HMT Exerts an Anticancer Effect by Targeting PAK-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HMT
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Crystal Violet Staining Assay, Cell Growth Assay
2.5. Western Blot Analysis
2.6. Wound Healing Assay
2.7. Invasion Assay Using the Boyden Chamber
2.8. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.9. siRNA Transfection
2.10. 3D Culture Tumor Organoids
2.11. TLC and HPLC Analysis
3. Results
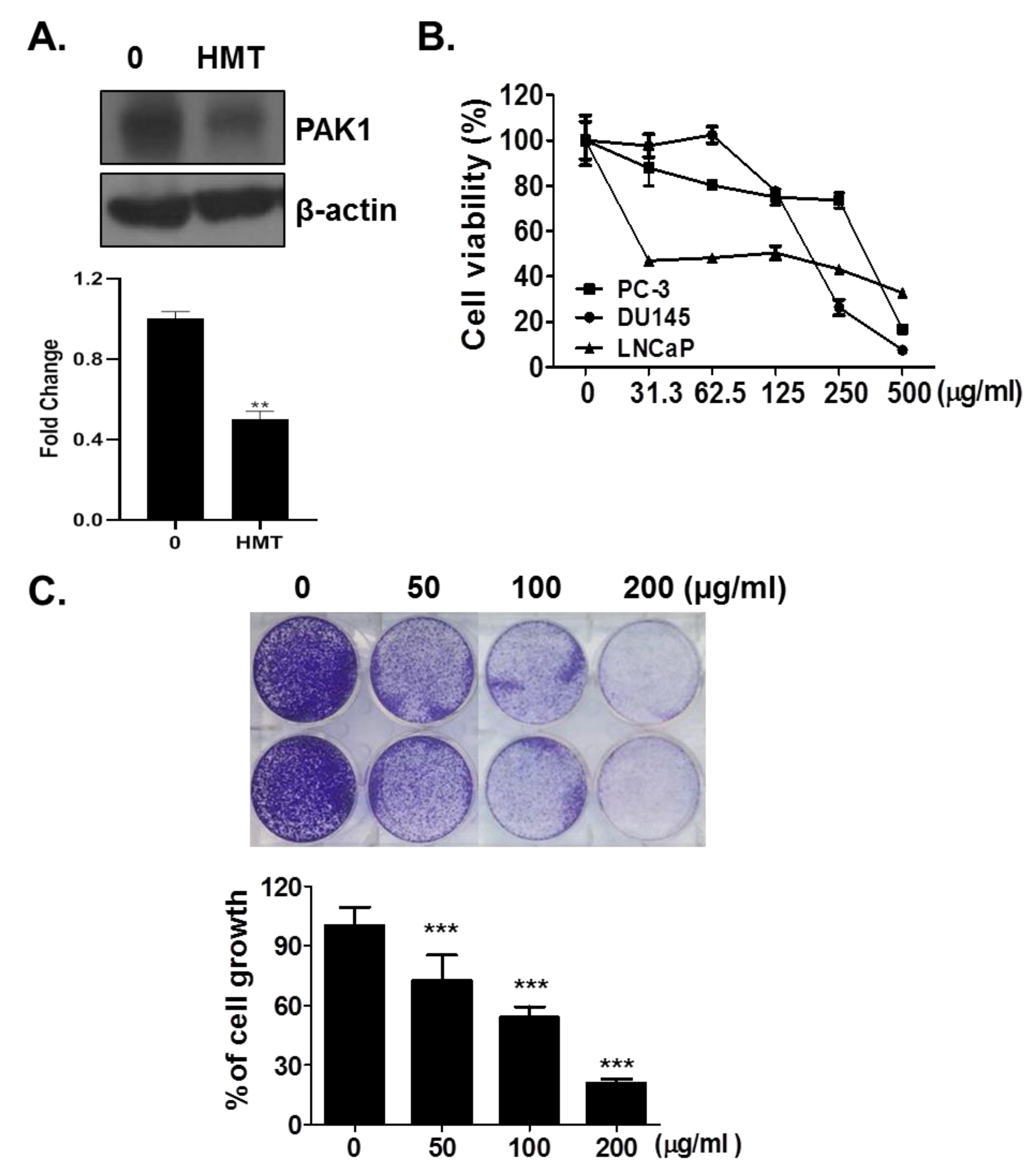
3.1. HMT Suppresses PAK-1
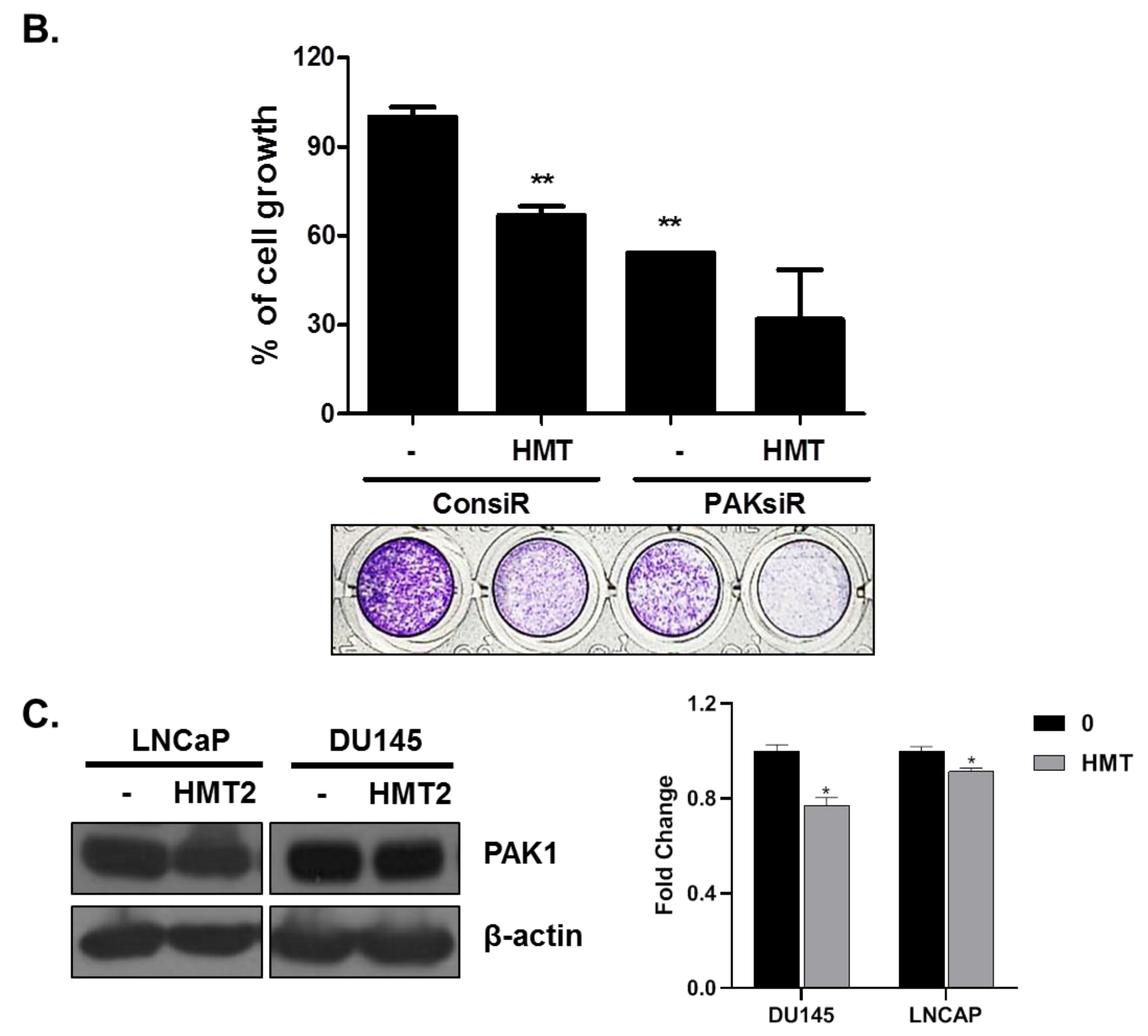
3.2. HMT Inhibits Cell Growth
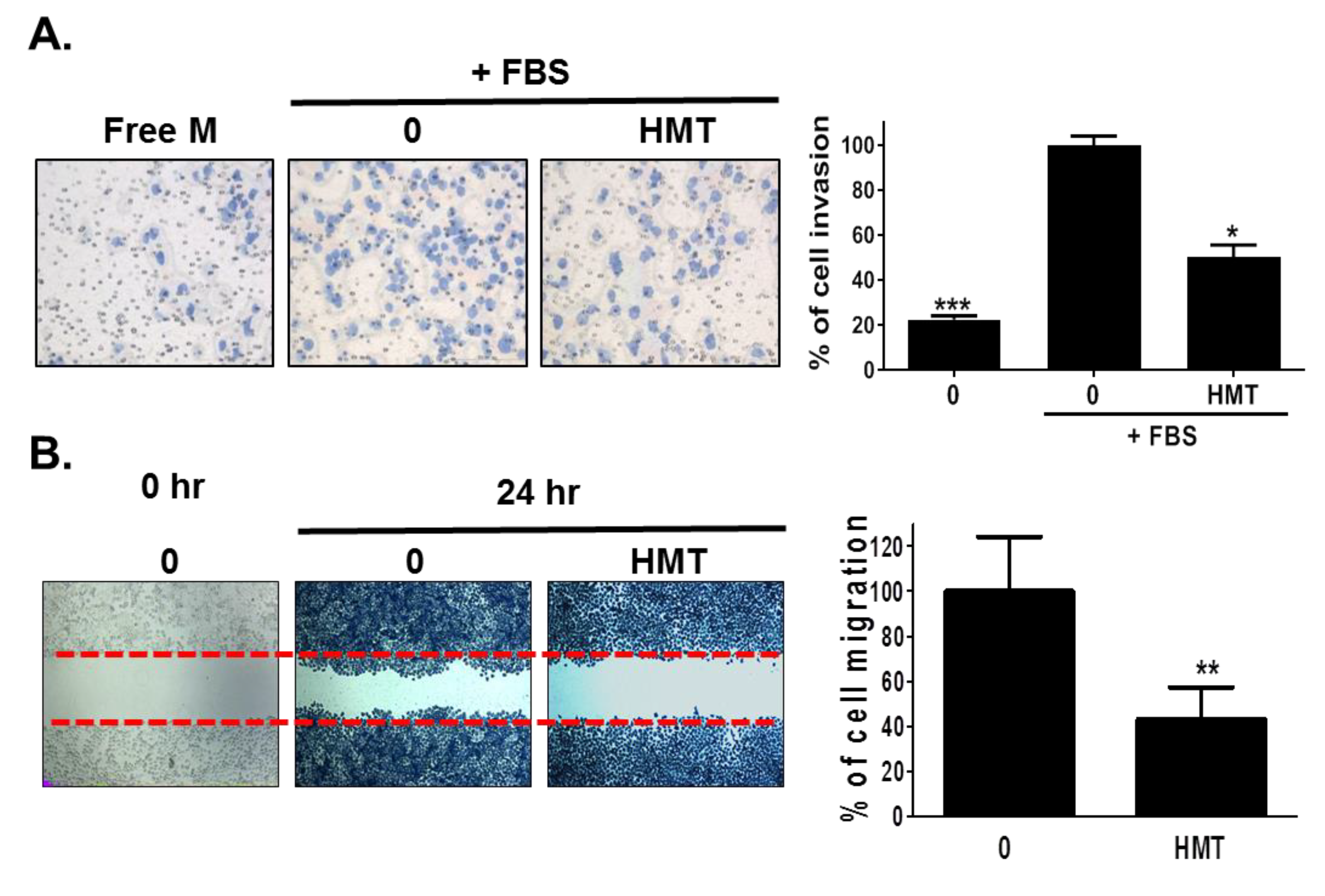
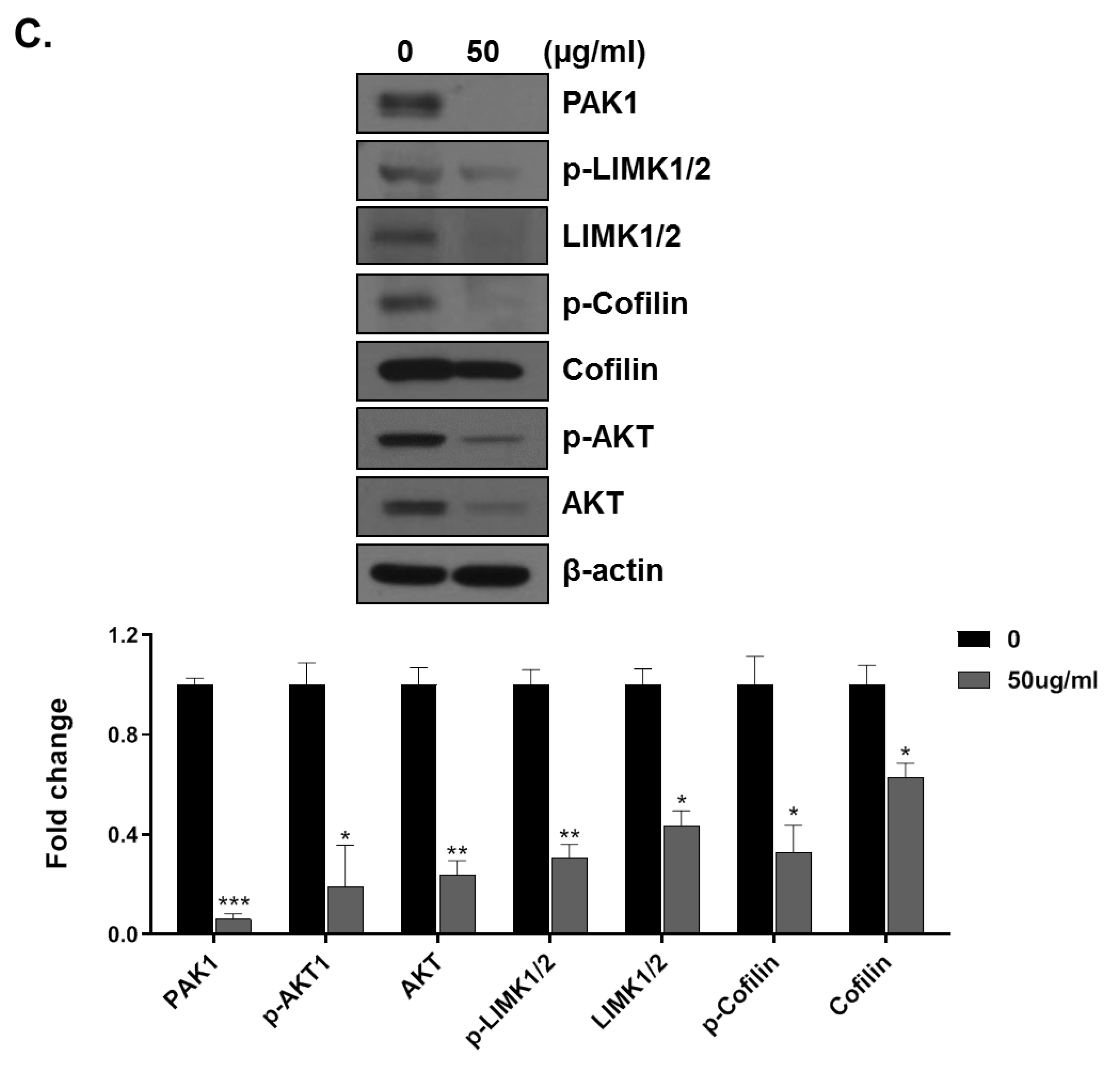
3.3. HMT Inhibits Cell Migration and Invasion by Inhibiting PAK-1 Pathway
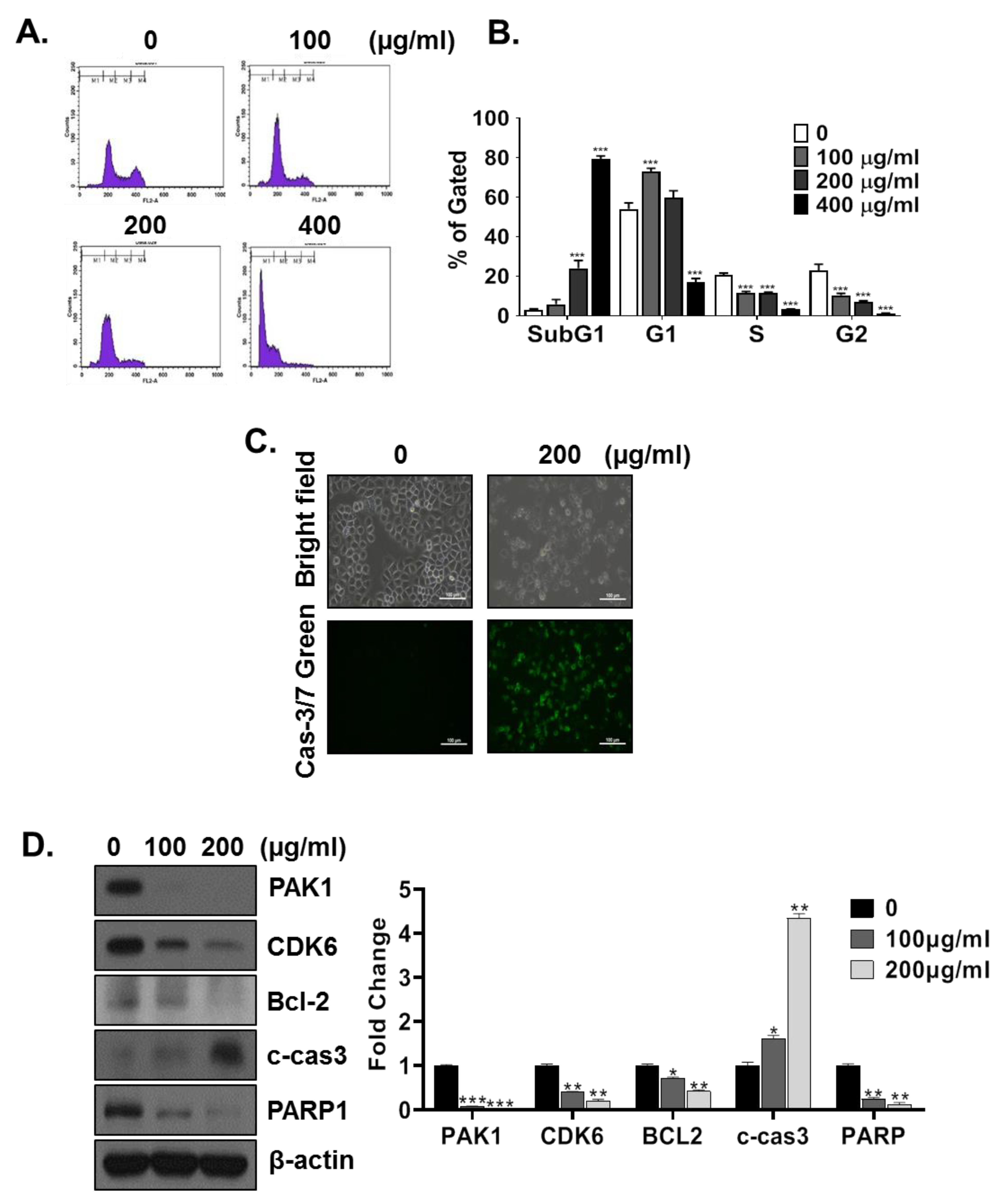
3.4. HMT Induces G1 and Sub-G1 Arrest
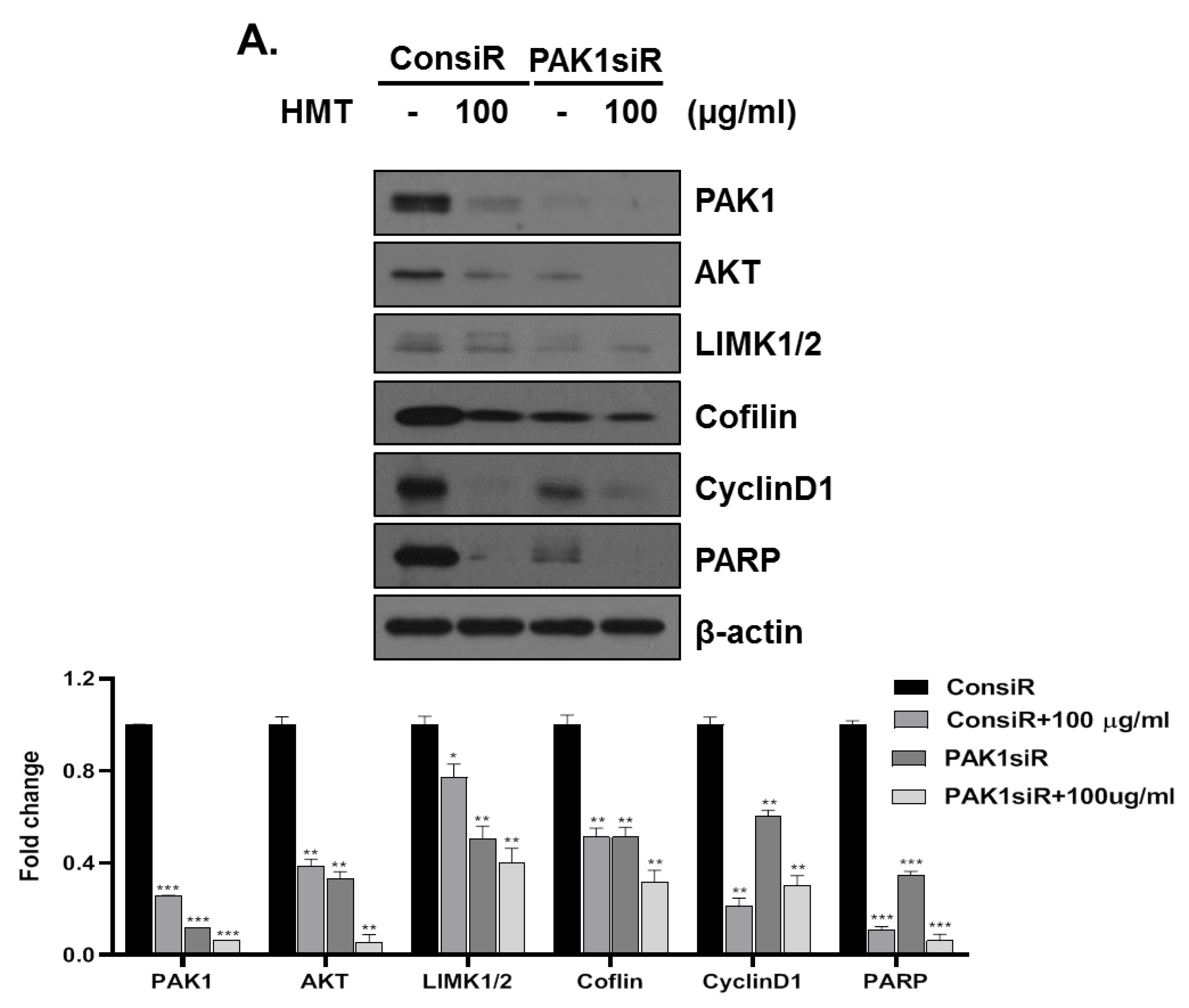
3.5. PAK-1 Mediates HMT-Induced G1 Arrest and Apoptosis and Suppresses Cell Proliferation and Cell Motility
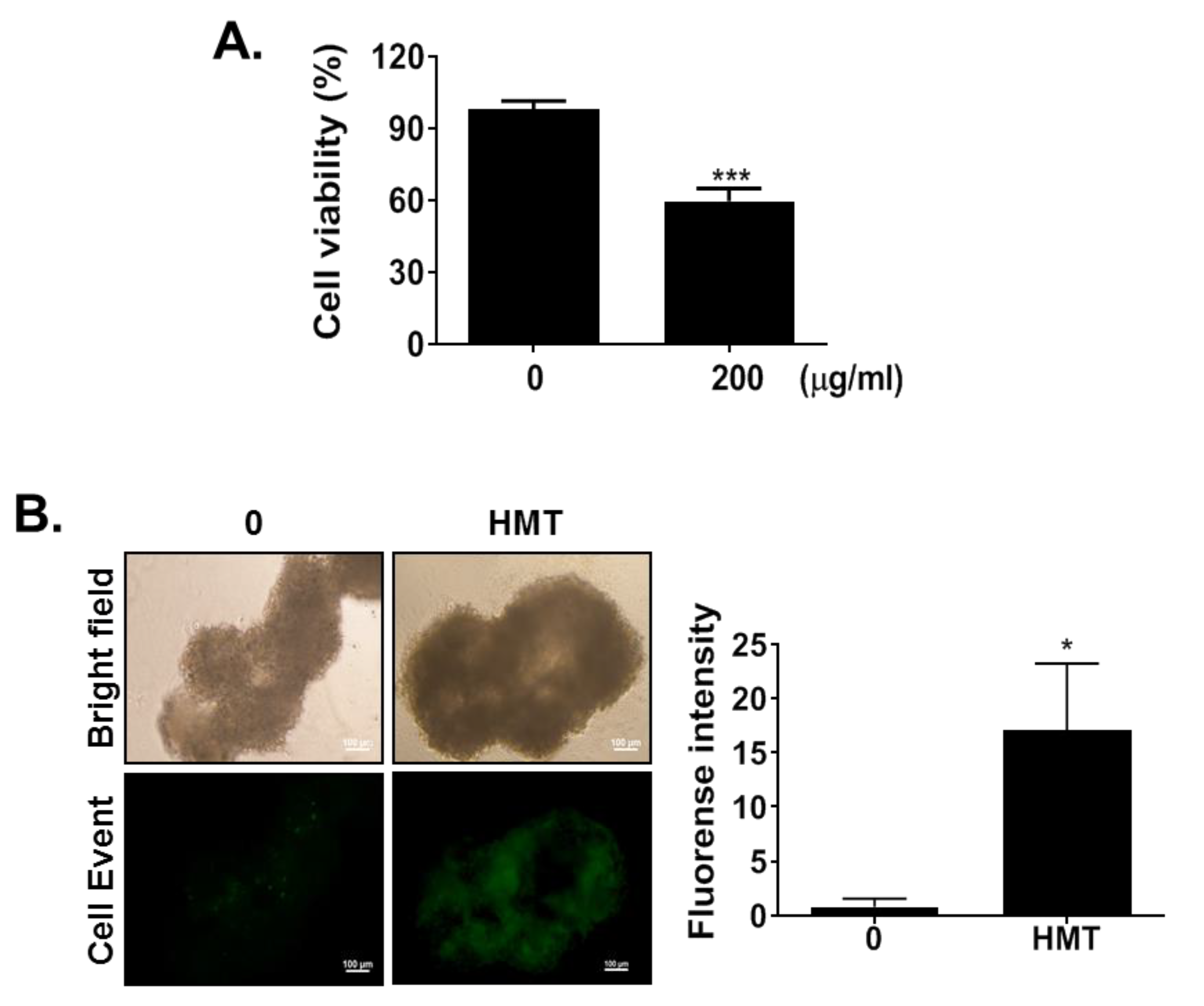
3.6. HMT Reduces Tumor Spheroid Viability
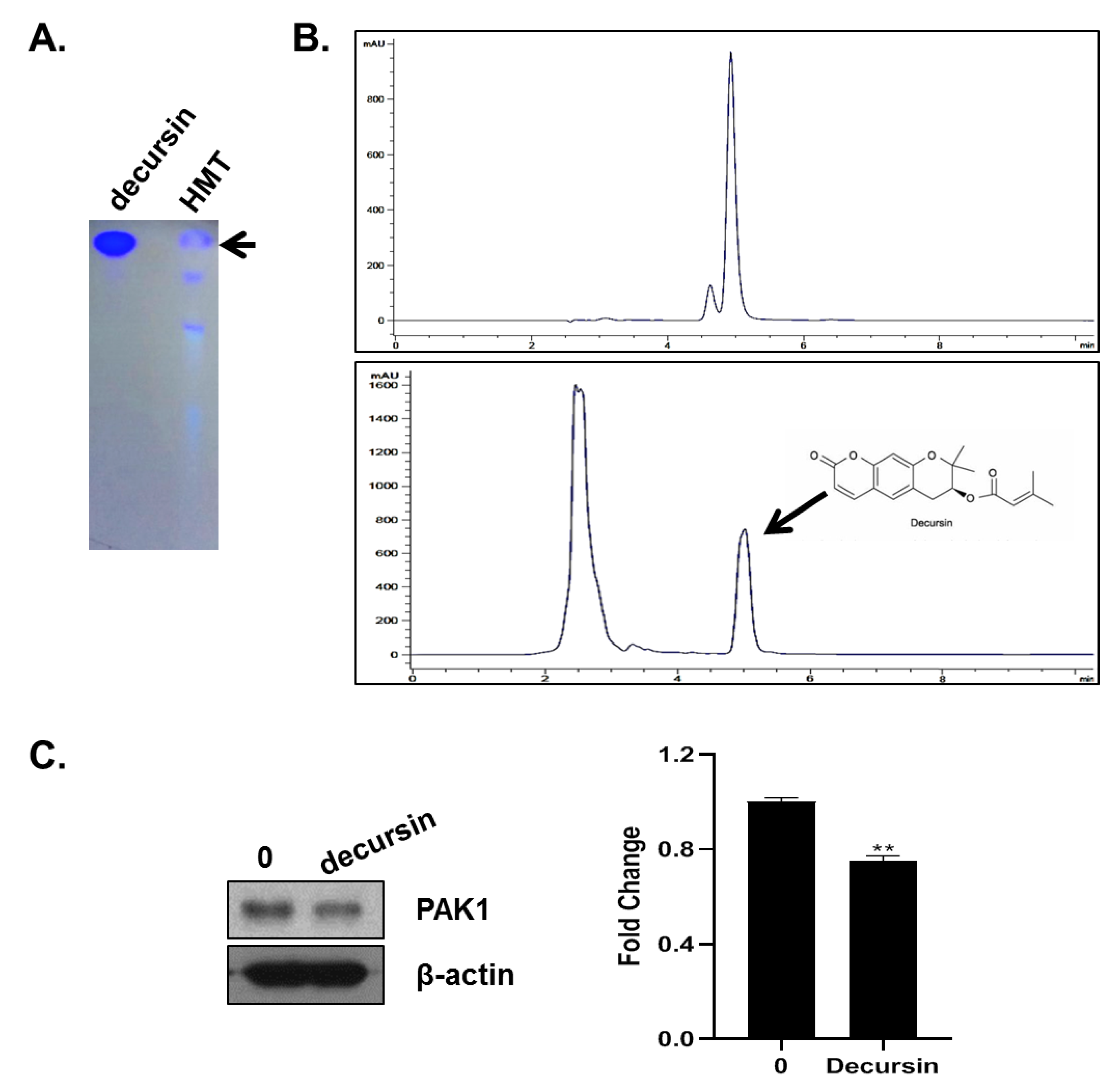
3.7. HMT Contains Decursin That Inhibits PAK-1
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087 (accessed on 28 February 2020).
- Mostaghel, E.A.; Page, S.T.; Lin, D.W.; Fazli, L.; Coleman, I.M.; True, L.D.; Knudsen, B.; Hess, D.L.; Nelson, C.C.; Matsumoto, A.M.; et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007, 67, 5033–5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, J.H.; Douglass, L.E.; Deddens, J.A.; Colligan, B.M.; Bhatt, T.R.; Pemberton, J.O.; Konicek, S.; Hom, J.; Marshall, M.; Graff, J.R. Pak-1 expression increases with progression of colorectal carcinomas to metastasis. Clin. Cancer Res. 2004, 10, 3448–3456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Jia, G.; Li, Y.; Liu, J.; Luo, J.; Zhang, J.; Xu, G.; Chen, G. Clinicopathological signature of p21-activated kinase 1 in prostate cancer and its regulation of proliferation and autophagy via the mTOR signaling pathway. Oncotarget 2017, 8, 22563–22580. [Google Scholar] [CrossRef] [Green Version]
- Manser, E.; Leung, T.; Salihuddin, H.; Zhao, Z.S.; Lim, L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 1994, 367, 40–46. [Google Scholar] [CrossRef]
- Stupack, D.G.; Cho, S.Y.; Klemke, R.L. Molecular signaling mechanisms of cell migration and invasion. Immunol. Res. 2000, 21, 83–88. [Google Scholar] [CrossRef]
- Ding, Y.; Milosavljevic, T.; Alahari, S.K. Nischarin inhibits LIM kinase to regulate cofilin phosphorylation and cell invasion. Mol. Cell. Biol. 2008, 28, 3742–3756. [Google Scholar] [CrossRef] [Green Version]
- Bokoch, G.M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003, 72, 743–781. [Google Scholar] [CrossRef]
- Chew, T.L.; Masaracchia, R.A.; Goeckeler, Z.M.; Wysolmerski, R.B. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J. Muscle Res. Cell Motil. 1998, 19, 839–854. [Google Scholar] [CrossRef]
- Daniels, R.H.; Bokoch, G.M. p21-activated protein kinase: A crucial component of morphological signaling? Trends Biochem. Sci. 1999, 24, 350–355. [Google Scholar] [CrossRef]
- Edwards, D.C.; Sanders, L.C.; Bokoch, G.M.; Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell. Biol. 1999, 1, 253–259. [Google Scholar] [CrossRef]
- Vadlamudi, R.K.; Kumar, R. P21-activated kinases in human cancer. Cancer Metastasis Rev. 2003, 22, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; McGough, A.; Ono, S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999, 9, 364–370. [Google Scholar] [CrossRef]
- Huynh, N.; Liu, K.H.; Baldwin, G.S.; He, H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim. Biophys. Acta 2010, 1803, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.-W.; Wu, T.-C.; Chen, C.-Y.; Lee, H. PAK1 Is a Novel Therapeutic Target in Tyrosine Kinase Inhibitor–Resistant Lung Adenocarcinoma Activated by the PI3K/AKT Signaling Regardless of EGFR Mutation. Clin. Cancer Res. 2016, 22, 5370–5382. [Google Scholar] [CrossRef] [Green Version]
- Kessel, K.A.; Lettner, S.; Kessel, C.; Bier, H.; Biedermann, T.; Friess, H.; Herrschbach, P.; Gschwend, J.E.; Meyer, B.; Peschel, C.J. Use of complementary and alternative medicine (CAM) as part of the oncological treatment: Survey about Patients’ attitude towards CAM in a university-based oncology Center in Germany. PLoS ONE 2016, 11, e0165801. [Google Scholar]
- Zaid, H.; Silbermann, M.; Amash, A.; Gincel, D.; Abdel-Sattar, E.; Sarikahya, N.B. Medicinal plants and natural active compounds for cancer chemoprevention/chemotherapy. Evid. Based Complement Alternat. Med. 2017, 2017, 7952417. [Google Scholar] [CrossRef]
- Shizhen, L. Bencao Gangmu; People’s Medical Publishing House Co., LTD: Beijing, China, 1975. [Google Scholar]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Cain, K. Chemical-induced apoptosis: Formation of the Apaf-1 apoptosome. Drug Metab. Rev. 2003, 35, 337–363. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326 Pt 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005, 12 (Suppl. 1), 942–961. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Nicholas, N.S.; Wells, C.M. Role of p-21-activated kinases in cancer progression. Int. Rev. Cell Mol. Biol. 2014, 309, 347–387. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.J.; McIntosh, E.D.; Martin, F.J.; Menser, M.A. A fifty-year follow-up of ocular defects in congenital rubella: Late ocular manifestations. Aust. N. Z. J. Ophthalmol. 1994, 22, 1–6. [Google Scholar] [CrossRef]
- Goc, A.; Al-Azayzih, A.; Abdalla, M.; Al-Husein, B.; Kavuri, S.; Lee, J.; Moses, K.; Somanath, P.R. P21 Activated kinase-1 (Pak1) Promotes Prostate Tumor Growth and Microinvasion via Inhibition of Transforming Growth Factor β Expression and Enhanced Matrix Metalloproteinase 9 Secretion. J. Biol. Chem. 2013, 288, 3025–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Magid, A.F. PAK1: A Therapeutic Target for Cancer Treatment. ACS Med. Chem. Lett. 2013, 4, 431–432. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.Y.; Dong, B.; Valencia, C.A.; Zeng, C.T.; Koch, J.N.; Prudnikova, T.Y.; Chernoff, J. Group I Paks are essential for epithelial- mesenchymal transition in an Apc-driven model of colorectal cancer. Nat. Commun. 2018, 9, 3473. [Google Scholar] [CrossRef]
- Bae, K.; Kim, E.; Choi, J.J.; Kim, M.K.; Yoo, H.S. The effectiveness of anticancer traditional Korean medicine treatment on the survival in patients with lung, breast, gastric, colorectal, hepatic, uterine, or ovarian cancer: A prospective cohort study protocol. Medicine 2018, 97, e12444. [Google Scholar] [CrossRef]
- Ye, L.; Jia, Y.; Ji, K.E.; Sanders, A.J.; Xue, K.; Ji, J.; Mason, M.D.; Jiang, W.G. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncol. Lett. 2015, 10, 1240–1250. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-Y.; Kim, J.-E.; Kim, M.; Kim, J.-H. A review of traditional Korean medical treatment for cancer-related cognitive impairment. J. Korean Med. 2016, 37, 74–86. [Google Scholar] [CrossRef]
- Xiang, Y.N.; Cuo, Z.M.; Zhu, P.F.; Chen, J.; Huang, Y.Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Gong, W.Y.; Wu, J.F.; Liu, B.J.; Zhang, H.Y.; Cao, Y.X.; Sun, J.; Lv, Y.B.; Wu, X.; Dong, J.C. Flavonoid components in Scutellaria baicalensis inhibit nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in vitro. Int. J. Oncol. 2014, 44, 1561–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yao, J.; Wu, X.P.; Zhao, L.; Zhou, Y.X.; Zhang, Y.; You, Q.D.; Guo, Q.L.; Lu, N. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL-6/STAT3 signaling pathway. Mol. Carcinog. 2015, 54 (Suppl. 1), E81–E93. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, H.; Chen, S.; Yang, R.; Li, H.; Zhang, G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J. Cell. Mol. Med. 2018, 22, 2478–2487. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Lee, E.O.; Kim, S.H.; Lee, H.J. Essential oil of Pinus koraiensis inhibits cell proliferation and migration via inhibition of p21-activated kinase 1 pathway in HCT116 colorectal cancer cells. BMC Complement. Altern. Med. 2014, 14, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menges, C.W.; Sementino, E.; Talarchek, J.; Xu, J.F.; Chernoff, J.; Peterson, J.R.; Testa, J.R. Group I p21-Activated Kinases (PAKs) Promote Tumor Cell Proliferation and Survival through the AKT1 and Raf-MAPK Pathways. Mol. Cancer Res. 2012, 10, 1178–1188. [Google Scholar] [CrossRef] [Green Version]
- Rane, C.K.; Minden, A. P21 activated kinase signaling in cancer. Proc. Semin. Cancer Biol. 2019, 54, 40–49. [Google Scholar] [CrossRef]
- Thullberg, M.; Gad, A.; Beeser, A.; Chernoff, J.; Stromblad, S. The kinase-inhibitory domain of p21-activated kinase 1 (PAK1) inhibits cell cycle progression independent of PAK1 kinase activity. Oncogene 2007, 26, 1820–1828. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.C.; Jubb, A.M.; Haverty, P.M.; Zhou, W.; Tran, V.; Truong, T.; Turley, H.; O’Brien, T.; Vucic, D.; Harris, A.L.; et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7177–7182. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Wu, X.; Wang, H.X.; Hou, G.W.; Han, X.; Song, W. PAK1 silencing is synthetic lethal with CDK4/6 inhibition in gastric cancer cells via regulating PDK1 expression. Hum. Cell 2020, 33, 377–385. [Google Scholar] [CrossRef]
- Li, W.; Ni, Q.; Zhao, Z.; Zhang, C. Inhibitory effects of seahorse treat S180 entity tumor in mic. Med. Pharmacacetical J. 1998, 20, 6–7. [Google Scholar]
- Aimin, Z. Pharmacologic Researches on Ethanol Extracts from Hippocampus. J. Chin. Pharm. Aff. 2005, 1, 23–25. [Google Scholar]
- Meng, X.; Xu, D.; Mei, X.; Xu, S.; Lv, J.; Li, B.J. Research on Hippocampus capsule therapy of experimental benign prostatic hyperplasia. Chin. Pharm. J. 2005, 40, 190–193. [Google Scholar]
- Xu, D.H.; Wang, L.H.; Mei, X.T.; Li, B.J.; Lv, J.L.; Xu, S.B. Protective effects of seahorse extracts in a rat castration and testosterone-induced benign prostatic hyperplasia model and mouse oligospermatism model. Environ. Toxicol. Pharmacol. 2014, 37, 679–688. [Google Scholar] [CrossRef]
- Cha, T.L.; Qiu, L.; Chen, C.T.; Wen, Y.; Hung, M.C. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005, 65, 2287–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.X.; Zhang, X.Q.; Kang, L.D.; Zhang, P.J.; Chen, W.W.; Liu, W.W.; Liu, Q.W.; Zhang, J.Y. Emodin induces apoptosis in human prostate cancer cell LNCaP. Asian J. Androl. 2008, 10, 625–634. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, H.M.; Ong, C.N. Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cell Mol. Life Sci. 2005, 62, 1167–1175. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Dou, Q.P. Molecular mechanisms of green tea polyphenols. Nutr. Cancer 2009, 61, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.J.; Chen, B.H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016, 11, 1907–1926. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.P.; Wang, A.; Ye, J.H.; Zheng, X.Q.; Polito, C.A.; Lu, J.L.; Li, Q.S.; Liang, Y.R. Suppressive Effects of Tea Catechins on Breast Cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- Rogovskii, V.S.; Popov, S.V.; Sturov, N.V.; Shimanovskii, N.L. The Possibility of Preventive and Therapeutic Use of Green Tea Catechins in Prostate Cancer. Anti-Cancer Agents Med. Chem. 2019, 19, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Turkekul, K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018, 6, 126–135. [Google Scholar] [CrossRef]
- Yim, D.; Singh, R.P.; Agarwal, C.; Lee, S.; Chi, H.; Agarwal, R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005, 65, 1035–1044. [Google Scholar]
- Jiang, C.; Lee, H.J.; Li, G.X.; Guo, J.M.; Malewicz, B.; Zhao, Y.; Lee, E.O.; Lee, H.J.; Lee, J.H.; Kim, M.S.; et al. Potent antiandrogen and androgen receptor activities of an Angelica gigas-containing herbal formulation: Identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res. 2006, 66, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Kim, S.H.; Jiang, C.; Lee, H.; Guo, J. Oriental herbs as a source of novel anti-androgen and prostate cancer chemopreventive agents. Acta Pharmacol. Sin. 2007, 28, 1365–1372. [Google Scholar] [CrossRef]
- Song, G.-Y.; Lee, J.-H.; Cho, M.; Park, B.-S.; Kim, D.-E.; Oh, S. Decursin suppresses human androgen-independent PC3 prostate cancer cell proliferation by promoting the degradation of β-catenin. Mol. Pharmacol. 2007, 72, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Lee, H.J.; Lee, E.O.; Lee, J.H.; Lee, K.S.; Kim, K.H.; Kim, S.H.; Lu, J. In vivo anti-cancer activity of Korean Angelica gigas and its major pyranocoumarin decursin. Am. J. Chin. Med. 2009, 37, 127–142. [Google Scholar] [CrossRef] [Green Version]
- Wartenberg, M.; Ling, F.C.; Müschen, M.; Klein, F.; Acker, H.; Gassmann, M.; Petrat, K.; Pütz, V.; Hescheler, J.; Sauer, H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor-1 and reactive oxygen species. FASEB J. 2003, 17, 1–22. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.; Jiang, W.G.; Mason, M.D. Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J. Urol. 2000, 163, 985–992. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Venkatasubramanian, R.; Forbes, N.S. Rapid uptake of glucose and lactate, and not hypoxia, induces apoptosis in three-dimensional tumor tissue culture. Integr. Biol. 2014, 6, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, C.; Riefke, B.; Grundemann, S.; Krebs, A.; Christian, S.; Prinz, F.; Osterland, M.; Golfier, S.; Rase, S.; Ansari, N.; et al. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp. Cell Res. 2014, 323, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fomin, M.A.; Dmitriev, R.I.; Jenkins, J.; Papkovsky, D.B.; Heindl, D.; Konig, B. Two-Acceptor Cyanine-Based Fluorescent Indicator for NAD(P)H in Tumor Cell Models. ACS Sens. 2016, 1, 702–709. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Cha, J.-S.; Lee, S.-O.; Ryu, S.-I.; Lee, Y.-K.; Han, H.; Kim, J.-E.; Lee, M.-H.; Lee, E.-O.; Lee, H.-J. HMT Exerts an Anticancer Effect by Targeting PAK-1. Appl. Sci. 2021, 11, 6034. https://doi.org/10.3390/app11136034
Xu Y, Cha J-S, Lee S-O, Ryu S-I, Lee Y-K, Han H, Kim J-E, Lee M-H, Lee E-O, Lee H-J. HMT Exerts an Anticancer Effect by Targeting PAK-1. Applied Sciences. 2021; 11(13):6034. https://doi.org/10.3390/app11136034
Chicago/Turabian StyleXu, Yinzhu, Jin-Sol Cha, Seon-Ok Lee, Soo-In Ryu, You-Kyung Lee, Hengmin Han, Jung-Eun Kim, Min-Ho Lee, Eun-Ok Lee, and Hyo-Jeong Lee. 2021. "HMT Exerts an Anticancer Effect by Targeting PAK-1" Applied Sciences 11, no. 13: 6034. https://doi.org/10.3390/app11136034
APA StyleXu, Y., Cha, J.-S., Lee, S.-O., Ryu, S.-I., Lee, Y.-K., Han, H., Kim, J.-E., Lee, M.-H., Lee, E.-O., & Lee, H.-J. (2021). HMT Exerts an Anticancer Effect by Targeting PAK-1. Applied Sciences, 11(13), 6034. https://doi.org/10.3390/app11136034





