Pharmacokinetics of Vancomycin Installation in Pleural Cavity—A Clinical Case with Animal Experiments
Abstract
:1. Introduction
2. Case Report
3. Materials and Methods
3.1. Animals
3.2. Pleura Thickness Rat Model Establishment
3.3. Experimental Procedure
3.4. Measuring Vancomycin Concentration Levels
3.5. Histology
3.6. Statistics
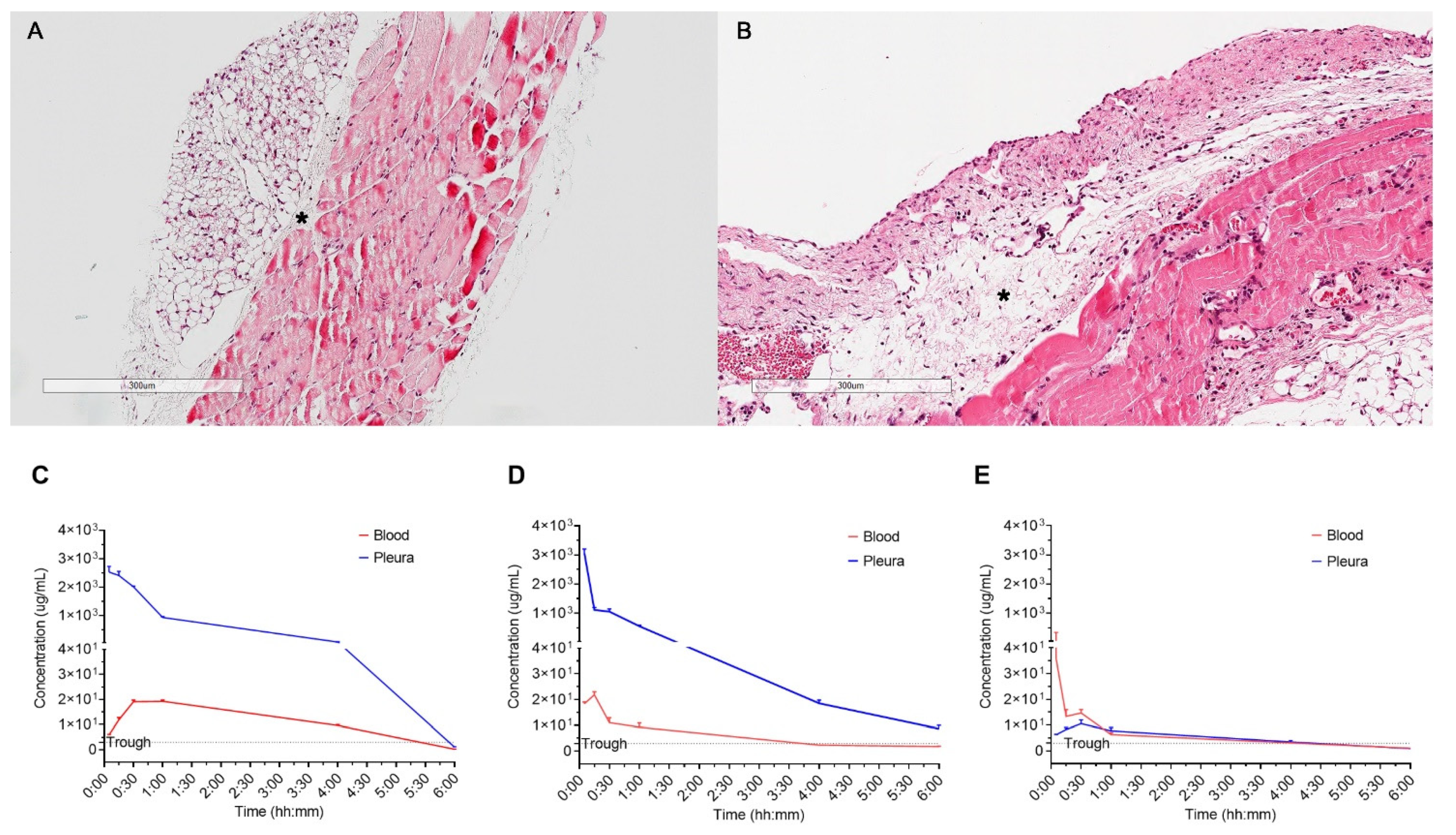
4. Results
4.1. Vancomycin Comcentration of Serum and Pleura
Vancomycin Injection to Pleural Cavity
- Compared with normal pleura, the concentration of injected vancomycin in the thickened pleura markedly decreased in 15 min, but was sustained above the trough level for four hours (Figure 2C,D)
- The peak concentration in the serum was the highest after 15 min in the thickened pleura, and 30 min in the normal pleura
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaheer, S.; Allen, M.S.; Cassivi, S.D.; Nichols, F.C.; Johnson, C.H.; Deschamps, C.; Pairolero, P.C. Postpneumonectomy empyema: Results after the clagett procedure. Ann. Thorac. Surg. 2006, 82, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Hicham, H.; Ibrahim, I.; Rabiou, S.; Marouane, L.; Yassine, O.; Mohamed, S. Postpneumonectomy empyema: Risk factors, prevention, diagnosis, and management. Asian Cardiovasc. Thorac. Ann. 2020, 28, 89–96. [Google Scholar] [CrossRef]
- Wilhelm, M.P. Vancomycin. Mayo Clin. Proc. 1991, 66, 1165–1170. [Google Scholar] [CrossRef]
- Albarellos, G.; Landoni, M. Current concepts on the use of antimicrobials in cats. Veter. J. 2009, 180, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-G. Pharmacokinetics and pharmacodynamics of antibiotics. Infect. Dis. Clin. N. Am. 2009, 23, 791. [Google Scholar]
- Suchánková, H.; Lečbychová, K.; Strojil, J.; Fürst, T. Individualized dosing of vancomycin in geriatric patients. Epidemiol. Mikrobiol. Imunol. 2020, 69, 172–180. [Google Scholar] [PubMed]
- Mierzejewski, M.; Korczynski, P.; Krenke, R.; Janssen, J.P. Chemical pluerodesis—A review of mechanisms involved in pleural space obliteration. Respir Res. 2019, 20, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Ahn, H.Y.; Kim, K.; Kim, J.H.; Moon, S.Y.; Kim, Y.D. Pharmacokinetics of Vancomycin Installation in Pleural Cavity—A Clinical Case with Animal Experiments. Appl. Sci. 2021, 11, 6456. https://doi.org/10.3390/app11146456
Lee S, Ahn HY, Kim K, Kim JH, Moon SY, Kim YD. Pharmacokinetics of Vancomycin Installation in Pleural Cavity—A Clinical Case with Animal Experiments. Applied Sciences. 2021; 11(14):6456. https://doi.org/10.3390/app11146456
Chicago/Turabian StyleLee, Soojin, Hyo Yeong Ahn, Keunyoung Kim, Jeong Hun Kim, Soo Young Moon, and Yeong Dae Kim. 2021. "Pharmacokinetics of Vancomycin Installation in Pleural Cavity—A Clinical Case with Animal Experiments" Applied Sciences 11, no. 14: 6456. https://doi.org/10.3390/app11146456
APA StyleLee, S., Ahn, H. Y., Kim, K., Kim, J. H., Moon, S. Y., & Kim, Y. D. (2021). Pharmacokinetics of Vancomycin Installation in Pleural Cavity—A Clinical Case with Animal Experiments. Applied Sciences, 11(14), 6456. https://doi.org/10.3390/app11146456




