Soft Bio-Integrated Multifunctional Devices Using an Intrinsically Stretchable Conducting Nanomembrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Au–SEBS Electrodes and Sensors
2.2. Mechanical and Electrical Characterizations of Au–SEBS Electrodes
2.3. Various Sensors and Heater Demonstration of the Au–SEBS Electrodes
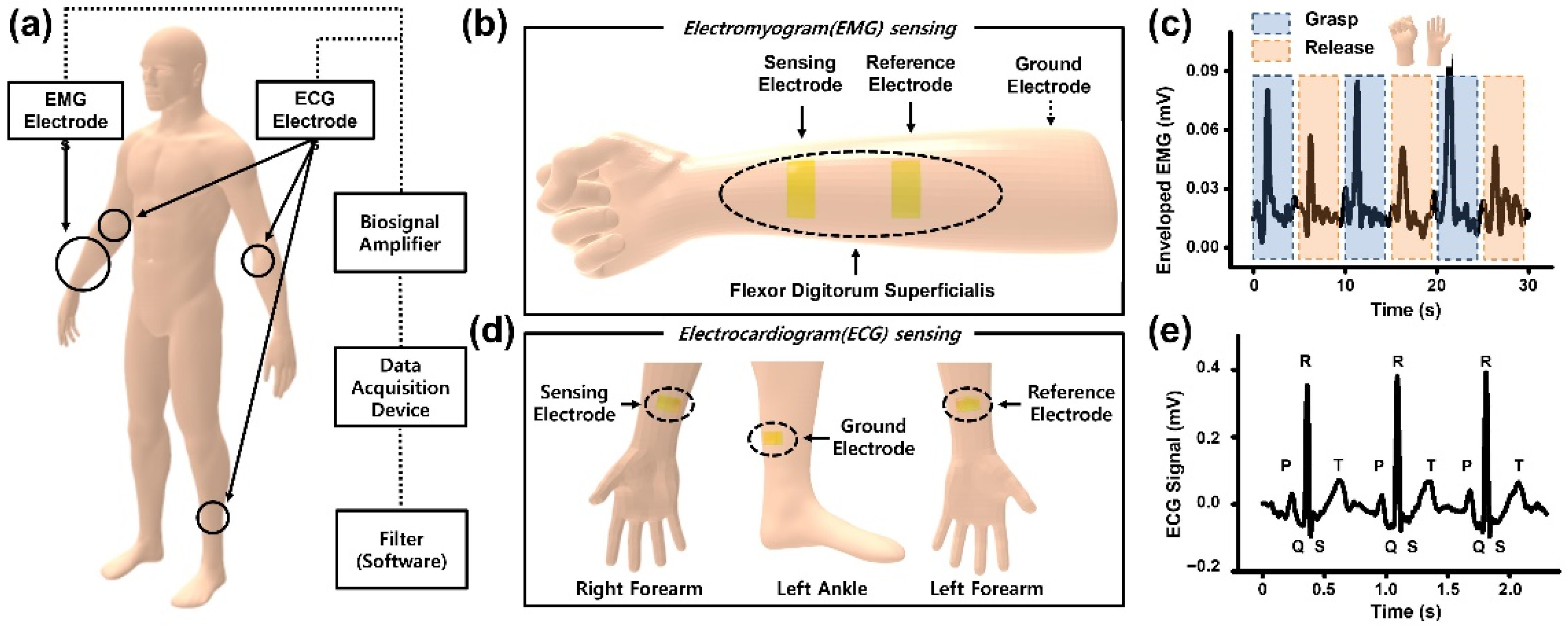
2.4. Measurement of Electrocardiogram and Electromyogram Signal from Human Skin
3. Results and Discussion
3.1. Stretching Test and Morphological Characterization of the Au–SEBS Electrodes
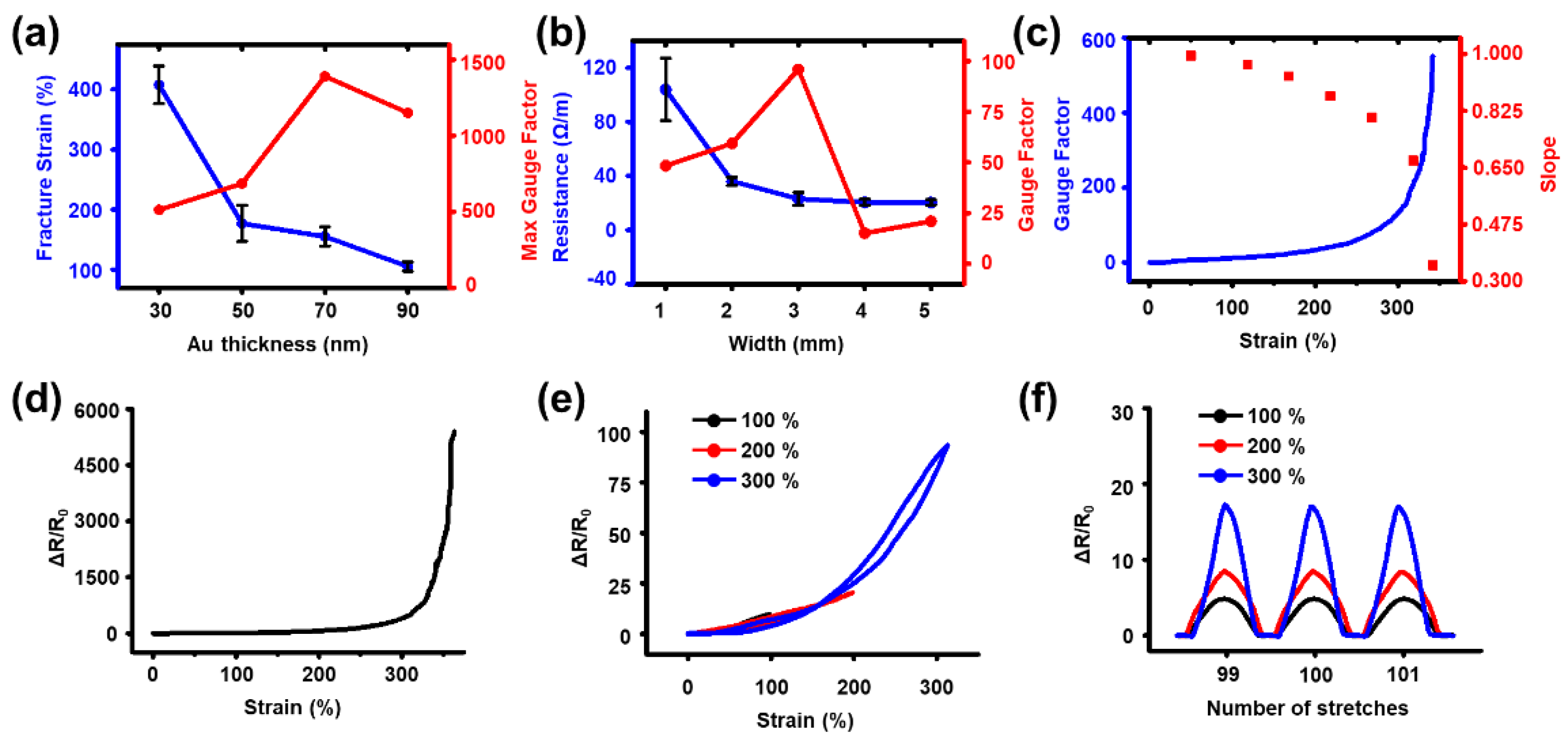
3.2. Strain Sensor
3.3. Electrochemical Sensor
3.4. Temperature Sensor
3.5. Heater
3.6. Electrophysiology Measurement of the Human Body
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Jiang, K.; Wei, Z.; Shen, G. Reviews of wearable healthcare systems: Materials, devices and system integration. Mater. Sci. Eng. R Reports 2020, 140, 100523. [Google Scholar] [CrossRef]
- Koydemir, H.C.; Ozcan, A. Wearable and Implantable Sensors for Biomedical Applications. Annu. Rev. Anal. Chem. 2018, 11, 127–146. [Google Scholar] [CrossRef]
- Ha, M.; Lim, S.; Ko, H. Wearable and flexible sensors for user-interactive health-monitoring devices. J. Mater. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zeng, X.; Xia, F.; Jin, W.; Liu, Y.; Hu, Y. Recent Advances in Flexible and Stretchable Sensing Systems: From the Perspective of System Integration. ACS Nano 2020, 14, 6449–6469. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C.F. Flexible Electronics: Stretchable Electrodes and Their Future. Adv. Funct. Mater. 2019, 29, 1805924. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Hou, Y.; Yin, C.; Yin, Z. A review on structures, materials and applications of stretchable electrodes. Front. Mater. Sci. 2021, 15, 54–78. [Google Scholar] [CrossRef]
- Park, M.; Park, J.; Jeong, U. Design of conductive composite elastomers for stretchable electronics. Nano Today 2014, 9, 244–260. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Nanomaterial-enabled stretchable conductors: Strategies, materials and devices. Adv. Mater. 2015, 27, 1480–1511. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.E. Recent Progress on Stretchable Electronic Devices with Intrinsically Stretchable Components. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Kim, Y.; Kweon, O.Y.; Won, Y.; Oh, J.H. Deformable and Stretchable Electrodes for Soft Electronic Devices. Macromol. Res. 2019, 27, 625–639. [Google Scholar] [CrossRef]
- Sun, Y.; Choi, W.M.; Jiang, H.; Huang, Y.Y.; Rogers, J.A. Controlled buckling of semiconductor nanoribbons for stretchable electronics. Nat. Nanotechnol. 2006, 1, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Song, J.; Won, M.C.; Kim, H.S.; Kim, R.H.; Liu, Z.; Huang, Y.Y.; Hwang, K.C.; Zhang, Y.W.; Rogers, J.A. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl. Acad. Sci. USA 2008, 105, 18675–18680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Xiao, J.; Song, J.; Huang, Y.; Rogers, J.A. Stretchable, curvilinear electronics based on inorganic materials. Adv. Mater. 2010, 22, 2108–2124. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef]
- Fan, J.A.; Yeo, W.H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Rondeau-Gagné, S.; Chiu, Y.C.; Chortos, A.; Lissel, F.; Wang, G.J.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef]
- Oh, J.Y.; Son, D.; Katsumata, T.; Lee, Y.; Kim, Y.; Lopez, J.; Wu, H.C.; Kang, J.; Park, J.; Gu, X.; et al. Stretchable self-healable semiconducting polymer film for active-matrix strain-sensing array. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Sim, K.; Rao, Z.; Kim, H.J.; Thukral, A.; Shim, H.; Yu, C. Fully rubbery integrated electronics from high effective mobility intrinsically stretchable semiconductors. Sci. Adv. 2019, 5, eaav5749. [Google Scholar] [CrossRef] [Green Version]
- Tien, H.C.; Huang, Y.W.; Chiu, Y.C.; Cheng, Y.H.; Chueh, C.C.; Lee, W.Y. Intrinsically stretchable polymer semiconductors: Molecular design, processing and device applications. J. Mater. Chem. C 2021, 9, 2660–2684. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Seo, H.; Kang, J.; Hong, J.; Seong, D.; Kim, H.J.; Kim, J.; Mun, J.; Youn, I.; Kim, J.; et al. An Ultrastretchable and Self-Healable Nanocomposite Conductor Enabled by Autonomously Percolative Electrical Pathways. ACS Nano 2019, 13, 6531–6539. [Google Scholar] [CrossRef]
- Lynch, P.J.; Ogilvie, S.P.; Large, M.J.; Graf, A.A.; O’Mara, M.A.; Taylor, J.; Salvage, J.P.; Dalton, A.B. Graphene-based printable conductors for cyclable strain sensors on elastomeric substrates. Carbon 2020, 169, 25–31. [Google Scholar] [CrossRef]
- Seyedin, S.; Uzun, S.; Levitt, A.; Anasori, B.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Garcia, J.R.; O’Suilleabhain, D.; Kaur, H.; Coleman, J.N. A Simple Model Relating Gauge Factor to Filler Loading in Nanocomposite Strain Sensors. ACS Appl. Nano Mater. 2021, 4, 2876–2886. [Google Scholar] [CrossRef]
- Jaymand, M. Recent progress in chemical modification of polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Lacour, S.P.; Chan, D.; Wagner, S.; Li, T.; Suo, Z. Mechanisms of reversible stretchability of thin metal films on elastomeric substrates. Appl. Phys. Lett. 2006, 88, 204103. [Google Scholar] [CrossRef]
- Graudejus, O.; Görrn, P.; Wagner, S. Controlling the morphology of gold films on poly(dimethylsiloxane). ACS Appl. Mater. Interfaces 2010, 2, 1927–1933. [Google Scholar] [CrossRef]
- Koshi, T.; Iwase, E. Crack-configuration analysis of metal conductive track embedded in stretchable elastomer. Micromachines 2018, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, X.; Qi, D.; Xu, C.; Yu, J.; Liu, Y.; Jiang, Y.; Liedberg, B.; Chen, X. High-Adhesion Stretchable Electrodes Based on Nanopile Interlocking. Adv. Mater. 2017, 29, 1603382. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Zhang, Q.; Lopez, J.; Wang, H.; Wu, H.C.; Niu, S.; Yan, H.; Wang, S.; Lei, T.; et al. Quadruple H-Bonding cross-linked supramolecular polymeric materials as substrates for stretchable, antitearing, and self-healable thin film electrodes. J. Am. Chem. Soc. 2018, 140, 5280–5289. [Google Scholar] [CrossRef]
- Song, K., II; Seo, H.; Seong, D.; Kim, S.; Yu, K.J.; Kim, Y.C.; Kim, J.; Kwon, S.J.; Han, H.S.; Youn, I.; et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Reddy, N.P.; Gupta, V. Toward direct biocontrol using surface EMG signals: Control of finger and wrist joint models. Med. Eng. Phys. 2007, 29, 398–403. [Google Scholar] [CrossRef]
- Mohideen, A.J.H.; Sidek, S.N. Development of EMG circuit to study the relationship between flexor digitorum superficialis muscle activity and hand grip strength. In Proceedings of the 2011 4th International Conference on Mechatronics (ICOM), Kuala Lumpur, Malaysia, 17–19 May 2011; pp. 17–19. [Google Scholar] [CrossRef]
- Gargiulo, G.D.; Bifulco, P.; Cesarelli, M.; McEwan, A.L.; Moeinzadeh, H.; O’loughlin, A.; Shugman, I.M.; Tapson, J.C.; Thiagalingam, A. On the einthoven triangle: A critical analysis of the single rotating dipole hypothesis. Sensors 2018, 18, 2353. [Google Scholar] [CrossRef] [Green Version]
- Kolanowska, A.; Herman, A.P.; Jȩdrysiak, R.G.; Boncel, S. Carbon nanotube materials for electrocardiography. RSC Adv. 2021, 11, 3020–3042. [Google Scholar] [CrossRef]
- Song, Z.; Li, W.; Bao, Y.; Han, F.; Gao, L.; Xu, J.; Ma, Y.; Han, D.; Niu, L. Breathable and Skin-Mountable Strain Sensor with Tunable Stretchability, Sensitivity, and Linearity via Surface Strain Delocalization for Versatile Skin Activities’ Recognition. ACS Appl. Mater. Interfaces 2018, 10, 42826–42836. [Google Scholar] [CrossRef]
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.Y.; Kim, T.I.; Choi, M. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 2014, 516, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, X.; Jiang, X.; Lin, S.; Lao, J.; Shi, J.; Zhen, Z.; Li, Z.; Zhu, H. Structural engineering of gold thin films with channel cracks for ultrasensitive strain sensing. Mater. Horiz. 2016, 3, 248–255. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.J.; Nguyen, T.; Chu, M.; Pegan, J.D.; Khine, M. Highly stretchable wrinkled gold thin film wires. Appl. Phys. Lett. 2016, 108. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Lee, J.; Lee, H.; Yeo, J.; Hong, S.; Nam, K.H.; Lee, D.; Lee, S.S.; Ko, S.H. Highly stretchable and highly conductive metal electrode by very long metal nanowire percolation network. Adv. Mater. 2012, 24, 3326–3332. [Google Scholar] [CrossRef]
- Liu, D.; Perdue, R.K.; Sun, L.; Crooks, R.M. Immobilization of DNA onto poly(dimethylsiloxane) surfaces and application to a microelectrochemical enzyme-amplified DNA hybridization assay. Langmuir 2004, 20, 5905–5910. [Google Scholar] [CrossRef]
- Nebling, E.; Grunwald, T.; Albers, J.; Schäfer, P.; Hintsche, R. Electrical Detection of Viral DNA Using Ultramicroelectrode Arrays. Anal. Chem. 2004, 76, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Varshney, M.; Li, Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009, 24, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Colin, S.; Krier, G.; Jolibois, H.; Hachimi, A.; Muller, J.F.; Chambaudet, A. Characterization of the corrosion layer of copper-nickel alloys in a synthetic sweat medium by FTMS and LAMMA laser microprobes. Appl. Surf. Sci. 1998, 125, 29–45. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Ariza, E.; Rocha, L.A.; Gomes, J.R.; Carvalho, P.; Vaz, F.; Fernandes, A.C.; Rebouta, L.; Cunha, L.; Alves, E.; et al. Tribocorrosion behaviour of ZrOxNy thin films for decorative applications. Surf. Coatings Technol. 2006, 200, 6634–6639. [Google Scholar] [CrossRef] [Green Version]
- Kandala, C.V.K.; Butts, C.L. Design and performance of a capacitor sensor and impedance analyzer for nondestructive moisture content determination. Sens. Instrum. Food Qual. Saf. 2008, 2, 240–246. [Google Scholar] [CrossRef]
- Blume, S.O.P.; Ben-Mrad, R.; Sullivan, P.E. Modelling the capacitance of multi-layer conductor-facing interdigitated electrode structures. Sens. Actuators B Chem. 2015, 213, 423–433. [Google Scholar] [CrossRef]
- Sathya, S.; Muruganand, S.; Manikandan, N.; Karuppasamy, K. Design of capacitance based on interdigitated electrode for BioMEMS sensor application. Mater. Sci. Semicond. Process. 2019, 101, 206–213. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.N.; Tao, X.M.; Ding, X. Review of Flexible Temperature Sensing Networks for Wearable Physiological Monitoring. Adv. Healthc. Mater. 2017, 6, 1601371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Poblete, F.R.; Zhu, Y. Tailoring the Temperature Coefficient of Resistance of Silver Nanowire Nanocomposites and their Application as Stretchable Temperature Sensors. ACS Appl. Mater. Interfaces 2019, 11, 17836–17842. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, Z.D. Stretchable sensors for environmental monitoring. Appl. Phys. Rev. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, M.; Yoon, Y.J.; Park, I. Ultra-stretchable and skin-mountable strain sensors using carbon nanotubes-Ecoflex nanocomposites. Nanotechnology 2015, 26. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Zhang, D.; Wang, M.; Bo, Y.; Fan, X.; Li, F.; Liang, J.; Huang, Y.; Ma, R.; et al. Highly Stretchable Carbon Nanotubes/Polymer Thermoelectric Fibers. Nano Lett. 2021, 21, 1047–1055. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, W.; Zhang, Q.; Zheng, K.; Xu, J.; Xu, W.; Shang, E.; Jiang, J.; Zhang, J.; Liu, Y. 3D-Printed Graphene/Polydimethylsiloxane Composites for Stretchable and Strain-Insensitive Temperature Sensors. ACS Appl. Mater. Interfaces 2019, 11, 1344–1352. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, H.; Islam, M.; Peng, S.; Wu, S.; Lim, S.; Zhou, Y.; Wang, C.H. Multi-modal strain and temperature sensor by hybridizing reduced graphene oxide and PEDOT:PSS. Compos. Sci. Technol. 2020, 187, 107959. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, Y.H.; Park, H.; Jin, S.W.; Jeong, Y.R.; Yun, J.; You, I.; Zi, G.; Ha, J.S. Stretchable Active Matrix Temperature Sensor Array of Polyaniline Nanofibers for Electronic Skin. Adv. Mater. 2016, 28, 930–935. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Shi, X.; Li, H.; Wang, C.; Zhang, Q.; Ma, R.; Liang, J. Printable and Stretchable Temperature-Strain Dual-Sensing Nanocomposite with High Sensitivity and Perfect Stimulus Discriminability. Nano Lett. 2020, 20, 6176–6184. [Google Scholar] [CrossRef]
- Youn, D.Y.; Jung, U.; Naqi, M.; Choi, S.J.; Lee, M.G.; Lee, S.; Park, H.J.; Kim, I.D.; Kim, S. Wireless Real-Time Temperature Monitoring of Blood Packages: Silver Nanowire-Embedded Flexible Temperature Sensors. ACS Appl. Mater. Interfaces 2018, 10, 44678–44685. [Google Scholar] [CrossRef]
- Han, S.; Kim, M.K.; Wang, B.; Wie, D.S.; Wang, S.; Lee, C.H. Mechanically Reinforced Skin-Electronics with Networked Nanocomposite Elastomer. Adv. Mater. 2016, 28, 10257–10265. [Google Scholar] [CrossRef]
- Brosseau, L.; Yonge, K.; Robinson, V.; Marchand, S.; Judd, M.; Wells, G.; Tugwell, P. Thermotherapy for treatment of osteo-arthritis. Physiotherapy 2003, 89, 694–695. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Hyun, W.; Kim, J.; Kim, J.; Lee, Y.B.; Song, C.; Hwang, H.J.; Kim, J.H.; Hyeon, T.; et al. Stretchable Heater Using Ligand-Exchanged Silver Nanowire Nanocomposite for Wearable Articular Thermotherapy. ACS Nano 2015, 9, 6626–6633. [Google Scholar] [CrossRef]
- An, B.W.; Gwak, E.J.; Kim, K.; Kim, Y.C.; Jang, J.; Kim, J.Y.; Park, J.U. Stretchable, Transparent Electrodes as Wearable Heaters Using Nanotrough Networks of Metallic Glasses with Superior Mechanical Properties and Thermal Stability. Nano Lett. 2016, 16, 471–478. [Google Scholar] [CrossRef]
- Huang, Q.; Al-Milaji, K.N.; Zhao, H. Inkjet Printing of Silver Nanowires for Stretchable Heaters. ACS Appl. Nano Mater. 2018, 1, 4528–4536. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Wang, J.; Zhang, M.; Zhou, Y.; Cheng, J.; Kong, D. Strain-invariant conductance in an elastomeric nanocomposite mesh conductor for stretchable electronics. J. Mater. Chem. C 2020, 8, 9440–9448. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, C.; Zhao, J.; Cai, Z.; Ge, F. Low-Voltage Electrical Heater Based on One-Step Fabrication of Conductive Cu Nanowire Networks for Application in Wearable Devices. Adv. Mater. Interfaces 2021, 8, 2001695. [Google Scholar] [CrossRef]
- Zhou, J.; Mulle, M.; Zhang, Y.; Xu, X.; Li, E.Q.; Han, F.; Thoroddsen, S.T.; Lubineau, G. High-ampacity conductive polymer microfibers as fast response wearable heaters and electromechanical actuators. J. Mater. Chem. C 2016, 4, 1238–1249. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Li, P.; Fan, Z.; Du, D.; Ouyang, J. Stretchable heaters with composites of an intrinsically conductive polymer, reduced graphene oxide and an elastomer for wearable thermotherapy. J. Mater. Chem. C 2017, 5, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.H.; Song, J.W.; Kim, D.; Kim, J.; Park, J.K.; Oh, S.K.; Han, C.S. Transparent film heater using single-walled carbon nanotubes. Adv. Mater. 2007, 19, 4284–4287. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhu, W.B.; Yu, X.G.; Huang, P.; Fu, S.Y.; Hu, N.; Liao, K. Multifunctional Wearable Device Based on Flexible and Conductive Carbon Sponge/Polydimethylsiloxane Composite. ACS Appl. Mater. Interfaces 2016, 8, 33189–33196. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.R.; Saito, T.; Nishi, Y. Ultra-thin and high-response transparent and flexible heater based on carbon nanotube film. Appl. Phys. Lett. 2017, 110, 153301. [Google Scholar] [CrossRef]
- Sun, W.J.; Xu, L.; Jia, L.C.; Zhou, C.G.; Xiang, Y.; Yin, R.H.; Yan, D.X.; Tang, J.H.; Li, Z.M. Highly conductive and stretchable carbon nanotube/thermoplastic polyurethane composite for wearable heater. Compos. Sci. Technol. 2019, 181, 107695. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Zheng, K.; Chen, S.; Zhu, L.; Zhao, J.; Guo, X. Large Area Solution Processed Poly (Dimethylsiloxane)-Based Thin Film Sensor Patch for Wearable Electrocardiogram Detection. IEEE Electron Device Lett. 2018, 39, 424–427. [Google Scholar] [CrossRef]
- Kisannagar, R.R.; Jha, P.; Navalkar, A.; Maji, S.K.; Gupta, D. Fabrication of Silver Nanowire/Polydimethylsiloxane Dry Electrodes by a Vacuum Filtration Method for Electrophysiological Signal Monitoring. ACS Omega 2020, 5, 10260–10265. [Google Scholar] [CrossRef]
- Peng, H.L.; Liu, J.Q.; Dong, Y.Z.; Yang, B.; Chen, X.; Yang, C.S. Parylene-based flexible dry electrode for bioptential recording. Sens. Actuators B Chem. 2016, 231, 1–11. [Google Scholar] [CrossRef]
- Portelli, A.J.; Nasuto, S.J. Design and development of non-contact bio-potential electrodes for pervasive health monitoring applications. Biosensors 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahed, M.A.; Das, P.S.; Maharjan, P.; Barman, S.C.; Sharifuzzaman, M.; Yoon, S.H.; Park, J. yeong Flexible and robust dry electrodes based on electroconductive polymer spray-coated 3D porous graphene for long-term electrocardiogram signal monitoring system. Carbon 2020, 165, 26–36. [Google Scholar] [CrossRef]
- Barbado, D.; Elvira, J.L.L. Medicina del Deporte. Rev. Andaluza Med. Deport. 2015, 8, 79–85. [Google Scholar]
- Zeng, X.; Dong, Y.; Wang, X. Flexible electrode by hydrographic printing for surface electromyography monitoring. Materials 2020, 13, 2339. [Google Scholar] [CrossRef]
- Roland, T.; Wimberger, K.; Amsuess, S.; Russold, M.F.; Baumgartner, W. An Insulated Flexible Sensor for Stable Electromyography Detection: Application to Prosthesis Control. Sensors 2019, 19, 961. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Parekh, U.; Pailla, T.; Garudadri, H.; Gilja, V.; Ng, T.N. Stretchable Dry Electrodes with Concentric Ring Geometry for Enhancing Spatial Resolution in Electrophysiology. Adv. Healthc. Mater. 2017, 6, 1700552. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, H.; Sheikhnejad, O.; Xiong, Y.; Gu, H.; Zhu, P.; Li, G.; Sun, R.; Wong, C.P. Stretchable and printable medical dry electrode arrays on textile for electrophysiological monitoring. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 243–248. [Google Scholar] [CrossRef]
- Koo, J.H.; Jeong, S.; Shim, H.J.; Son, D.; Kim, J.; Kim, D.C.; Choi, S.; Hong, J.I.; Kim, D.H. Wearable Electrocardiogram Monitor Using Carbon Nanotube Electronics and Color-Tunable Organic Light-Emitting Diodes. ACS Nano 2017, 11, 10032–10041. [Google Scholar] [CrossRef]
- Liu, B.; Tang, H.; Luo, Z.; Zhang, W.; Tu, Q.; Jin, X. Wearable carbon nanotubes-based polymer electrodes for ambulatory electrocardiographic measurements. Sens. Actuators A Phys. 2017, 265, 79–85. [Google Scholar] [CrossRef]
- Pani, D.; Achilli, A.; Bonfiglio, A. Survey on Textile Electrode Technologies for Electrocardiographic (ECG) Monitoring, from Metal Wires to Polymers. Adv. Mater. Technol. 2018, 3, 1800008. [Google Scholar] [CrossRef]
- Näbauer, M.; Callewaert, G.; Cleemann, L.; Morad, M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science 1989, 244, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Hwang, S.W.; Lee, C.H.; Cheng, H.; Jeong, J.W.; Kang, S.K.; Kim, J.H.; Shin, J.; Yang, J.; Liu, Z.; Ameer, G.A.; et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 2015, 15, 2801–2808. [Google Scholar] [CrossRef]
- Zhou, W.; Yao, S.; Wang, H.; Du, Q.; Ma, Y.; Zhu, Y. Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 2020, 14, 5798–5805. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kang, K.; Choi, H.; Yoon, J.; Kim, Y.; An, S.; Jung, H.; Seong, D.; Park, K.; Baac, H.; et al. Soft Bio-Integrated Multifunctional Devices Using an Intrinsically Stretchable Conducting Nanomembrane. Appl. Sci. 2021, 11, 6562. https://doi.org/10.3390/app11146562
Lee S, Kang K, Choi H, Yoon J, Kim Y, An S, Jung H, Seong D, Park K, Baac H, et al. Soft Bio-Integrated Multifunctional Devices Using an Intrinsically Stretchable Conducting Nanomembrane. Applied Sciences. 2021; 11(14):6562. https://doi.org/10.3390/app11146562
Chicago/Turabian StyleLee, Sangkyu, Kyumin Kang, Heewon Choi, Jiyong Yoon, Yewon Kim, Soojung An, Hyunjin Jung, Duhwan Seong, Kyuha Park, Hyoungwon Baac, and et al. 2021. "Soft Bio-Integrated Multifunctional Devices Using an Intrinsically Stretchable Conducting Nanomembrane" Applied Sciences 11, no. 14: 6562. https://doi.org/10.3390/app11146562
APA StyleLee, S., Kang, K., Choi, H., Yoon, J., Kim, Y., An, S., Jung, H., Seong, D., Park, K., Baac, H., & Son, D. (2021). Soft Bio-Integrated Multifunctional Devices Using an Intrinsically Stretchable Conducting Nanomembrane. Applied Sciences, 11(14), 6562. https://doi.org/10.3390/app11146562






