Manufacture of 2D-Printed Precision Drug-Loaded Orodispersible Film Prepared from Tamarind Seed Gum Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gum Powder
2.3. Preparation of the Theophylline Solution (Ink Solution)
2.4. Printing Substrate Preparation
2.5. Drug Printing
2.6. Assessment of Physicochemical Properties and Morphology of the Printing Substrate
2.6.1. Mechanical Properties
2.6.2. Morphology of the Printing Substrate
2.6.3. Thickness and Moisture Content of the Printing Substrate
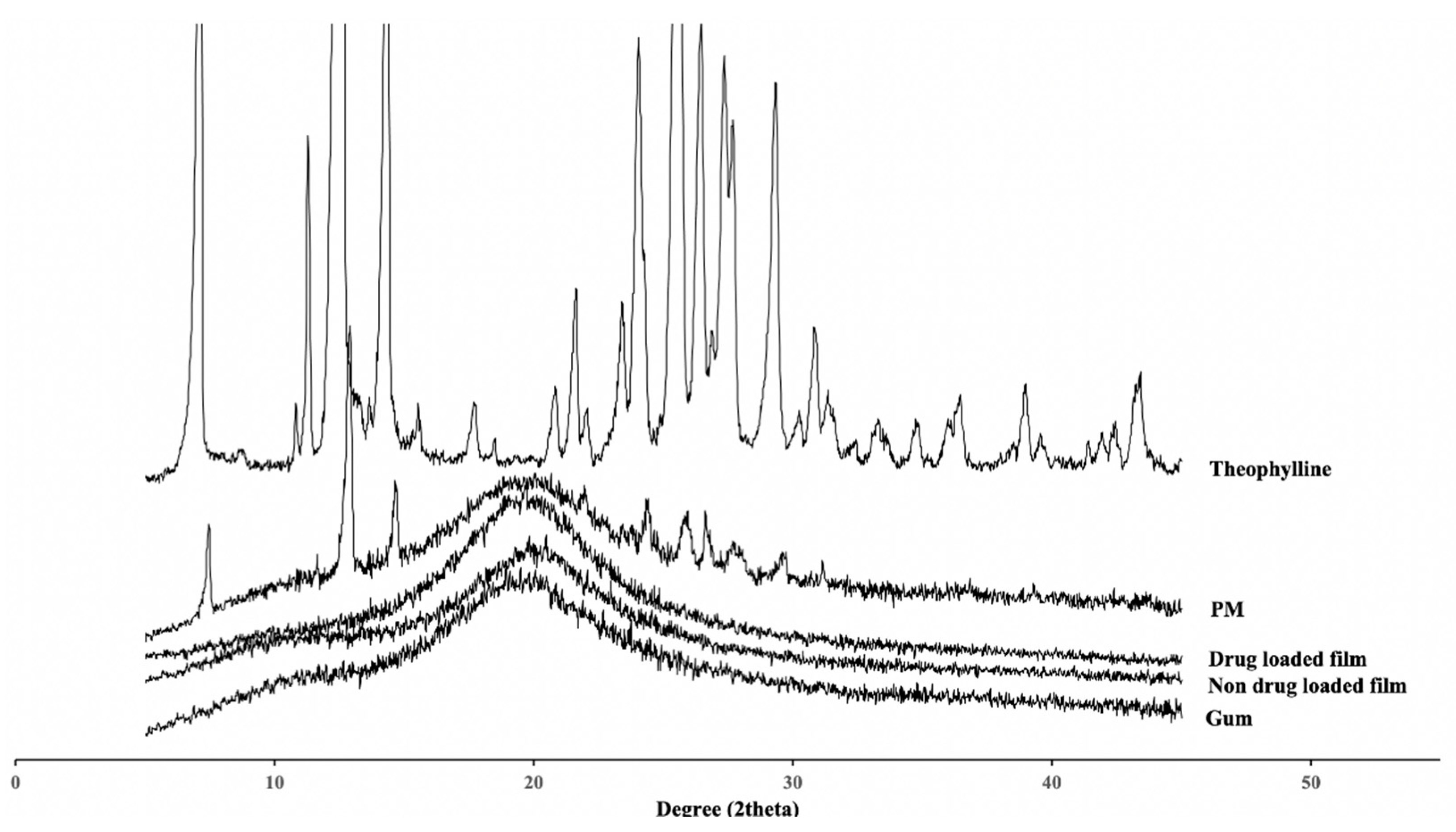
2.6.4. Powder X-ray Diffraction
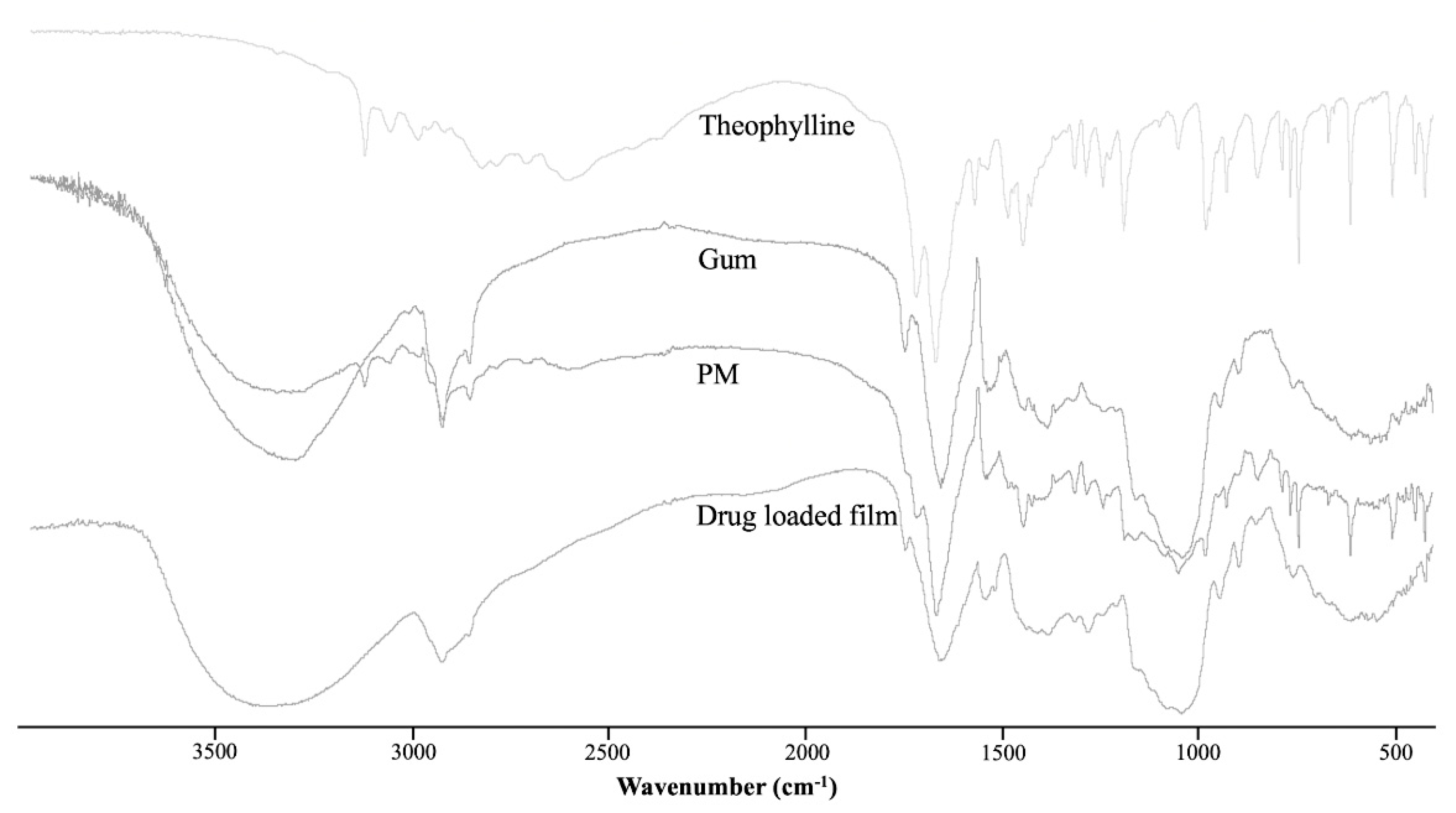
2.6.5. Fourier Transform Infrared Spectrometer
2.7. In Vitro Disintegration Study
2.8. Printed Drug Amount and Drug Content Analysis
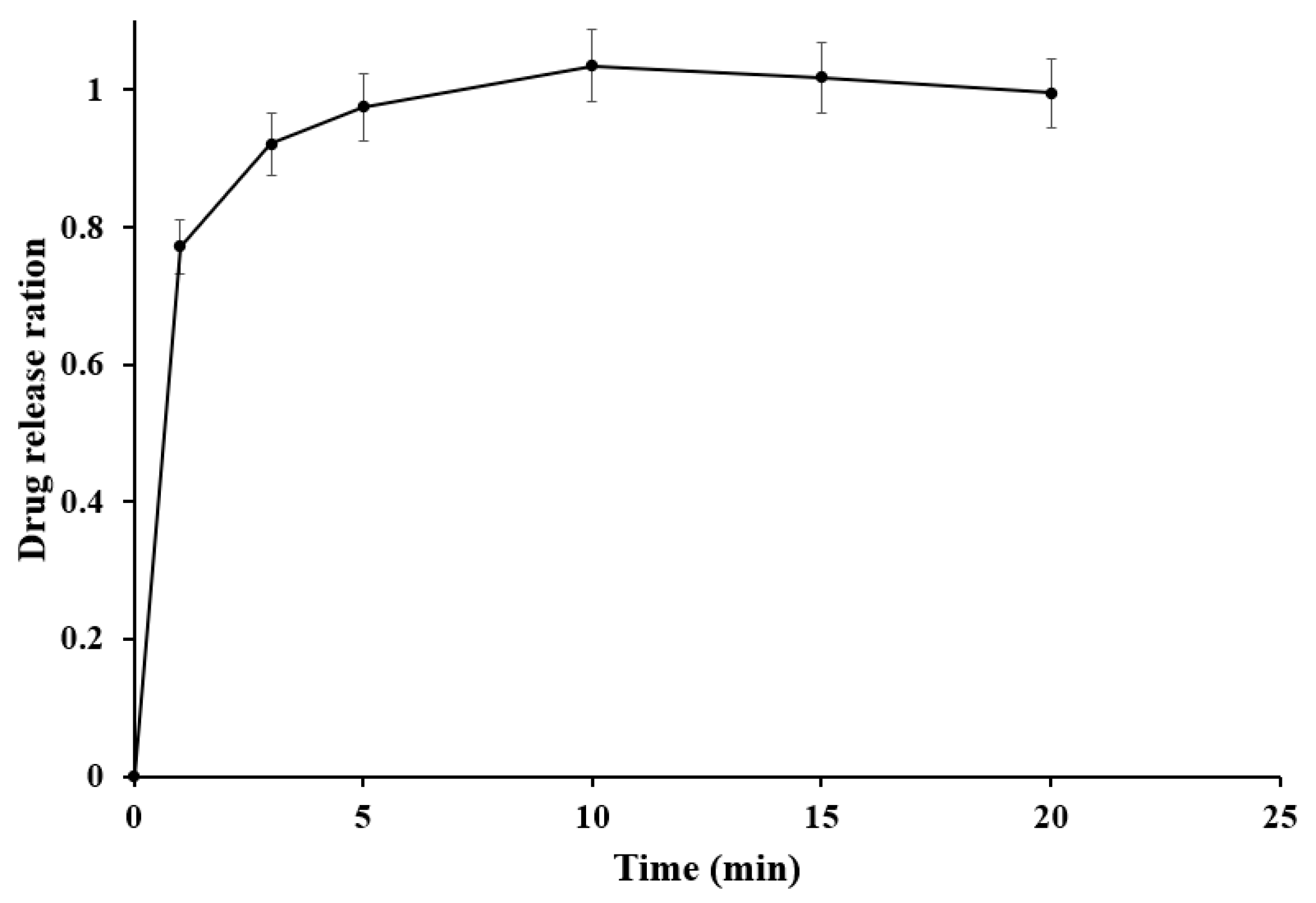
2.9. In Vitro Dissolution Study
2.10. Statistical Analysis
3. Results
3.1. Assessment of Physicochemical Properties and Morphology of the Printing Substrate
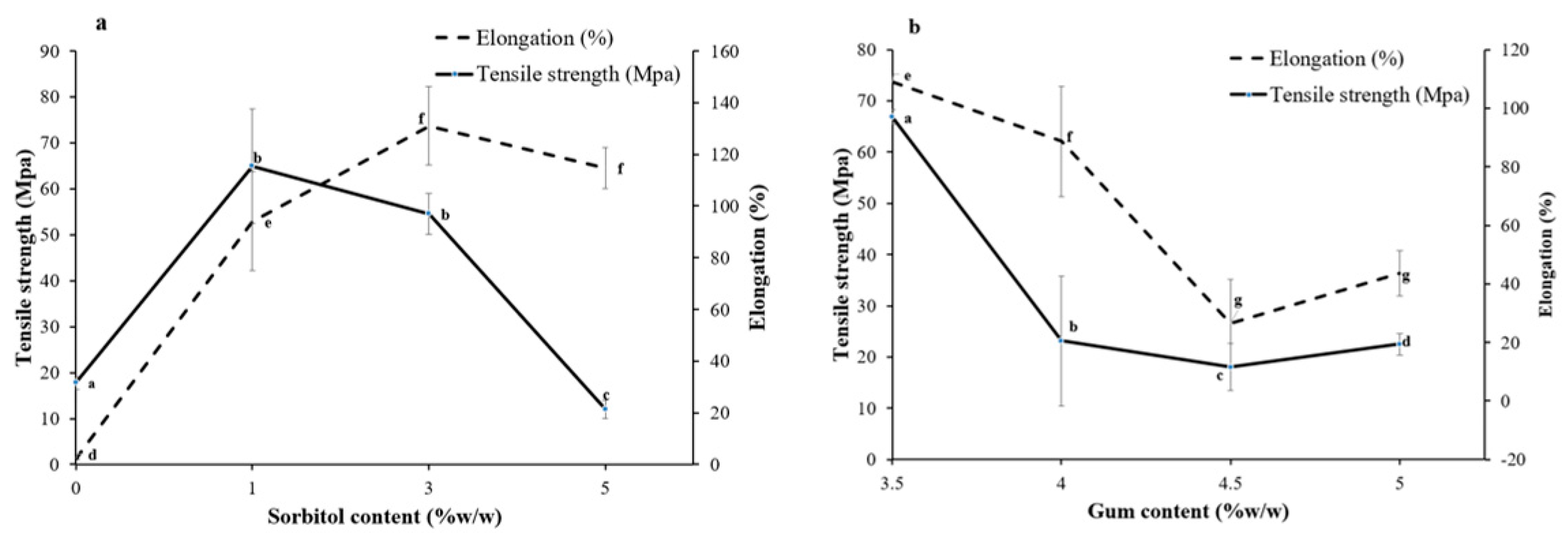
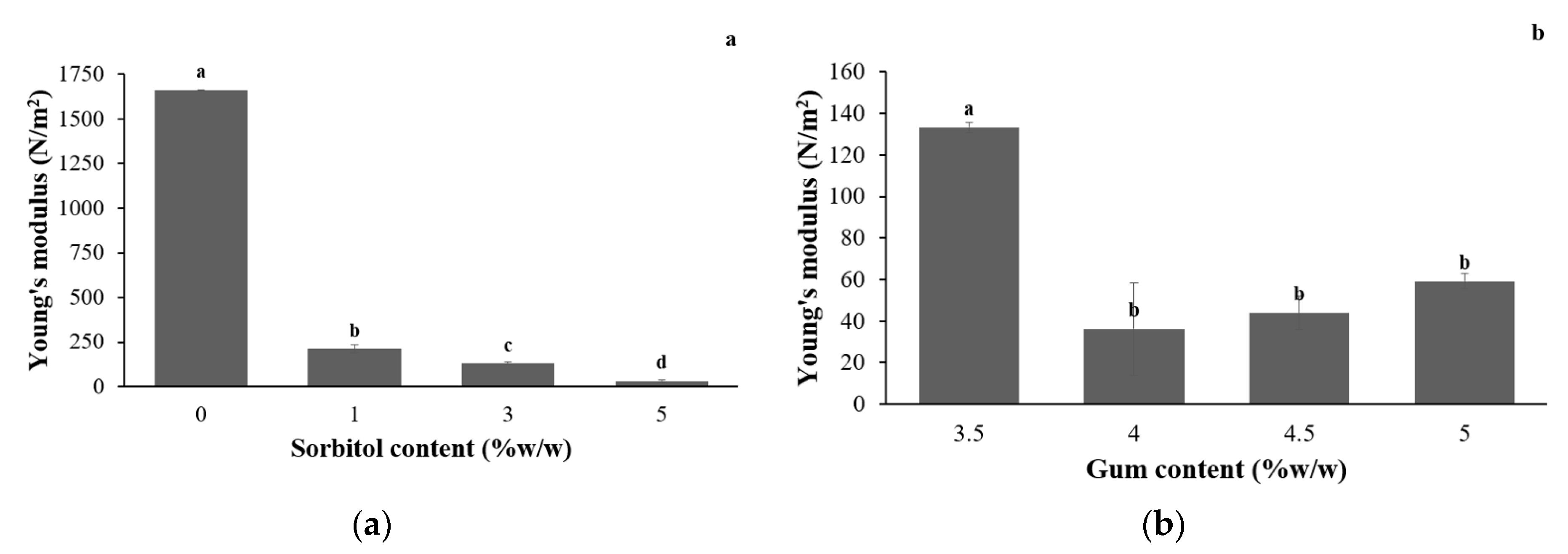
3.1.1. Mechanical Properties
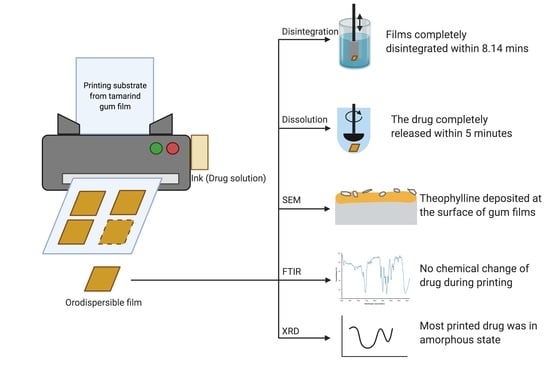
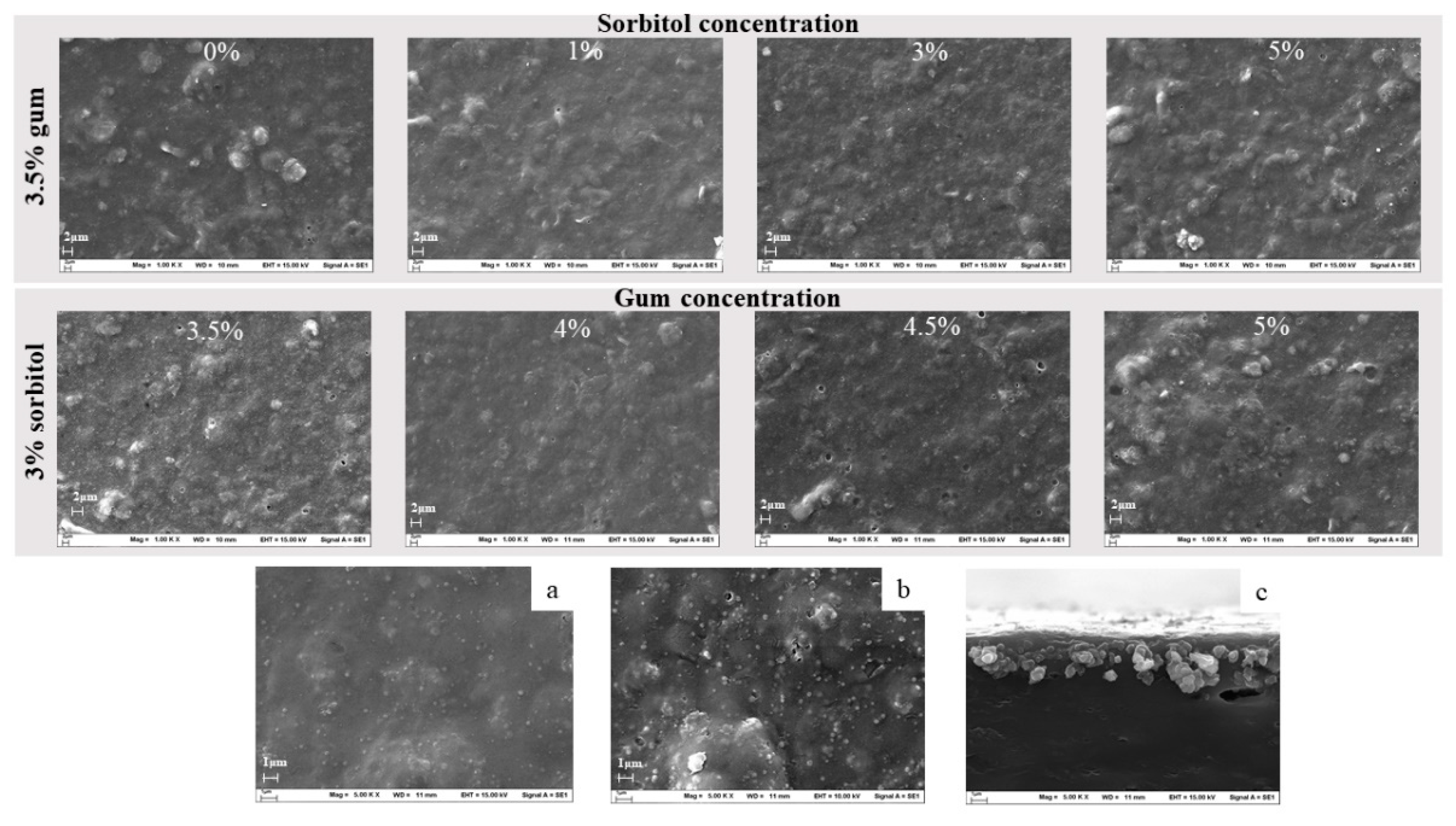
3.1.2. Morphology of the Printing Substrate
3.1.3. Thickness and Moisture Content of the Printed Substrate
3.1.4. Powder X-ray Diffraction
3.1.5. Fourier Transform Infrared Spectrometer
3.2. In Vitro Disintegration Study
3.3. Printed Drug Amount and Drug Content Analysis
3.4. In Vitro Dissolution Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Issa, A.M. Personalized medicine and the practice of medicine in the 21st century. McGill J. Med. 2007, 10, 53. [Google Scholar]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef]
- Buanz, A.B.M.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Alomari, M.; Dodoo, C.C.; Trenfield, S.J.; Velaga, S.; Basit, A.W.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards printed pediatric medicines in hospital pharmacies: Comparison of 2D and 3D-printed orodispersible warfarin films with conventional oral powders in unit dose sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef]
- Gans, B.D.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Slavkova, M.; Breitkreutz, J. Orodispersible drug formulations for children and elderly. Eur. J. Pharm. Sci. 2015, 75, 2–9. [Google Scholar] [CrossRef]
- Scarpa, M.; Stegemann, S.; Hsiao, W.-K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef]
- El-Setouhy, D.A.; Abd El-Malak, N.S. Formulation of a novel tianeptine sodium orodispersible film. AAPS PharmSciTech 2010, 11, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Dinge, A.; Nagarsenker, M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech 2008, 9, 349–356. [Google Scholar] [CrossRef]
- Vasvári, G.; Kalmár, J.; Veres, P.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Haimhoffer, Á.; Rusznyák, Á.; Fenyvesi, F. Matrix systems for oral drug delivery: Formulations and drug release. Drug Discov. Today Technol. 2018, 27, 71–80. [Google Scholar] [CrossRef]
- Jani, R.; Patel, D. Hot melt extrusion: An industrially feasible approach for casting orodispersible film. Asian J. Pharm. Sci. 2015, 10, 292–305. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. SOFTs–Structured orodispersible film templates. Eur. J. Pharm. Biopharm. 2019, 137, 209–217. [Google Scholar] [CrossRef]
- Buanz, A.B.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, M.O.; Gaisford, S. Ink-jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 494, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wickström, H.; Nyman, J.O.; Indola, M.; Sundelin, H.; Kronberg, L.; Preis, M.; Rantanen, J.; Sandler, N. Colorimetry as quality control tool for individual inkjet-printed pediatric formulations. AAPS PharmSciTech 2017, 18, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Azizi Machekposhti, S.; Mohaved, S.; Narayan, R.J. Inkjet dispensing technologies: Recent advances for novel drug discovery. Expert Opin. Drug Discov. 2019, 14, 101–113. [Google Scholar] [CrossRef] [PubMed]
- SantoshNaidu, M.; Radha, G.; Girish, P.; Lohithasu, D. Comparison studies on transdermal films of natural tamarind seed polysaccharide extract containing anti hypertension drug with PVA. HPMC Guar Gum World J. Pharm. Res. 2014, 3, 753–763. [Google Scholar]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Combining inkjet printing and amorphous nanonization to prepare personalized dosage forms of poorly-soluble drugs. Eur. J. Pharm. Biopharm. 2015, 96, 314–321. [Google Scholar] [CrossRef]
- Jonkman, J.H.G. Therapeutic consequences of drug interactions with theophylline pharmacokinetics. J. Allergy Clin. Immunol. 1986, 78, 736–742. [Google Scholar] [CrossRef]
- Huanbutta, K.; Sangnim, T.; Sittikijyothin, W. Development of tamarind seed gum as dry binder in formulation of diclofenac sodium tablets. Walailak J. Sci. Technol. 2016, 13, 863–874. [Google Scholar]
- Bin Liew, K.; Tan, Y.T.F.; Peh, K.K. Characterization of oral disintegrating film containing donepezil for Alzheimer disease. AAPS PharmSciTech 2012, 13, 134–142. [Google Scholar] [CrossRef]
- The National Formulary, USP 40 NF. 2017. Available online: https://search.library.wisc.edu/catalog/999509774402121 (accessed on 12 January 2021).
- El Meshad, A.N.; El Hagrasy, A.S. Characterization and optimization of orodispersible mosapride film formulations. AAPS PharmSciTech 2011, 12, 1384–1392. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H.J.; Zhong, F. Physicochemical and thermomechanical characterization of tara gum edible films: Effect of polyols as plasticizers. Carbohydr. Polym. 2014, 111, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Sittikijyothin, W.; Sangnim, T. Development of topical natural based film forming system loaded propolis from stingless bees for wound healing application. J. Pharm. Investig. 2020, 50, 625–634. [Google Scholar] [CrossRef]
- Shaw, N.B.; Monahan, F.J.; O’riordan, E.D.; O’sullivan, M. Physical properties of WPI films plasticized with glycerol, xylitol, or sorbitol. J. Food Sci. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Buanz, A.B.; Telford, R.; Scowen, I.J.; Gaisford, S. Rapid preparation of pharmaceutical co-crystals with thermal ink-jet printing. CrystEngComm 2013, 15, 1031–1035. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of glycerol and sorbitol plasticizers on physical and thermal properties of sugar palm starch based films. In Proceedings of the 13th International Conference on Environment, Ecosystems and Development (EED ‘15), Kuala Lumpur, Malaysia, 23–25 April 2015; p. 157. [Google Scholar]
- Nunes, C.; Mahendrasingam, A.; Suryanarayanan, R. Investigation of the multi-step dehydration reaction of theophylline monohydrate using 2-dimensional powder X-ray diffractometry. Pharm. Res. 2006, 23, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Takahashi, H.; Okamoto, H.; Tanino, H.; Danjo, K. Theophylline particle design using chitosan by the spray drying. Int. J. Pharm. 2004, 270, 167–174. [Google Scholar] [CrossRef]
- Huanbutta, K.; Terada, K.; Sriamornsak, P.; Nunthanid, J. Simultaneous X-ray Diffraction-Differential Scanning Calorimetry and Physicochemical Characterizations of Spray Dried Drugs and Chitosan Microspheres. Walailak J. Sci. Technol. 2016, 13, 849–861. [Google Scholar]
- Huanbutta, K.; Sangnim, T.; Sittikijyothin, W. Physicochemical characterization of gum from tamarind seed: Potential for pharmaceutical application. Asian J. Pharm. Sci. 2016, 11. [Google Scholar] [CrossRef]
- Kesavan, J.G.; Peck, G.E. Solid-state stability of theophylline anhydrous in theophylline anhydrous-polyvinylpyrrolidone physical mixtures. Drug Dev. Ind. Pharm. 1996, 22, 189–199. [Google Scholar] [CrossRef]
- Aguilar, J.E.; Montoya, E.G.; Lozano, P.P.; Negre, J.M.S.; Carmona, M.M.; Grau, J.R.T. New sedem-odt expert system: An expert system for formulation of orodispersible tablets obtained by direct compression. In Formulation Tools for Pharmaceutical Development; Elsevier: Amsterdam, The Netherlands, 2013; pp. 137–154. [Google Scholar]
- Ruiz-Picazo, A.; Colón-Useche, S.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I.; Bermejo, M.; Langguth, P. Effect of thickener on disintegration, dissolution and permeability of common drug products for elderly patients. Eur. J. Pharm. Biopharm. 2020, 153, 168–176. [Google Scholar] [CrossRef]
- Castro, P.M.; Sousa, F.; Magalhães, R.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Incorporation of beads into oral films for buccal and oral delivery of bioactive molecules. Carbohydr. Polym. 2018, 194, 411–421. [Google Scholar] [CrossRef] [PubMed]
| Sample No. | Printing Size (cm2) | Overprinting Repeat |
|---|---|---|
| 1 | 2 × 2 | 1 |
| 2 | 2 × 2 | 3 |
| 3 | 2 × 2 | 5 |
| 4 | 4 × 3 | 1 |
| 5 | 4 × 5 | 1 |
| Gum Concentration (% w/w) | Sorbitol Percent from Gum | Moisture Content (%) | Thickness (μm) | Disintegration Time (min) |
|---|---|---|---|---|
| 3.5 | 0 | 9.30 ± 1.04 | 109.11 ± 23.24 | 19.31 ± 0.88 |
| 3.5 | 1 | 9.29 ± 0.25 | 106.56 ± 30.63 | 11.16 ± 1.76 |
| 3.5 | 3 | 8.31 ± 0.36 | 106.89 ± 10.47 | 8.14 ± 0.98 |
| 3.5 | 5 | 7.49 ± 0.62 | 109.11 ± 18.74 | 9.33 ± 0.13 |
| 4 | 3 | 9.40 ± 1.12 | 117.22 ± 21.28 | 17.57 ± 3.24 |
| 4.5 | 3 | 9.71 ± 1.57 | 131.33 ± 30.71 | >30 |
| 5 | 3 | 9.38 ± 0.32 | 151.33 ± 18.21 | >30 |
| Printing Area (cm2) | Printing Repeat | Actual Drug Content (μg) | Drug Content Analysis (%) |
|---|---|---|---|
| 2 × 2 | 1 | 28.05 ± 1.01 | 96.68 ± 3.47 |
| 2 × 2 | 3 | 121.21 ± 3.19 | 101.54 ± 2.67 |
| 2 × 2 | 5 | 219.97 ± 4.52 | 99.58 ± 2.04 |
| 4 × 3 | 1 | 123.80 ± 5.21 | 89.72 ± 3.77 |
| 4 × 5 | 1 | 181.58 ± 6.65 | 103.42 ± 3.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huanbutta, K.; Sriamornsak, P.; Singh, I.; Sangnim, T. Manufacture of 2D-Printed Precision Drug-Loaded Orodispersible Film Prepared from Tamarind Seed Gum Substrate. Appl. Sci. 2021, 11, 5852. https://doi.org/10.3390/app11135852
Huanbutta K, Sriamornsak P, Singh I, Sangnim T. Manufacture of 2D-Printed Precision Drug-Loaded Orodispersible Film Prepared from Tamarind Seed Gum Substrate. Applied Sciences. 2021; 11(13):5852. https://doi.org/10.3390/app11135852
Chicago/Turabian StyleHuanbutta, Kampanart, Pornsak Sriamornsak, Inderbir Singh, and Tanikan Sangnim. 2021. "Manufacture of 2D-Printed Precision Drug-Loaded Orodispersible Film Prepared from Tamarind Seed Gum Substrate" Applied Sciences 11, no. 13: 5852. https://doi.org/10.3390/app11135852
APA StyleHuanbutta, K., Sriamornsak, P., Singh, I., & Sangnim, T. (2021). Manufacture of 2D-Printed Precision Drug-Loaded Orodispersible Film Prepared from Tamarind Seed Gum Substrate. Applied Sciences, 11(13), 5852. https://doi.org/10.3390/app11135852