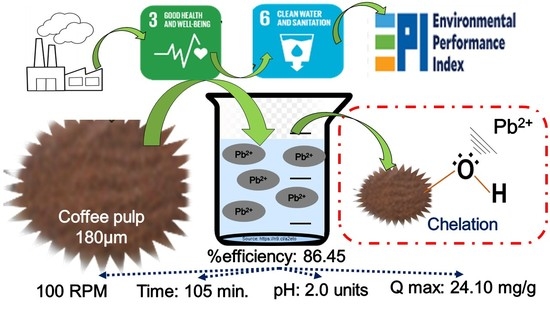
Biosorption of Pb(II) Using Coffee Pulp as a Sustainable Alternative for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Site and Physical Treatment of the CP
2.2. Cellulose and Lignin Content in the CP
2.3. Quantification of Pb(II)
2.4. Determination of the Optimum pH of Adsorption of Pb(II)
2.5. Determination of the Adsorption Kinetics for Pb(II)
2.6. Determination of the Adsorption Isotherm for Pb(II)
2.7. Determination of the Point of Zero Charge (pHpzc) in the CP
3. Results and Discussion
3.1. Lignocellulosic Content of the CP
3.2. Determination of Optimum pH of Pb(II) with CP
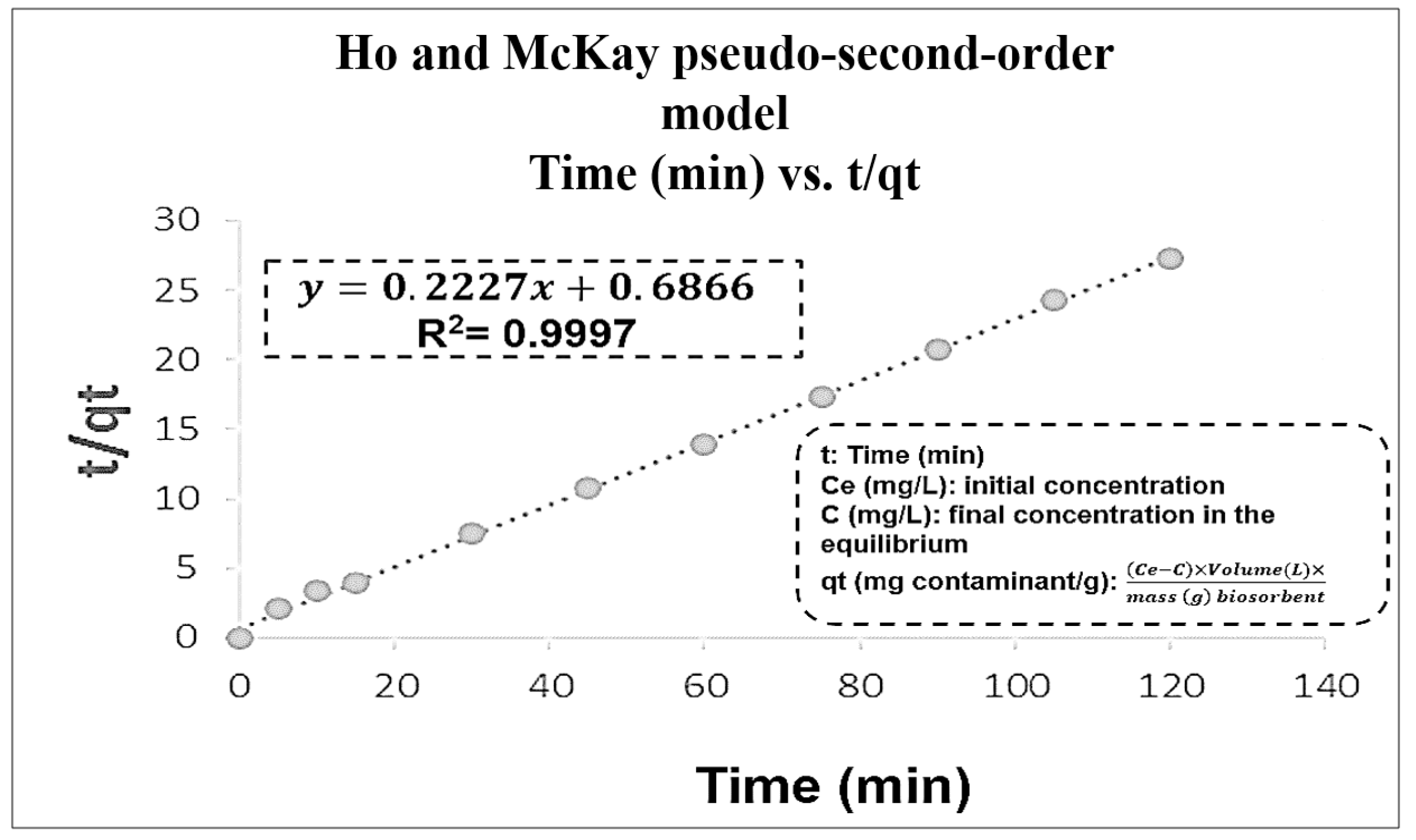
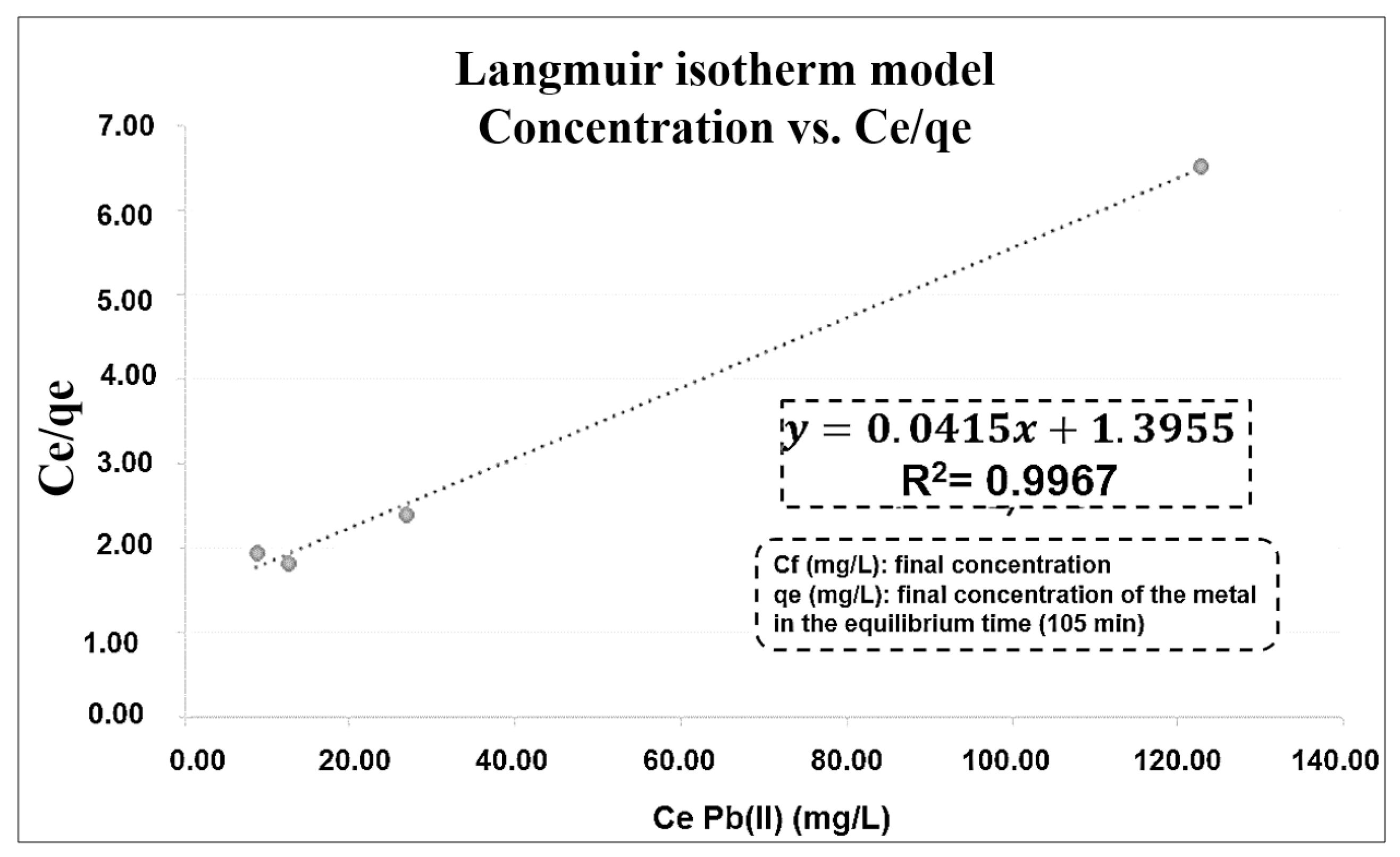
3.3. Kinetics and Adsorption Isotherm
3.4. Determination of Point of Zero Charge (pHpzc) for the CP
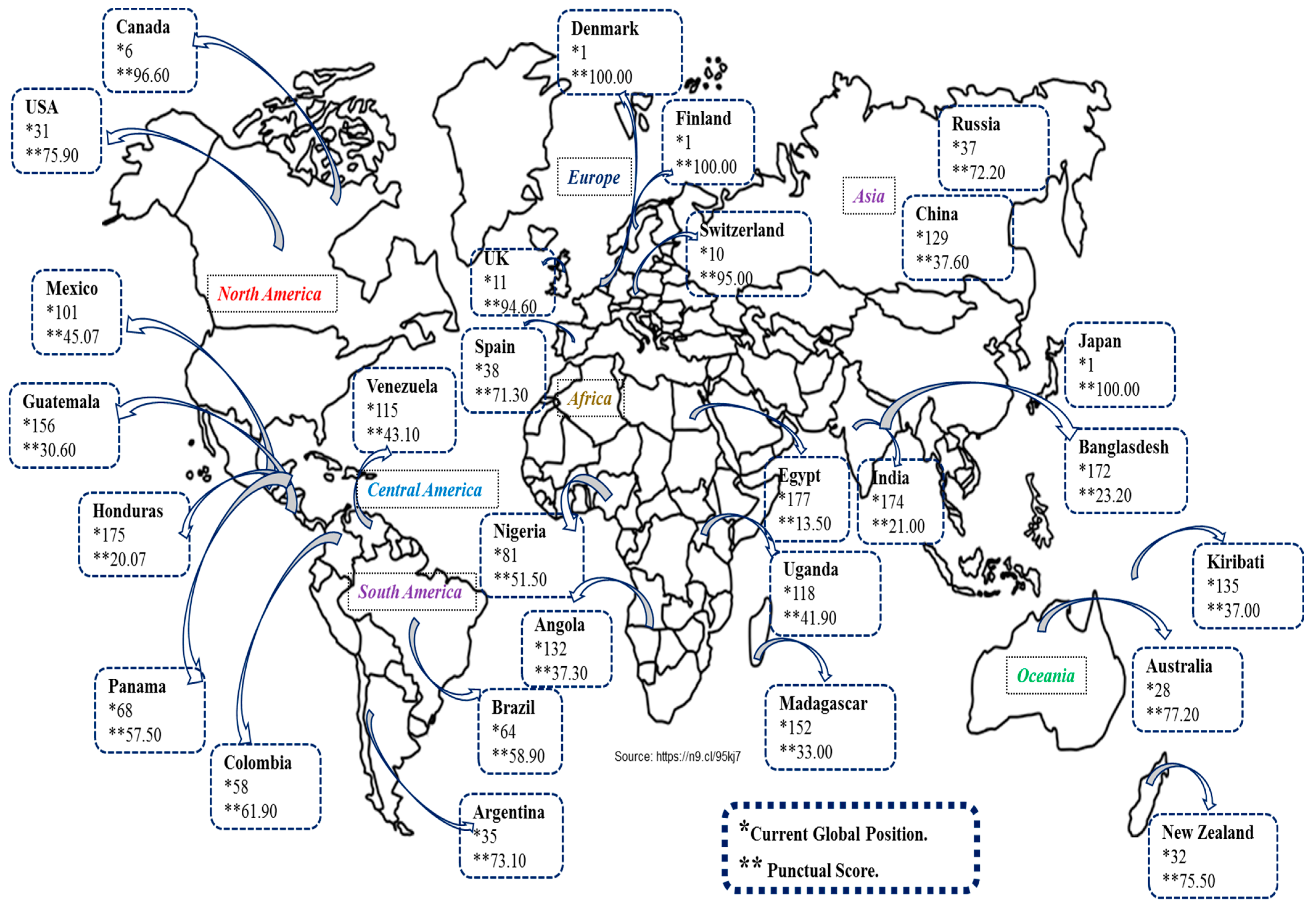
3.5. Techno-Economics Aspects and Future Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Wendling, Z.A.; Emerson, J.W.; Esty, D.C.; Levy, M.A.; de Sherbinin, A.; Esty, D.; Hoving, K.; Ospina, C.; Murray, J.; Jacob, M.; et al. Report 2020-EPI for Heavy Metals. 2020. Available online: https://epi.yale.edu/downloads/epi2020report20210112.pdf (accessed on 10 July 2019).
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2017, 391, 462–512. [Google Scholar] [CrossRef]
- Gómez Aguilar, D.L.; Esteban Muñoz, J.A.; Baracaldo Guzmán, D. Tecnologías no convencionales para la remoción de plomo presente en aguas residuales: Una revisión bibliográfica 2010–2019. Tecnura 2020, 24, 97–116. [Google Scholar] [CrossRef]
- Yoshinaga, J. Lead in the Japanese living environment. Environ. Health Prev. Med. 2012, 17, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Gottesfeld, P. Time to Ban Lead in Industrial Paints and Coatings. Front. Public Health 2015, 3, 144. [Google Scholar] [CrossRef]
- Meyer, P.A.; Brown, M.J.; Falk, H. Global approach to reducing lead exposure and poisoning. Mutat. Res. 2008, 659, 166–175. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheet: Lead Poisoning and Health. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs379/en/ (accessed on 10 July 2019).
- Londoño Franco, L.F.; Londoño Munoz, P.T.; Muñoz Garcia, F.G. Los riesgos de los metales pesados en la salud humana y animal. Biotechnol. Sec. Agropecu. Agroind. 2016, 14, 145–153. [Google Scholar] [CrossRef]
- Valdivia, M.M. Intoxicación por plomo. Rev. Soc. Per. Med. Interna 2005, 18, 22–27. [Google Scholar]
- Moreno, A.; Granada, J. Intoxicación por Plomo: Diagnóstico Diferencial de Dolor Abdominal Crónico. Reporte de Caso y Revisión de Tema. Univ. Med. 2012, 53, 199–207. Available online: http://www.redalyc.org/resumen.oa?id=231024382008 (accessed on 19 July 2019). [CrossRef]
- World Health Organization (WHO). Adverse Health Effects of Heavy Metals in Children. 2011. Available online: https://www.who.int/ceh/capacity/heavy_metals.pdf (accessed on 10 July 2019).
- Caviedes Rubio, D.I.; Muñoz Calderón, R.A.; Perdomo Gualtero, A.; Rodríguez Acosta, D.; Sandoval Rojas, I.J. Tratamientos para la remoción de metales pesados comúnmente presentes en aguas residuales industriales. Una Revisión. Ing. Reg. 2015, 13, 73–90. [Google Scholar] [CrossRef]
- Atabani, A.E.; Al-Muhtaseb, A.H.; Kumar, G.; Saratale, G.D.; Aslam, M.; Khan, H.A.; Said, Z.; Mahmoud, E. Valorization of spent coffee grounds into biofuels and value-added products: Pathway towards integrated bio-refinery. Fuel 2019, 254, 115640. [Google Scholar] [CrossRef]
- Cerino-Córdova, F.; Dávila-Guzmán, N.; García León, A.; Salazar-Rabago, J.; Soto-Regalado, E. Revalorization of Coffee Waste. In Coffee—Production and Research; Castanheira, D.T., Ed.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/books/coffee-production-and-research/revalorization-of-coffee-waste (accessed on 14 June 2021).
- Espitia, N. Determinación de metales pesados en sedimentos superficiales en cuerpos de agua del canal del dique en las poblaciones de Gambote y Soplaviento (Bolívar). Rev. Inst. Investig. Fac. Ing. Geo. Min. Metal. Geo. 2014, 17. [Google Scholar] [CrossRef]
- John Jairo, F.; José Luis, M.; Humberto, G. Heavy Metals in Sinú River, Department of Córdoba, Colombia, South America. Rev. Fac. Ing. Univ. Antioq. 2010, 55, 35–44. Available online: http://www.scielo.org.co/pdf/rfiua/n55/n55a04.pdf (accessed on 19 July 2019).
- Iordache, M.; Iordache, A.M.; Sandru, C.; Voica, C.; Zgavarogea, R.; Miricioiu, M.G.; Ionete, R.E. Assessment of Heavy Metals Pollution in Sediments from Reservoirs of the Olt River as Tool for Environmental Risk Management. Rev. Chim. 2019, 70, 4153–4162. Available online: https://revistadechimie.ro/pdf/5%20IORDACHE%20M%2012%2019.pdf (accessed on 19 July 2019).
- Quintero Rendón, L.A.; Agudelo, E.A.; Quintana Hernández, Y.A.; Cardona Gallo, S.A.; Osorio Arias, A.F. Determinación de Indicadores Para la Calidad de Agua, Sedimentos y Suelos, Marinos y Costeros en Puertos Colombianos. Gest. Ambiente 2010, 13, 51–64. Available online: https://revistas.unal.edu.co/index.php/gestion/article/view/25414 (accessed on 19 July 2019).
- United Nations (UN). Goal 3: Ensure a Healthy Life and Promote Well-Being for All People at All Ages. 2017. Available online: https://www.un.org/sustainabledevelopment/es/health/ (accessed on 19 July 2019).
- United Nations (UN). Goal 6: Ensure Water Availability, Sustainable Management and Sanitation for All. 2017. Available online: https://www.un.org/sustainabledevelopment/es/water-and-sanitation/ (accessed on 10 July 2019).
- Hidalgo, S. Reutilización de Residuos de Rapa Para la Eliminación de Metales Tóxicos en Efluentes Líquidos. 2004. Available online: https://upcommons.upc.edu/handle/2099.1/3673 (accessed on 14 June 2021).
- Carvajal-Flórez, E.; Marulanda-Giraldo, L. Uso de residuos de café como biosorbente para la remoción de metales pesados en aguas residuales. Ing. USBMed 2020, 11, 44–55. [Google Scholar] [CrossRef]
- Pintado, K. Comparación de la Biosorción y Desorción de Metales Pesados Mediante el uso de Marlo de Maíz (Zea mays) en Aguas Contaminadas. Ph.D. Thesis, Universidad Politécnica Salesiana, Cuenca, Ecuador, 2018. Available online: https://dspace.ups.edu.ec/bitstream/123456789/16226/1/UPS-CT007883.pdf (accessed on 19 July 2019).
- Oré Jiménez, F.; Lavado Meza, C.; Bendezú Montes, S. Biosorción de Pb (II) de Aguas Residuales de Mina Usando el Marlo de Maíz (Zea mays). Rev. Soc. Quim. Peru 2015, 81, 122–134. Available online: http://www.scielo.org.pe/pdf/rsqp/v81n2/a05v81n2.pdf (accessed on 19 July 2019). [CrossRef]
- Duarte Zaragoza, V.M.; Carrillo, R.; Gutiérrez Castorena, C.M. Lead sorption–desorption from organic residues. Environ Technol. 2011, 32, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Alazaba, A.; Amin, M. Removal of Heavy Metals from Wastewater using Date Palm as a Biosorbent: A Comparative Review. Sains Malays. 2018, 47, 35–49. [Google Scholar] [CrossRef]
- Acosta, I.; Sandoval, P.; Bautista, D.; Hernández, N.; Cárdenas, J.; Martínez, V. Bioadsorción de cromo (VI) por la cáscara de Mamey (Mammea americana L.). Av. Cienc. Ing. 2012, 3, 1–9. [Google Scholar] [CrossRef]
- Gómez Aguilar, D.L.; Rodríguez Miranda, J.P.; Esteban Muñoz, J.A.; Betancur, P.; Fredy, J. Coffee Pulp: A Sustainable Alternative Removal of Cr (VI) in Wastewaters. Process 2019, 7, 403. [Google Scholar] [CrossRef]
- Ahmed, H. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2016, 229, 555–565. [Google Scholar] [CrossRef]
- Palomino, C.C.; Vargas Parker, R.; Visitación Figueroa, L. Aprovechamiento de pelos de vacuno del proceso de pelambre enzimático de las curtiembres en la remoción de plomo. Rev. Soc. Quim. Peru 2016, 82, 183–195. [Google Scholar] [CrossRef][Green Version]
- ASTM International. ANSI/ASTM D1106-56: Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM International (ASTM): West Conshohocken, PA, USA, 2001. [Google Scholar]
- Salmones, D.; Mata, G.; Waliszewski, K. Comparative culturing of spp. on coffee pulp and wheat straw: Biomass production and substrate biodegradation. Bioresour. Technol. 2005, 96, 537–544. [Google Scholar] [CrossRef]
- Menezes, E.G.T.; do Carmo, J.R.; Alves, J.G.L.F.; Menezes, A.G.T.; Guimarães, I.C.; Queiroz, F.; Pimenta, C.J. Optimization of alkaline pretreatment of coffee pulp for production of bioethanol. Biotechnol. Prog. 2014, 30, 451–462. [Google Scholar] [CrossRef]
- Juliastuti, S.R.; Widjaja, T.; Altway, A.; Sari, V.A.; Arista, D.; Iswanto, T. The effects of microorganism on coffee pulp pretreatment as a source of biogas production. MATEC Web Conf. 2018, 156, 2–10. [Google Scholar] [CrossRef]
- Phuong, D.V.; Quoc, L.P.T.; Tan, P.V.; Duy, L.N.D. Production of bioethanol from Robusta coffee pulp (Coffea robusta L.) in Vietnam. Foods Raw Mater. 2019, 7, 10–17. [Google Scholar] [CrossRef]
- ASTM International. ANSI/ASTM D1103-60: Method of Test for Alpha-Cellulose in Wood; ASTM International (ASTM): West Conshohocken, PA, USA, 1960. [Google Scholar]
- Yazán, D. Descomposición Microbiológica de Desechos Orgánicos Vegetales Originados en la Universidad Central del Ecuador. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2013. Available online: http://www.dspace.uce.edu.ec/bitstream/25000/2122/1/T-UCE-0017-49.pdf (accessed on 19 July 2019).
- Gómez, D. Remoción de Manganeso en Aguas Residuales Industriales, Empleando Pulpa de Café; Resumen de Presentación Oral, 33° Congreso Latinoamericano de Química (CLAQ), X Congreso de Ciencias Químicas, Tec-nología e Innovación; Químicuba: Manizales, Colombia, 2018; Available online: https://drive.google.com/file/d/1tQHEi__y16Q-vPaCl4cXERVWVVNFGZlZ/view (accessed on 19 July 2019).
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Moreno, J. Importancia y Aplicaciones de la Adsorción en Fase Líquida. In Sólidos Porosos; Universidad de los Andes: Bogotá, Colombia, 2007; pp. 155–207. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2007, 3039817. [Google Scholar] [CrossRef]
- Dávila-Guzmán, N.E.; Cerino-Córdova, F.; Soto-Regalado, E.; Rangel-Méndez, J.R.; Díaz-Flores, P.E.; Garza-González, M.T.; Loredo-Medrano, J.A. Copper Biosorption by Spent Coffee Ground: Equilibrium, Kinetics, and Mechanism. J. Clean Soil Air Water 2013, 41, 557–564. [Google Scholar] [CrossRef]
- Cerino-Córdova, F.J.; Díaz-Flores, P.E.; García-Reyes, R.B.; Soto-Regalado, E.; Gómez-González, R.; Garza-González, M.T.; Bustamante-Alcántara, E. Biosorption of Cu (II) and Pb (II) from aqueous solutions by chemically modified spent coffee grains. Int. J. Environ. Sci. Technol. 2013, 10, 611–622. [Google Scholar] [CrossRef]
- Bustamante, E. Adsorción de Metales Pesados en Residuos de Café Modificados Químicamente. Universidad Autónoma de Nuevo León, 2011. Available online: http://eprints.uanl.mx/2323/1/1080223849.pdf (accessed on 10 July 2019).
- Wu, C.H.; Kuo, C.Y.; Guan, S.S. Adsorption kinetics of lead and zinc ions by coffee residues. Pol. J. Environ. Stud. 2016, 24, 761–767. [Google Scholar] [CrossRef]
- Papa, E.F. Batch and Fixed Bed Column Studies on Cadmium (I) and Lead (II) Adsorption from Aqueous Solution by Coffee Pulp Biochar. In Proceedings of the 5th International Conference on Civil, Architecture, Environment and Waste Management (CAEWM-17), Singapore, 29–30 March 2017; pp. 284–289. [Google Scholar]
- Alhogbi, B.G. Potential of coffee husk biomass waste for the adsorption of Pb (II) ion from aqueous solutions. Sustain. Chem. Pharm. 2017, 6, 21–25. [Google Scholar] [CrossRef]
- Barón, L.J. Evaluación de la Cascarilla de Café Como Material Adsorbente Para la Remoción de Iones Plomo Pb2+ Presente en Soluciones Acuosas. 2014. Available online: http://repository.unilibre.edu.co/bitstream/handle/10901/11234/ (accessed on 10 July 2019).
- Naga, A.; Reddy, D.S.; Kumar, G.S.; Ravindhranath, K.; Krishna Mohan, G.V. Removal of lead and fluoride from contaminated water using exhausted coffee grounds based bio-sorbent. J. Environ. Manag. 2018, 218, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Gómez-González, R.; Cerino-Córdova, F.J.; García-León, A.M.; Soto-Regalado, E.; Dávila-Guzmán, N.E.; Salazar-Rabago, J.J. Lead biosorption onto coffee grounds: Comparative analysis of several optimization techniques using equilibrium adsorption models and ANN. J. Taiwan Inst. Chem. Eng. 2016, 68, 201–210. [Google Scholar] [CrossRef]
- Seniūnaitė, J.; Vaiškūnaitė, R.; Bolutienė, V. Coffee grounds as an adsorbent for copper and lead removal from aqueous solutions. In Proceedings of the 9th International Conference “Environmental Engineering”, Vilnius, Lithuania, 22–23 May 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd, K.; Wan, W.; Jume, B. Equilibrium, Kinetic and Mechanism Studies of Cu(II) and Cd(II) Ions Adsorption by Modified Chitosan Beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef]
- Thomassen, G.; Van Dael, M.; Van Passel, S.; You, F. How to assess the potential of emerging green technologies? Towards a prospective environmental and techno-economic assessment framework. Green Chem. 2019, 21, 4868–4886. [Google Scholar] [CrossRef]

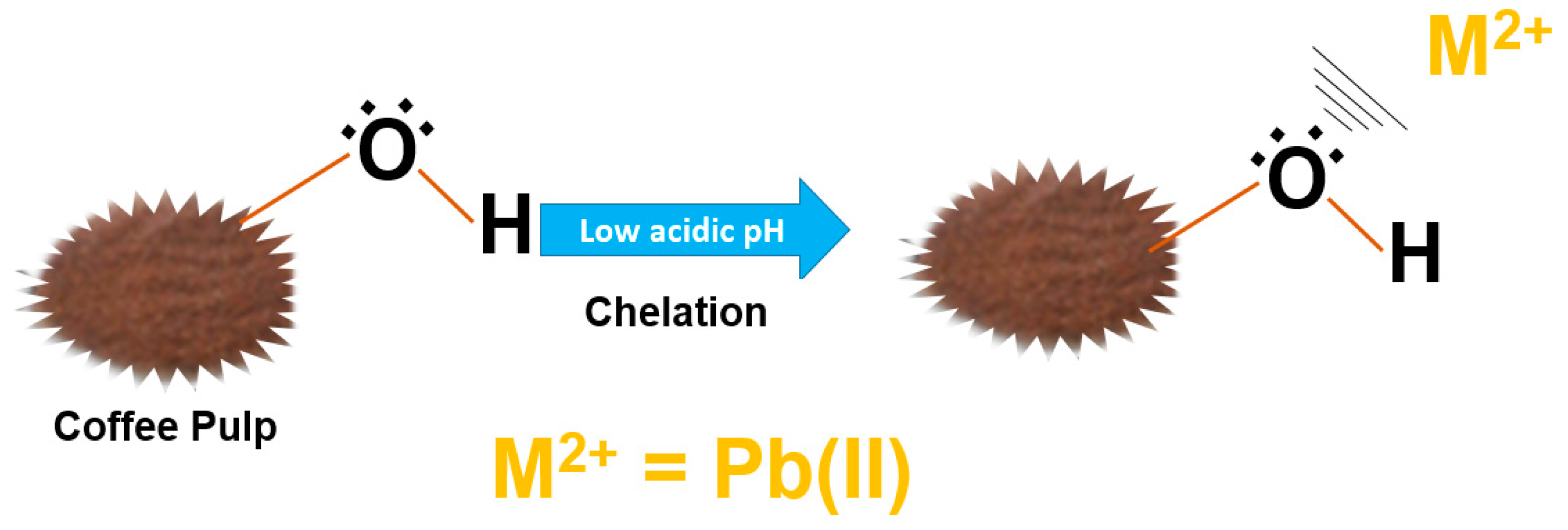
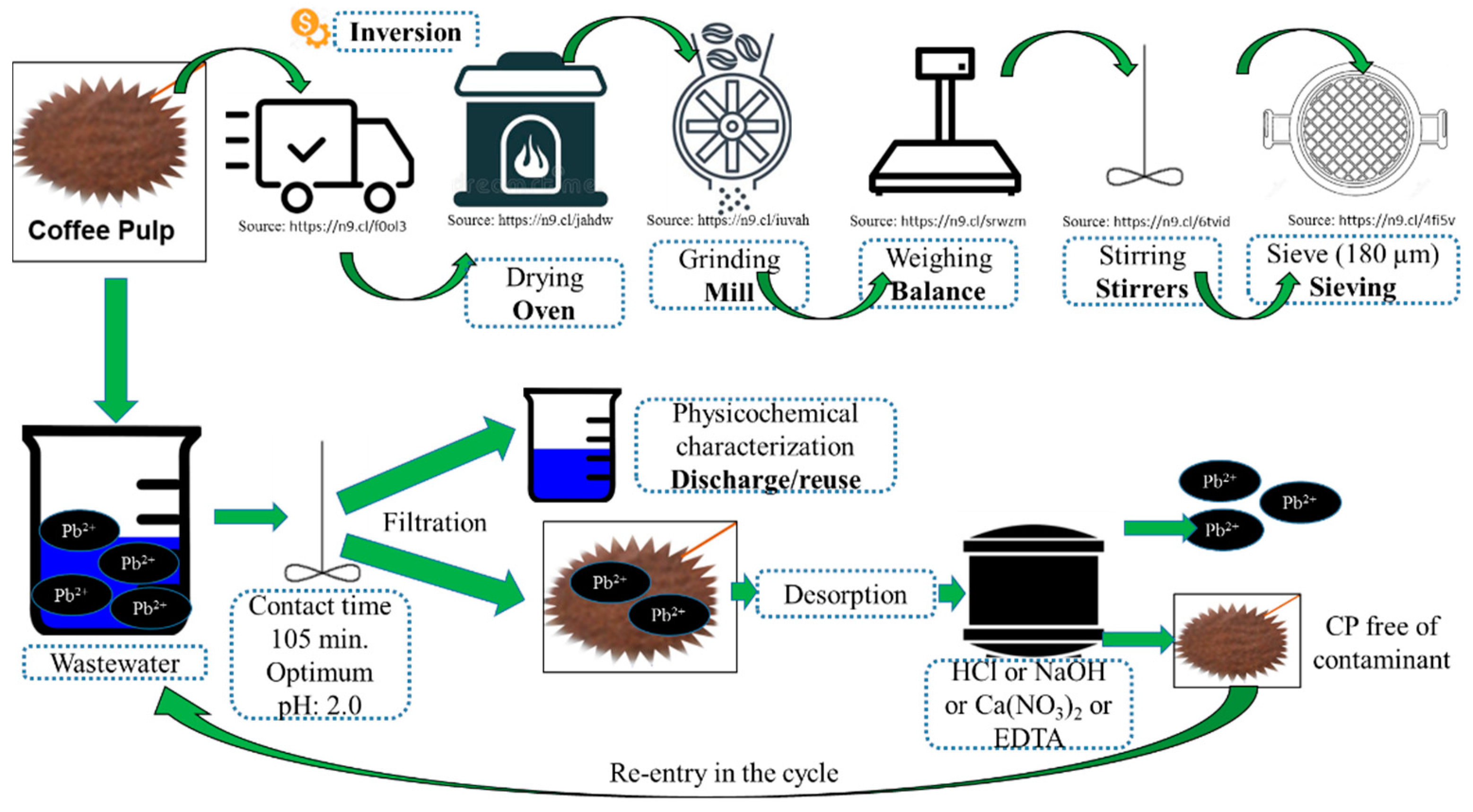
| Parameter | Value of This Research n = 3 | Method Used | Bibliographic Report and Reference | Interval |
|---|---|---|---|---|
| Lignin (%m/m) | 19.25 ± 0.16 | ANSI/ASTM D1106-56 [33] | 25–26 [34] | 5.21–26 |
| 23.8–17.4 [35] | ||||
| 5.21 [36] | ||||
| 9.0–22.0 [37] | ||||
| Cellulose (%m/m) | 29.93 ± 0.21 | ANSI/ASTM D1103-60 [38] | 23–24.5 [34] | 13–57.9 |
| 18–13 [35] | ||||
| 57.9 [36] | ||||
| 16.0–25.88 [37] |
| Order Kinetic | Kinetic Parameters | R2 |
|---|---|---|
| 0 | Time (t) vs. Concentration Pb(II) (C) | 0.4725 |
| 1 | t vs. Ln C | 0.6868 |
| 2 | t vs. | 0.8469 |
| Pseudo-first order | t vs. Log (qe – qt) | 0.5112 |
| Pseudo-second order | t vs. | 0.9997 |
| Isotherms Models | Henry | Freundlich | Langmuir |
|---|---|---|---|
| R2 | 0.8288 | 0.9416 | 0.9967 |
| Constant (K) | 0.134 L·g−1 | 3.683 mg·L−1 | 0.030 L·mg−1 |
| Lignocellulosic Wastes Derived from Coffee Crops | Biosorption Characteristics | Q max. Interval | Reference | |
|---|---|---|---|---|
| pH (pH Units) | Capacity of Maximum Adsorption (Q max.) (mg·g−1 ) | |||
| Spent coffee powder/coffee grounds | 4.5 | 66.30 | 49.73–159.50 | [44] |
| 3.5 | 49.73 | [45] | ||
| 3.5 | 159.50 | |||
| 4.0 | 158.70 | [46] | ||
| Coffee waste (instant coffee beans) | 5. 0 | 9.70 | 9. 70 | [47] |
| Coffee shell/pulp | 5.0 | 54.05 | 4.80–230.00 | [48] |
| N/A | 37.04 | [49] | ||
| 4.5 | 4.80 | [50] | ||
| 0.5–9.0 | 7.20 | [31] | ||
| 0.5–9.0 | 230.00 | |||
| 2.0–9.0 | 50.80 | |||
| 2.0 | 24.10 | This study | ||
| Raw coffee beans | 6.0 | 61.60 | 22.90–61.60 | [51] |
| 5.0 | 22.90 | [52] | ||
| 3.0–4.0 | 96% | [50] | ||
| N/A | 87.20 | 87.02–159.54 | [53] | |
| Spent coffee beans | 3.0–4.0 | 87.02 | [50] | |
| 2.0–12.0 | 93.24 | |||
| 2.0–12.0 | 159.54 | |||
| Lignocellulosic Waste Derived from Coffee | Biosorption Characteristics | Reference | ||
|---|---|---|---|---|
| pH (pH units) | Q max. (mg·g−1) | Chemical Modification | ||
| Spent coffee powder/coffee grounds | 3.5 | 159.50 | Citric acid | [45] |
| 4.0 | 158.70 | Citric acid | [46] | |
| Coffee shell/pulp | 5.0 | 54.05 | Pyrolysis | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Aguilar, D.L.; Rodríguez-Miranda, J.P.; Baracaldo-Guzmán, D.; Salcedo-Parra, O.J.; Esteban-Muñoz, J.A. Biosorption of Pb(II) Using Coffee Pulp as a Sustainable Alternative for Wastewater Treatment. Appl. Sci. 2021, 11, 6066. https://doi.org/10.3390/app11136066
Gómez-Aguilar DL, Rodríguez-Miranda JP, Baracaldo-Guzmán D, Salcedo-Parra OJ, Esteban-Muñoz JA. Biosorption of Pb(II) Using Coffee Pulp as a Sustainable Alternative for Wastewater Treatment. Applied Sciences. 2021; 11(13):6066. https://doi.org/10.3390/app11136066
Chicago/Turabian StyleGómez-Aguilar, Dora Luz, Juan Pablo Rodríguez-Miranda, Deisy Baracaldo-Guzmán, Octavio José Salcedo-Parra, and Javier Andrés Esteban-Muñoz. 2021. "Biosorption of Pb(II) Using Coffee Pulp as a Sustainable Alternative for Wastewater Treatment" Applied Sciences 11, no. 13: 6066. https://doi.org/10.3390/app11136066
APA StyleGómez-Aguilar, D. L., Rodríguez-Miranda, J. P., Baracaldo-Guzmán, D., Salcedo-Parra, O. J., & Esteban-Muñoz, J. A. (2021). Biosorption of Pb(II) Using Coffee Pulp as a Sustainable Alternative for Wastewater Treatment. Applied Sciences, 11(13), 6066. https://doi.org/10.3390/app11136066