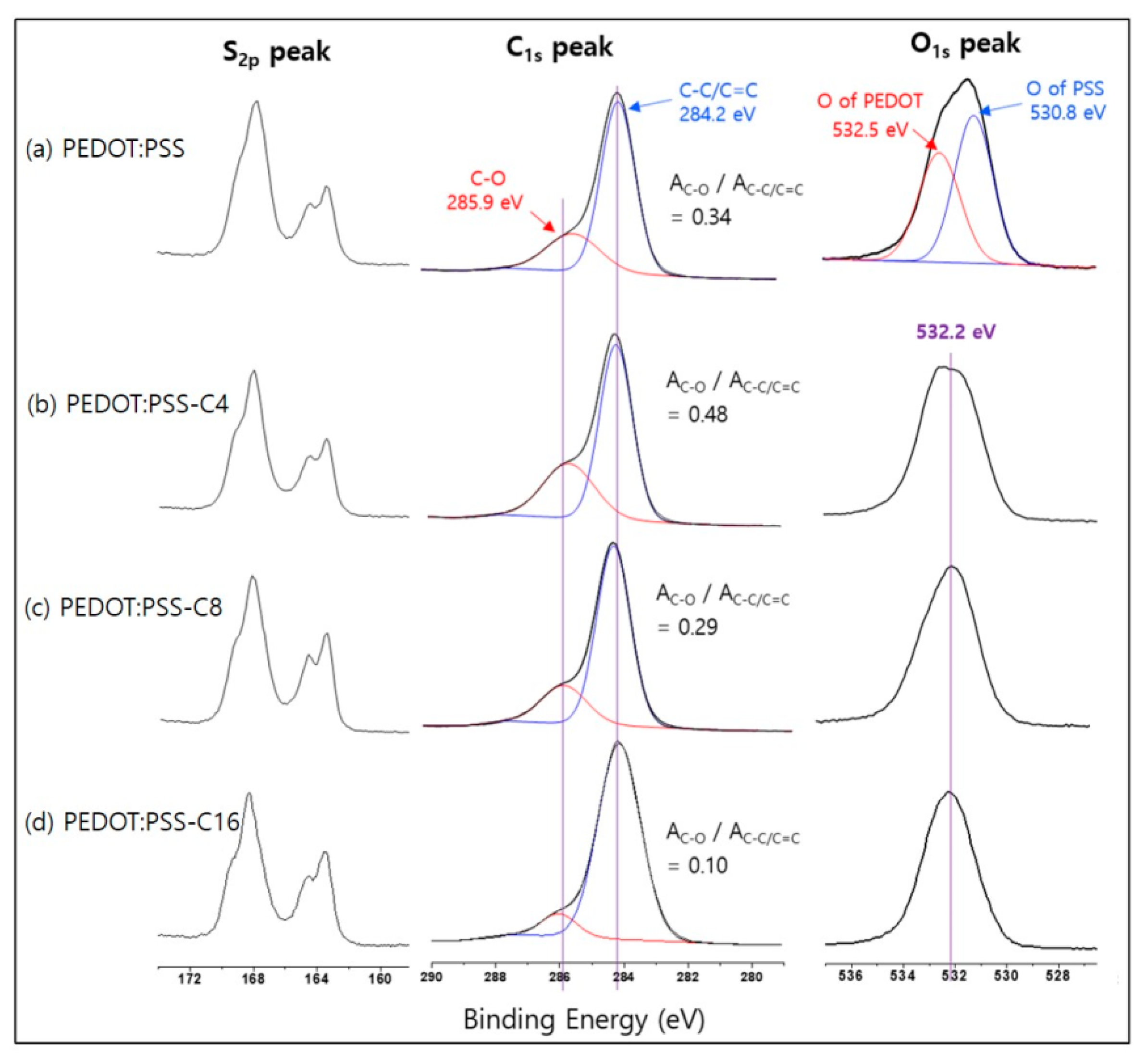


| Code | Carbon Numbers of Epoxyalkanes | Reaction Conditions | Conductivity b) (S·m−1) | |||
|---|---|---|---|---|---|---|
| Weight of PEDOT:PSS Dispersion a) (g) | Weight of Epoxyalkanes (g) | Washing Solvent | Weight of Products Obtained (g) | |||
| PEDOT:PSS | - | 50 g | - | IPA | 0.51 | 132 ± 31 |
| PEDOT:PSS-C4 | 4 | 50 g | 1.19 | IPA | 0.46 | 350 ± 57 |
| PEDOT:PSS-C6 | 6 | 50 g | 1.65 | IPA | 0.55 | 268 ± 16 |
| PEDOT:PSS-C8 | 8 | 50 g | 2.11 | IPA | 0.62 | 114 ± 15 |
| PEDOT:PSS-C12 | 12 | 50 g | 3.03 | IPA | 0.58 | 94 ± 39 |
| PEDOT:PSS-C16 | 16 | 50 g | 3.96 | Acetone | 0.48 | 55 ± 11 |
| Code | DMSO | Contact Angle (°) | Surface Energy (mN/m) | Transmittance (%) | Surface Resistance a) (10 × Ω/sq) | Conductivity b) (S/m) | Surface Roughness. RMS (nm) | |
|---|---|---|---|---|---|---|---|---|
| (I) | PEDOT:PSS | - | 49.9 | 52.4 | 97.5 | 6.2 | 155±59 | 2.539 |
| (II) | PEDOT:PSS | 2 wt% | - | - | 98.2 | 3.1 | - | 3.207 |
| (III) | PEDOT:PSS-C6 | - | 83.0 | 34.6 | 97.7 | 5.6 | 354±81 | 5.141 |
| (IV) | PEDOT:PSS-C6 | 2 wt% | - | - | 97.5 | 3.1 | - | 5.757 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, I.-S.; Park, C.-W.; Kang, H.-I.; Joe, S.-y.; Pak, N.-Y.; Chung, D.-w. Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode. Appl. Sci. 2021, 11, 6605. https://doi.org/10.3390/app11146605
Hwang I-S, Park C-W, Kang H-I, Joe S-y, Pak N-Y, Chung D-w. Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode. Applied Sciences. 2021; 11(14):6605. https://doi.org/10.3390/app11146605
Chicago/Turabian StyleHwang, In-Seong, Chul-Woo Park, Hye-In Kang, Sung-yoon Joe, Na-Young Pak, and Dae-won Chung. 2021. "Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode" Applied Sciences 11, no. 14: 6605. https://doi.org/10.3390/app11146605
APA StyleHwang, I.-S., Park, C.-W., Kang, H.-I., Joe, S.-y., Pak, N.-Y., & Chung, D.-w. (2021). Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode. Applied Sciences, 11(14), 6605. https://doi.org/10.3390/app11146605





