Abstract
The 1,2,3-thiadiazole moiety occupies a significant and prominent position among privileged heterocyclic templates in the field of medicine, pharmacology and pharmaceutics due to its broad spectrum of biological activities. The 1,2,3-thiadiazole hybrid structures showed myriad biomedical activities such as antifungal, antiviral, insecticidal, antiamoebic, anticancer and plant activators, etc. In the present review, various synthetic transformations and approaches are highlighted to furnish 1,2,3-thiadiazole scaffolds along with different pharmaceutical and pharmacological activities by virtue of the presence of the 1,2,3-thiadiazole framework on the basis of structure–activity relationship (SAR). The discussion in this review article will attract the attention of synthetic and medicinal researchers to explore 1,2,3-thiadiazole structural motifs for future therapeutic agents.
1. Introduction
The fascinating aromatic 1,2,3-thiadiazole is a structurally active pharmacophore and great interest for researchers due to its versatile and wide array of biological activities in the field of medicine, pharmacology and pharmaceutics. Thiadiazoles occur naturally in four different isomeric forms, having one sulfur and two nitrogen atoms with hydrogen binding domain as presented in Figure 1 [1,2,3,4].
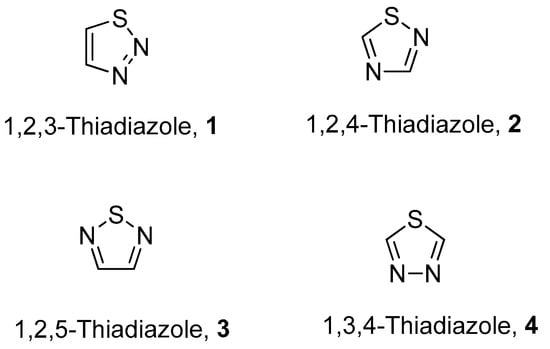
Figure 1.
Structures of different isomeric forms of thiadiazole (1–4).
Thiadiazole scaffold emerges as an interesting structural motif due to the presence of different heteroatoms in its structure and its promising therapeutic activities in the treatment and cure of several diseases. Different substitutions on the thiadiazole nucleus improve the structure–activity relationship (SAR) of thiadiazole hybrid structures to enhance its therapeutic efficacy against a wide variety of pathogens [1,5]. Thiadiazole scaffolds exhibit a wide range of biological activities including antimicrobial [6,7,8,9,10,11], carbonic anhydrase inhibitors [12,13,14], antifungal [15,16,17,18,19], antibacterial [20,21,22,23,24,25], systemic acquired resistance [26,27], insecticidal activity [28,29], antiviral [30,31,32,33], antioxidant [34,35], anticonvulsant [36,37,38,39,40,41,42], antihypertensive activity [43,44], anticancer and antitumor [45,46,47,48,49,50,51], analgesic [52,53], anti-inflammatory [54,55,56,57,58], plant activator [59,60], antidiabetic [61], antileishmanial activity [62,63,64,65] and antituberculosis [66,67,68,69,70,71]. The different marketed drugs of thiadiazole moiety (5–14) are given below [26,72,73,74,75,76] (Figure 2).
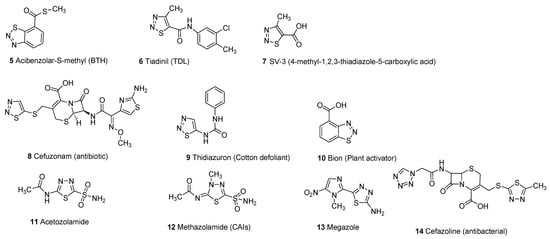
Figure 2.
Commercial drugs based on different thiadiazole scaffolds (5–14).
The bioactive 1,2,3-thiadiazole analogues display excellent and outstanding new profiles of medicinal, pharmacological and pharmaceutical activities [1,77]. In this review article, the most recent and relevant synthetic approaches and pharmacological activities of 1,2,3-thiadiazole analogues are discussed and can be classified into antiviral, insecticidal, antifungal and anticancer activities.
2. Synthetic Approaches
The different synthetic approaches and methodologies such as Hurd–Mori cyclization, ultrasound, microwave, multi-component reaction, metal-free conditions, [4 + 1] annulation, oxidative coupling intermolecular [3 + 2] heterocyclizations and heterogeneous catalysis are listed to furnish bioactive 1,2,3-thiadiazoles.
2.1. Hurd–Mori and Lalezari Cyclization
Pyrazolyl-1,2,3-thiadiazole scaffolds were afforded through carbon-sulfur, carbon double bonded with nitrogen and nitrogen-sulfur bonds formation by applying Hurd–Mori cyclization conditions as described in Scheme 1. In this efficient synthetic approach, various pyrazolyl-phenylethanones (15) were reacted with semicarbazide (16) to generate corresponding semicarbazone (17) intermediate that cyclized into substituted 1,2,3-thiadiazoles (18) after treating with thionyl chloride in good to excellent yield (Scheme 1) [72].
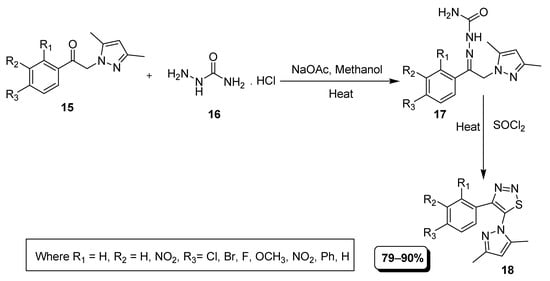
Scheme 1.
Construction of pyrazole-based thiadiazoles 18.
A similar one-pot simple synthetic strategy is presented in Scheme 2. Ketones having alkyl and aryl ring substituents (19) were treated with semicarbazide to afford appropriate semicarbazones (21), which underwent cyclization in excess of thionyl chloride via the Hurd-Mori process to achieve 1,2,3-thiadiazole hybrids (21) [78].
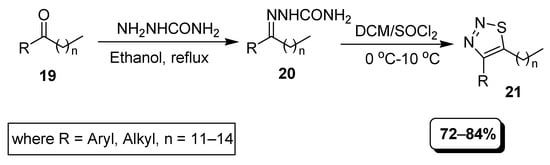
Scheme 2.
Synthesis of 1,2,3-thiadiazoles 21 from semicarbazone.
In another example, semicarbazide hydrochloride was reacted with 2-oxoallobetulin (22) to furnish semicarbazone (23) intermediate which cyclized into thiadiazole (24) using SOCl2 via Hurd-Mori protocol (Scheme 3). The regioselectivity of the functionalization was mostly centered at the C-3 position for the product [79].
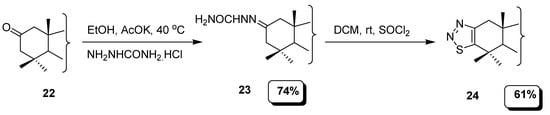
Scheme 3.
Synthesis of 1,2,3-thiadiazole derivatives from 2-oxoallobetulin.
Kumar et al. reported a simple and easy approach for the synthesis of thiadiazole derivatives, accomplished under mild reaction conditions. In their pathway, ionic liquids sulfonyl hydrazine (25) reacted with ketones or diketones (26) to provide ionic liquid hydrazone (27) that further treated with SOCl2 furnished substituted 1,2,3-thiadiazoles (28) in excellent (80–91%) yield (Scheme 4) [80].
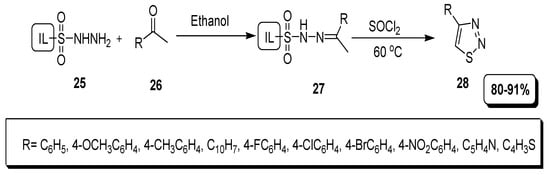
Scheme 4.
Synthesis of 1,2,3-thiadiazoles via ionic liquid support.
A facile, practical and improved Hurd–Mori approach to obtain 1,2,3-thiadiazoles in 44–98% yield range was presented by Chen et al. (Scheme 5). In this metal-free methodology, N-tosylhydrazones (29) and sulfur (30) were reacted in the presence of TBAI as a catalyst to achieve substituted aryl 1,2,3-thiadiazoles (31) [81].
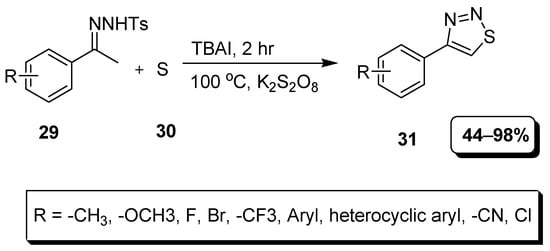
Scheme 5.
Reaction of N-tosylhydrazones (29) with elemental sulfur for the synthesis of thiadiazole (31).
2.2. Microwave-Assisted Synthesis of 1,2,3-Thiadiazoles
Sun et al. described a multi-step and microwave-assisted technique in which hydrazine and the diethyl carbonate (32) were combine to achieve hydrazide intermediate (33) which treated with ethyl 3-oxobutanoate (34) produced suitable Schiff base derivative (35). The substituted 1,2,3-thiadiazole-5-carboxylate scaffold (36) was provided by cyclization of SOCl2 with derivative (35) via a Hurd–Mori reaction. In the next step, the reaction of compound (36) with N2H4·H2O provided 1,2,3-thiadiazole acetanilide derivative (37) which was combined with substituted isothiocyanate to afford a substituted isothiocyanic ester (38). In the alkaline conditions, isothiocyanic ester (38) cyclized to 1,2,4-triazole with 1,2,3-thiadiazole substituent (39). The SH moiety on triazole was reacted with different alkyl or benzyl chlorides under microwave conditions to obtain the final products (40) in high yields within 15 min at 90 °C (Scheme 6) [82].
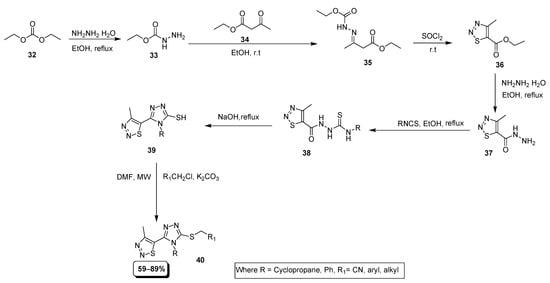
Scheme 6.
Synthesis of triazole-based 1,2,3-thiadiazole derivatives (49).
2.3. Multi Component Synthetic Strategies
Zheng et al. developed a simple, convenient and one-step multi-component synthetic approach of 1,2,3-thiadiazole via Ugi four-component reaction (U-4CR) (Scheme 7). The first step of this strategy was the generation of imine intermediate by mixing an amine (42) derivative with aldehyde (43). In the second step, imine intermediate was treated with thiadiazole (41) and isocyanide component (44) to obtain 5-methyl substituted thiadiazole derivatives (45) in low to excellent (6–98%) yield [83].
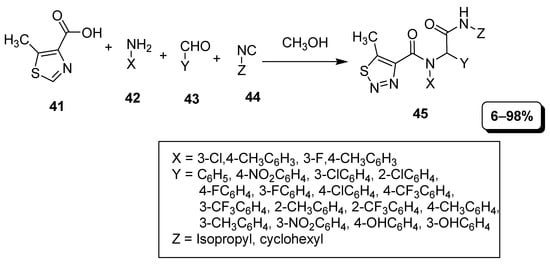
Scheme 7.
5-methyl substituted thiadiazole derivatives (45) synthesis via U-4CR.
Wang et al. utilized sodium borohydride for the reduction of the 5-carboxylate group of thiadiazole (46) to corresponding alcohol (47) which readily combined with pyridinium chlorochromate (PCC) to achieve 5-carbaldehyde substituted thiadiazole (48). The substituted amines, cyclohexyl isocyanide and azidotrimethylsilane were treated with (48) scaffold in methanol via Ugi-4CR to obtain tetrazole moiety containing 4-methyl substituted 1,2,3-thiadiazoles (49) in moderate yield (Scheme 8) [84].
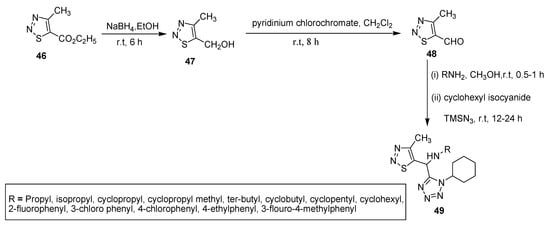
Scheme 8.
Preparation of tetrazole ring containing thiadiazole derivatives (49).
The reaction of methyl ketones (50) with p-toluenesulfonyl hydrazide (51) and KSCN in DMSO solvent afforded corresponding thiadiazoles (53) as reported by Wang et al. This reaction was facilitated by I2 and CuCl2, resultantly obtained 71–89% yield range in case of aryl-substituted thiadiazoles while 48–74% yield range was reserved for alkyl-substituted thiadiazoles (Scheme 9) [85].
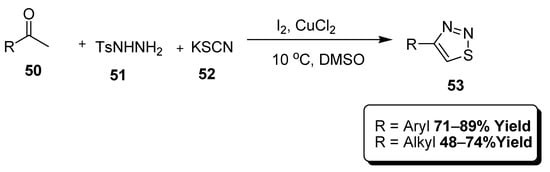
Scheme 9.
I2/CuCl2 mediated catalysis for the construction of thiadiazoles (53).
2.4. Ultrasonic Assisted Synthesis of 1,2,3-Thiadiazoles
An interesting example of the use of the ultrasonic-assisted technique is the synthesis described by Dong et al. (Scheme 10). In this multi-step synthetic route, the 5-carboxylic acid substituted thiadiazole (54) was reacted with thionyl chloride to obtain its corresponding 5-carbonyl chloride derivative (55), which in the next step was combined with glycine to afford carboxamide derivative (56). The last one (56) was reacted with substituted benzaldehyde and Ac2O under the ultrasonic-assisted irradiations conditions to furnish 4-benzylideneoxazole moiety containing 4-methyl-1,2,3-thiadiazole (57). In further steps, the oxazole derivative (57) was subjected to reaction with piperidine to obtain the intermediate (58) which further halogenated in chloroform to afford final substituted phenyl acrylamide 1,2,3-thiadiazole derivatives (59) in 73.9–99% yield [86].
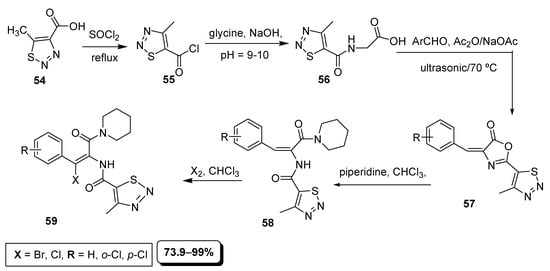
Scheme 10.
Synthesis of acrylamide derivatives of thiadiazoles (59).
2.5. Multi-Step Synthesis of 1,2,3-Thiadiazole from Quinolin-8-ol
Hayat et al. reported the synthetic route to achieve substituted 1,2,3-thiadiazole derivatives and investigated their pharmacological profile (Scheme 11). Ethyl substituted quinolineoxy acetate (61) was afforded by treating ethyl chloroacetate with quinolin-8-ol (60) in refluxing conditions which upon reaction with N2H4·H2O furnished 2-(quinolin-8-yloxy)aceto hydrazide (62). Next, the condensation reaction between substituted aromatic aldehydes and (62) to afford quinolinloxy acetohydrazone (63), which in the presence of SOCl2 cyclized into targeted thiadiazole derivatives (64) [87].
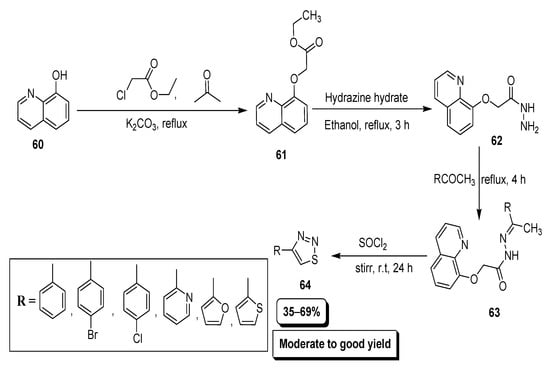
Scheme 11.
Acetohydrazone as starting precursor for the preparation of thiadiazoles (64).
2.6. Hydrolization and Esterification Approach
The simple synthetic route for carboxylate derivatives of thiadiazoles (67) is outlined in Scheme 12 and include two steps, i.e., first hydrolysis of thiadiazole (65) to 6-carboxylic acid derivative of thiadiazole (66), followed by esterification with various substituted alcohols afforded desired derivatives (67) [88].
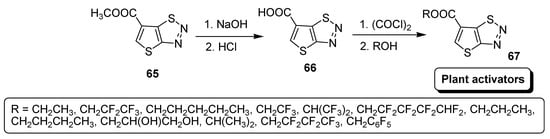
Scheme 12.
Synthesis of new carboxylate derivatives of thiadiazoles (67).
2.7. Transition Metal-Free Synthetic Approach
The 5-acyl-1,2,3-thiadiazoles scaffolds (71) were afforded by I2/DMSO-mediated cross-coupling reaction of three components, i.e., enaminones (68), elemental sulfur (69) and tosylhydrazine (70) (Scheme 13). Broad functional groups tolerance with targeted compounds up to 92% yield are the advantages of this methodology [89].
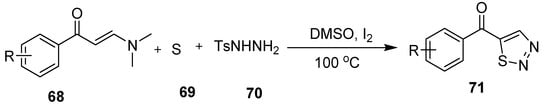
Scheme 13.
Synthesis of substituted 1,2,3-thiadiazole (71) via cross-coupling mediated by I2/DMSO.
2.8. Nucleophilic Addition
A new simple operational and inexpensive strategy was developed to construct thiadiazole motifs (75) by the reaction of α-diazo carbonyl compounds (72) with BnBr (74) and CS2 (73) as depicted in Scheme 14 [90].
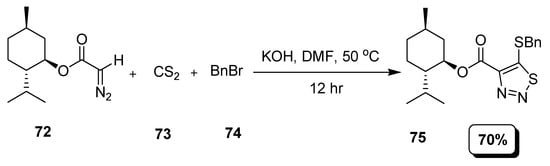
Scheme 14.
Synthesis of 1,2,3-thiadiazoles (75) via nucleophilic addition.
2.9. [4 + 1]. Annulation of Azoalkenes
A new sustainable, regioselective, environmentally friendly and broad functional-group compatibility synthetic strategy for thiadiazoles was described by Zhang et al. In their protocol, photocatalysis reaction of azoalkenes (76) with KSCN in the presence of cercosporin and tBuOK afforded desired thiadiazoles (77). Electron donating groups showed maximum yield, week EWD groups displayed good yield. Strong EWD groups afforded moderate yield, heterocyclic moiety such as furan showed the least yield while pyridine did not furnish yield under the conditions as mentioned in Scheme 15 [91].

Scheme 15.
Photocatalysis reaction of azoalkenes (76) with KSCN to afford 1,2,3-thiadiazoles (77).
2.10. Intramolecular Oxidative Nucleophilic Substitution of Hydrogen (ONSH)
In this basic media strategy in Scheme 16, thiadiazoles (78) with I2 as an oxidant in the presence of 1.1 equivalent of tBuOK provided 1,2,3-thiadiazoles (79) [92].
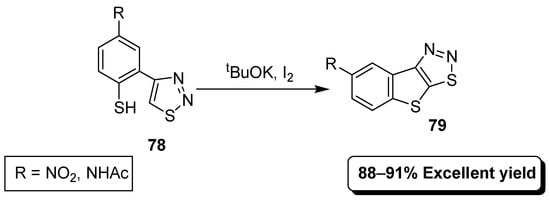
Scheme 16.
Preparation of fused 1,2,3-thiadiazoles via ONSH mechanism.
2.11. Intermolecular [3 + 2] Heterocyclization
Fan et al. presented a metal-free, eco-friendly, flexible structural modifications and mild reaction conditions methodology in which combination of I2 and O2 promoted intermolecular [3 + 2] heterocyclization between aryl hydrazines (80) and triethylammonium thiolates (81) furnished 1,2,3-thiadiazle derivatives (82) up to 92% yield (Scheme 17) [93].
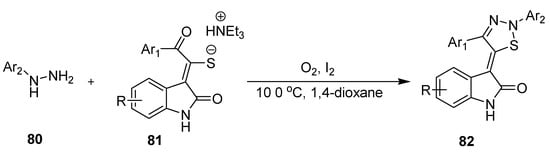
Scheme 17.
I2/O2 mediated synthesis of 1,2,3-thiadiazoles (82).
2.12. Cornforth Rearrangement Approach
In this domino type reaction, N-heteroarylamidines based thiadiazole analogues (85) were furnished in moderate to good (58–76%) yield by treating acetamides (83) with imidazole (84) in EtOH/EtONa mixture (Scheme 18). This domino reaction mechanism is analogous to Cornforth rearrangement [94].
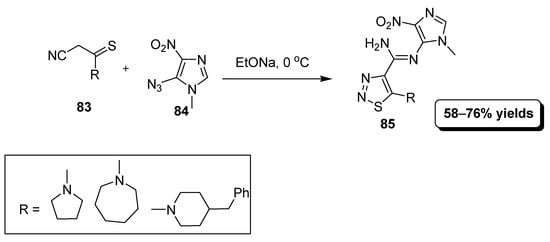
Scheme 18.
N-Heteroarylamidines based thiadiazole analogues (85).
2.13. Cascade Process
Liu et al. reported a simple and facile synthetic route for thiadiazoles involving reaction of chlorinated ketones (86) with tosylhydrazine (70) to obtain intermediate (87) which further cyclized with sulfur reagent in the presence of basic medium to yield (38–68%) disubstituted 1,2,3-thiadiazoles (88). Bromophenyl displayed the maximum 68% yield in substrate scope, methoxycarbonyl phenyl afforded least 38% yield, while dihyroxyphenyl and nitrophenyl failed to give desired product in 0.0% yield (Scheme 19) [95].
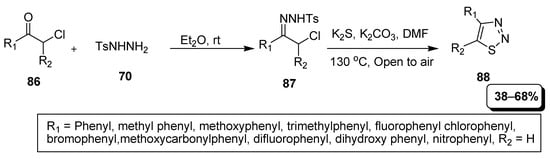
Scheme 19.
Synthesis of 1,2,3-thiadiazoles from in situ generated azoalkenes with S3•− via a cascade process.
3. Biological Activities of 1,2,3-Thiadiazole Derivatives
The main objective of the present section is the search and presentation of the 1,2,3-thiadiazole based most potent therapeutic agents for a variety of biological and pharmacological applications with little or no side effects. In this section, 1,2,3-thiadiazole derivatives have been divided into classes according to their biological activity.
3.1. Antiviral Agents
Pawar et al. described antiviral drugs as such bioactive compounds which are used for the treatment of viral infections [96]. Rossignol et al. reported that most of the antiviral agents are specifically targeted oriented while some other antivirals have a broad spectrum against a vast variety of viral infections [97]. Some antiviral drugs such as ribavirin (89; Figure 3) are being used against both RNA and DNA viruses [98,99,100], efavirenz (90; Figure 3) used for the treatment of HIV/AIDS [101,102,103] and arbidol (91; Figure 3) was used against influenza viruses [104,105].
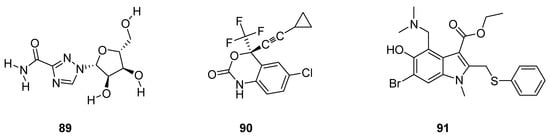
Figure 3.
Structures of antiviral drugs 89–91.
Zhan et al. prepared 1,2,3-thiadiazole thioacetanilide by adopting a multi-step methodology and exhibited their anti-HIV (Human Immunodeficiency Virus) activity against MT-4 cells. Among all synthesized derivatives, the thioacetanilide based 1,2,3-thiadiazole scaffold (92; Figure 4) showed remarkably the best anti-HIV activity in terms of EC50 value 0.059 ± 0.02 µM, CC50 > 283.25 µM, SI > 4883 µM compared to the reference compounds such as NVP (nevirapine) DLV (delaviridine), EFV (Efavirenz), AZT (azidothymidine) and VRX-480773. SAR displayed that the significant enhancement in the antiviral efficacy of compound 92 was due to the introduction of the nitro group on the phenyl ring. Anilide scaffold having NO2 and halogen-substituted aryl ring at o-position took great part to enhance the anti-HIV potential, however, the case was the difference for fluorine atom which decreased activity at a certain level [106].
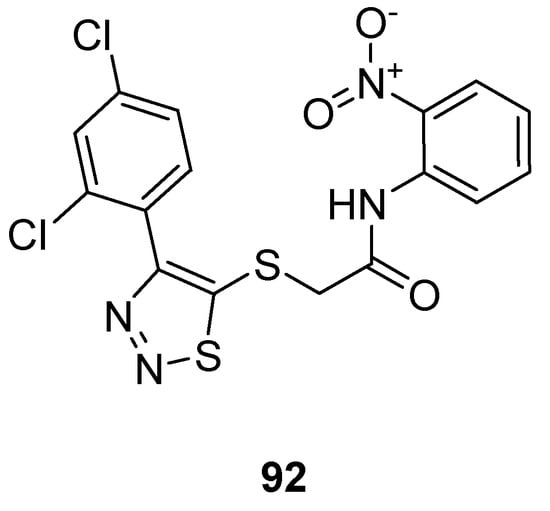
Figure 4.
Thioacetanilide based 1,2,3-thiadiazole scaffold 92 exhibiting maximum anti-HIV potential.
Zhan et al. prepared potent 1,2,3-thiadiazole derivatives against HIV-1. The new 1,2,3-thiadiazole (93; Figure 5) proved as the most active anti HIV-1 agent (EC50 value 0.0364 ± 0.0038 µM, CC50 > 240.08 µM and SI > 6460 µM) against MT-4 cells among the synthesized derivatives in comparison with the reference compounds NVP (EC50 0.208 µM) and DLV (EC50 0.320 µM) EFV (EC50 0.00440 µM) and AZT (EC50 0.0151 µM). Phenyl ring substituted with 2,4-Br2 group significantly increased the antiviral potential of compound 93 than all other substituents on the phenyl ring. SAR studies indicated decreasing order of antiviral strength, i.e., 2,4-Br2 > 2,4-Cl2 > 2,4-F2 [107].
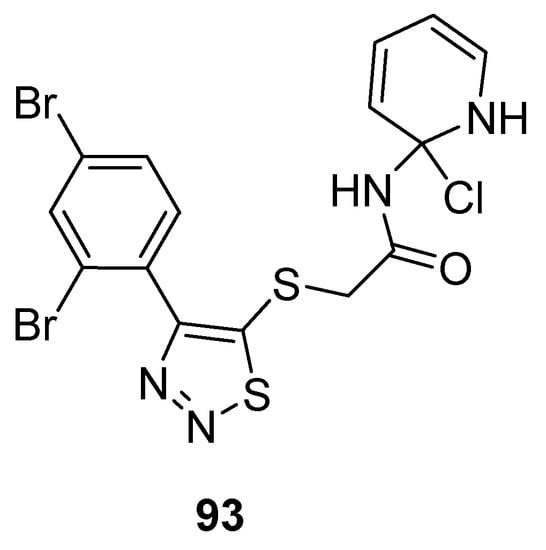
Figure 5.
Anti-HIV activity of 5-(2,4-dibromophenyl)-1,2,3-thiadiazole 93.
Dong et al. prepared potent antiviral piperidine-based thiadiazole derivatives, and the SAR study showed that chlorine atom substituted compounds exhibited good antiviral activity and the substitution on phenyl ring affects inhibitory action. 1,2,3-thiadiazole (94; Figure 6) displayed excellent potency with IC50 3.59 μg/mL as compared to lamivudine (IC50 value 14.8 μg/mL). It was observed that compounds having p-substituents exhibited more cytotoxic potency than the o-substituted derivatives. Moreover, lowering of the selective index with higher anti-HBV potencies of the synthesized compounds was observed when comparing the results with lamivudine. Compound 94 proved to be the most active one as compared to the other derivatives [86].
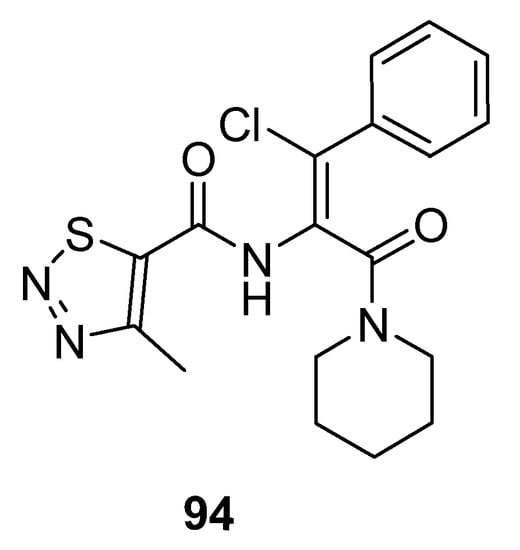
Figure 6.
Antiviral activity of 1,2,3-thiadiazole 94.
Synthesis and screening of antiviral potency of tetrazole-based 1,2,3-thiadiazoles structural against tobacco mosaic virus (TMV, anti-TMV) were evaluated [84]. The studies indicated that some obtained compounds (95–98; Figure 7) have higher anti-TMV activity (33.75–48.73%) than ribavirin at 100mg/mL concentration (33.23%) and comparable potency to ribavirin at 100 μg/mL. Structure 97 displayed the best protection effect among the synthesized derivatives as well as from the ribavirin—reference drug. Anti-TMV activity of synthesized scaffolds and reference drugs decreases in order of 98 > ninamycin >97 > 95 > 96 > ribavirin while the protection effect decreased in order of ninamycin > 97 > 96 > 95> ribavirin > 98. Compound 98 display a higher inhibition potential, i.e., 48.73% as compared to ninamycin and ribavirin. The SAR study showed that the 2-fluorophenyl substituent derivative 97 displayed the best protection effect among the synthesized congeners as well as from the ribavirin reference drug. The protection effect decreased in order of ninamycin > 2-fluorophenyl > cyclopropyl > isopropyl > ribavirin > 4-ethylphenyl. Overall the scaffold 97 proved to be a promising therapeutic agent, which has a better protection effect as well as the best anti-TMV activity [84].
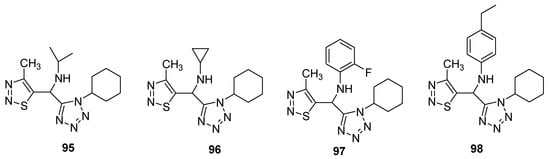
Figure 7.
Antiviral activity of terazole derivatives 95–98.
Mao et al. reported the synthetic protocol to afford substituted methyl carbohydrazide-based 1,2,3-thiadiazoles and determined the anti-TMV activity. The thiophene containing carbohydrazide 1,2,3-thiadiazole (99; Figure 8) showed potent direct anti-TMV activity with 58.72% and 61.03% induction potencies at 50 µg/mL when compared with reference drugs, ninamycin and tiadinil. Both the standard reference drugs, ninamycin and tiadinil, exhibited anti-TMV activity (54.93% and 7.94%) and induction activity (18.58% and 59.25%) respectively in vivo at 50 µg/mL concentration. The scaffold 99 showed remarkably and significant higher antiviral potential than the standard reference drugs. The excellent anti-TMV activity of analogue 99 was due to the presence of thiophene moiety as compare to all the other tested compounds. The structural motif 99 showed significantly higher induction activity (61.03%) more than the induction activities of reference drugs, ninamycin and tiadinil (18.58%) and (59.25%), respectively. So the derivative 99 proved to be a plant elicitor and anti-TMV agent simultaneously [108].
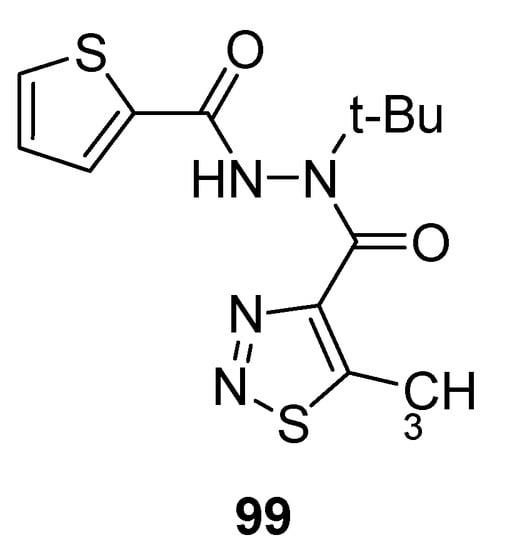
Figure 8.
Antiviral 1,2,3-thiadiazole scaffold 99.
Another example of compounds with anti-TMV activity are structures 100 and 101 with a 1,3,4-oxadiazole ring (Figure 9) [109]. They showed potent curative, inactivation and protection effects against TMV. The derivative 100 displayed curative rate 54.1%, inactivation rate 90.3% and protection rate 52.8% while the compound 101 showed 47.1, 85.5 and 46.4% curative, inactivation and protection rates, respectively. The ningnanmycin was used as a standard reference drug, exhibiting a 56.1% curative rate, 92.5% inactivation rate and 59.3% protection rate. The incorporation of 1,3,4-oxadiazole moiety in the skeleton of 1,2,3-thiadiazoles significantly increased the anti-TMV activity [109].
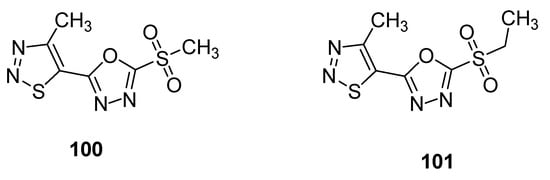
Figure 9.
1,2,3-thiadiazoles 100 and 101 with anti-TMV activity.
Other promising anti-TMV agents 102 and 103 were presented by Zheng et al. (Figure 10) [83]. The substituted 1,2,3-thiadiazole-4-carboxamide scaffold 102 exhibited the best antiviral curative activity of 60 and 47%, at 500 and 100 µg/mL concentrations, while the standard tiadinil showed curative activity 58 and 46% at 500 and 100 µg/mL concentrations, respectively. Compound 103 showed its potent protective effect 76 and 71% at 500 and 100 µg/mL concentrations in comparison with the standard drug tiadinil, which displayed protective 75 and 57% at 500 and 100 µg/mL. The structure 103 marked a higher protective effect than the tiadinil and 102 at 100 µg/mL concentration. The enhanced protective potential of 103 was dose-dependent and due to the presence of hyroxyphenyl moiety. The fluorophenyl, chlorophenyl and hyroxyphenyl functionalities contributed to better efficacy that led to a future investigation to develop promising anti-TMV agents [83].
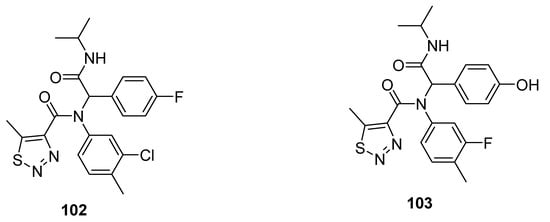
Figure 10.
1,2,3-Thiadiazole scaffolds 102 and 103 with anti-TMV activity.
1,2,3-Thiadiazole acetanilide hybrid structures prepared by Zhan et al. were screened for their antiviral activity against HIV-1 [110]. In the group of achieved derivatives structure (104; Figure 11) exhibited moderate antiviral activity (EC50 value 0.95 ± 0.33 µM) against HIV in comparison with the reference standard drugs such as: NVP (EC50 value 0.208 µM), DLV (EC50 value 0.32 µM), EFV (EC50 value 0.00440 µM) and zidovudine (EC50 value 0.0151 µM). The scaffold 104 displayed remarkable high and excellent anti-HIV-1 activity than all standards. The reason for the highest anti-HIV-I activity of structural motif 104 among the synthesized congeners was due to the 4-acetyl substituted anilide phenyl ring of 1,2,3-thiadiazole [110].
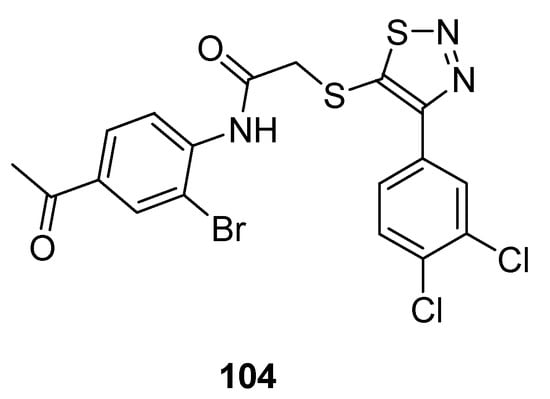
Figure 11.
Antiviral activity of 4-(3,4-dichlorophenyl)-1,2,3-thiadiazole scaffold 104.
3.2. Anticancer Agents
Cancer has drawn the attention of today’s medical science all over the world because it is the lethal, notably complex, most prominent and serious threat to human health. It forms the lump of uncontrolled growth cells called tumors or neoplasm tumor cells. The neoplasm malignant are diversified heterogeneous cells which have properties of rapid proliferation and capability to invade or spread to other parts of the body through the bloodstream and lymphatic system. Most researchers have devoted their extensive research to developing effective anticancer agents, along with the employment and application of integrated surgical procedures, radiation therapy and chemotherapy [111,112]. Great advances and many discoveries in the development of a large quantity of anticancer therapeutics such as carboplatin (105) cisplatin (106) [113,114,115], anastrozole (107) [116,117] and doxorubicin (108) (Figure 12), etc., have been made over the past 60 years [118,119,120,121].
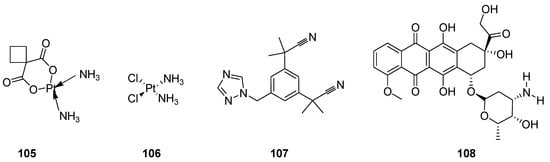
Figure 12.
Clinical anticancer drugs 105–108.
Noteworthy are the 4,5-diaryl-1,2,3-thiadiazole derivatives obtained by Wu et al. due to their method of synthesis and anticancer properties [122], and results pointed out that compounds 109 and 110 are the lead compounds by exhibiting favorable activity (Figure 13). Growth inhibition rate of tumor cells (obtained from mice S180) by derivative 109 at a dose of 40 mg/kg administrated for 5 days reached 81.0%, while compound 110 displayed a tumor suppression efficacy of 64.2% (at 100 mg/kg of dose administered to mice for five days). These effects were compared with the standard reference CA-4 (combretastatin A-4), which showed a 64.2% antitumor effect at a dose of 40 mg/kg used for 4 days in a mouse model. The SAR study indicated that the reason of the highest antitumor activity of structural motif 109 than reference drug CA-4 and derivative 110 was because of OMe group at C-4 and H at C-3 of 5-phenyl ring of 1,2,3-thiadiazole, while the replacement of hydrogen at C-3 with the nitro group dropped the antitumor activity of derivative 110. The scaffold 109 could be a promising candidate due to its lower cytotoxicity and excellent antitumor effect [122].
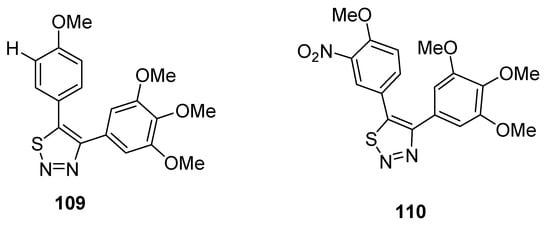
Figure 13.
Antitumor effect of 1,2,3-thiadiales 109 and 110.
New 1,2,3-thiadiazole derivatives (111 and 112; Figure 14) obtained by Hosny et al. via Hurd–Mori cyclization were tested against MCF-7 tumor cells. The IC50 values of compounds 111 and 112 (IC50 = 12.8 µg/mL and 8.1 µg/mL, respectively) were comparable to doxorubicin as the standard anticancer drug with IC50 = 3.13 µg/mL. The derivative 111 showed the highest anti-breast cancer activity than the scaffold 112 and reference drug doxorubicin [123].
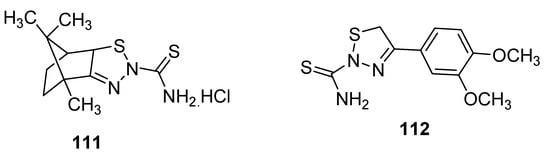
Figure 14.
Anticancer activity of 2-thioamide-1,2,3-thiadiazole 111 and 112.
Cikotiene et al. reported the synthetic protocol to achieve the substituted 4,5-diaryl-1,2,3-thiadiazoles as Hsp90 chaperone protein inhibitors. This protein plays a significant part in developing tumor cells, so its inhibition is the main target of many anticancer drugs. The Hsp90 chaperone stabilizes a number of proteins that are essential for the growth of tumors, due to which researchers all over the world are interested in the development of Hsp90 inhibitors which would be investigated as anticancer agents. In the group of tested derivatives, structure 113 deserves special attention as an inhibitor of cancer cells with GI50 value of 0.69 µM for U2OS (osteosarcoma) cells and 0.70 µM for HeLa (cervical carcinoma) cells (Figure 15) [124]. Isothermal titration calorimetry was used to determine the binding affinity of compound (113) to Hsp90F and Hsp90N. The derivative (39) acted as a binder to both Hsp90N andHsp90F with the observed Kd of about 42 nM and 37 nM, while for the reference compound17-AAG [17-(allylamino)-17-demethoxygeldanamycin] Kd was 200 nM and 240 nM, respectively [124].
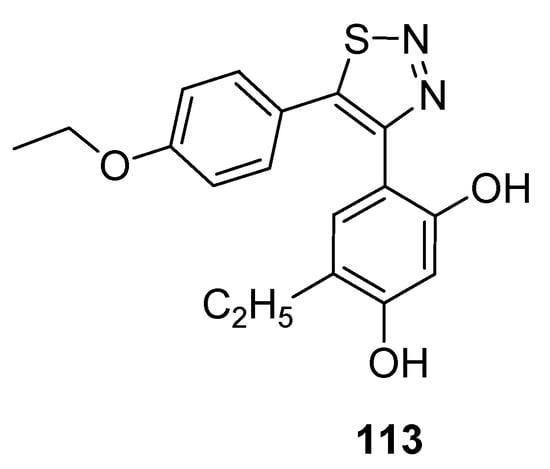
Figure 15.
Anticancer activity of 1,2,3-thiadiazole derivative 113 against U2OS and HeLa cell lines.
Synthesized by Cui et al., dehydroepiandrosterone derivatives of thiadiazoles were investigated against T47D and HAF cell lines for their antitumor activity applying the sulforhodamine B (SRB) assay. In this group of compounds, derivative 114 (Figure 16) focuses particular attention, due to its strong antitumor and antimetastatic activities, in vitro and in vivo. Its IC50 value for T47D cells was 0.058 ± 0.016 µM and for HAF cells IC50 = 21.1 ± 5.06 µM. Moreover, the selectivity of compound 114 (SI = 364) was 214 folds better than adriamycin (positive control) (ADM; SI = 1.7). The SAR study showed that the introduction of 1,2,3-thiadiazole and D-proline chemical entities in derivative 114 significantly enhanced antiproliferative activity than the parent DHEA. The SI of scaffold 114 was 214 folds better than SI of ADM standard drugs. The derivative 114 could be used as the promising and novel class of anticancer agent due to its low cytotoxicity, high selectivity index and excellent antitumor and antiproliferative activities (Figure 16) [125].
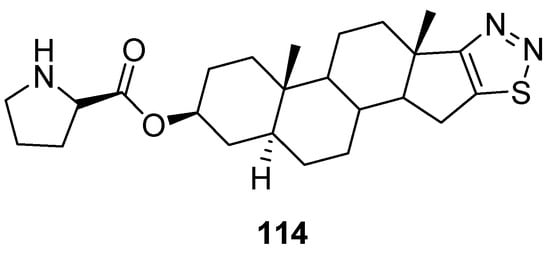
Figure 16.
1,2,3-Thiadiazole dehydroepiandrosterone derivative 114 as new antitumor agents.
3.3. Insecticidal Agents
Insecticides are the designed substances of either chemical or biological origin that used to regulate the insect behaviour, control insects, cause death, dysfunction and moving away [126,127]. Some of the insecticidal agents, such as tebufenozide (115) act as molting hormones primarily against caterpillar pests [128]; imidacloprid (116) is the most well-known broad spectrum insecticide, which used as an insect neurotoxin [129,130,131], and neuroactive pymetrozine (117) is a novel class of pyridineazomethine insecticide used for the control of aphids and whiteflies in crop fields (Figure 17) [132].
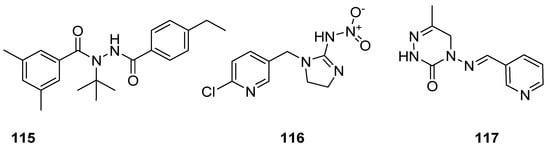
Figure 17.
Known insecticidal agents 115–117.
An interesting example of 1,2,3-thiadiazole derivatives with insecticidal properties are two groups of N-tert-butyl-N,N′-diacylhydrazines obtained by Wang et al. [29] and tested their activity against Plutella xylostella L. and Culex pipiens pallens. Derivative 118 (Figure 18) displayed remarkable and significantly the highest insecticidal potential (79% mortality at 200 μg/mL) against Plutella xylostella L., while 119 exhibited 68% mortality against the same insects at concentration 200 μg/mL. Insecticidal activity of reference agent, i.e., tebufenozide, was 40%. The scaffold 118 displayed excellent and the highest insecticidal potency, 79% more than the insecticidal potencies of reference drug tebufenozide (40%) and derivative 119 (68%). The EWD group chloro increased the insecticidal activity of scaffold 118, while ED group methyl decreased the insecticidal potency of scaffold 119 [29].
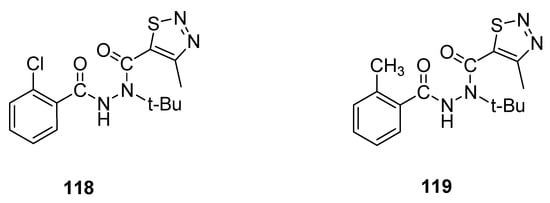
Figure 18.
Insecticidal activity of N-tert-butyl-N,N′-diacylhydrazines 118 and 119.
Zhang et al. designed the (E)-β-farnesene based carboxamides of thiadiazoles and checked their aphicidal behavior against Myzus persicae [133]. The three compound 120–122 (Figure 19) exhibited LC50 values of 33.4 μg/mL, 50.2 μg/mL and 61.8 μg/mL, respectively. The 1,2,3-thiadiazole carboxamide analogues showed significantly higher aphicidal activity than (E)-ß-farnesene, but lesser insecticidal activity than pymetrozine insecticide (LC50 = 7.1 μg/mL). It was observed that fluoro or difluoro groups on phenyl moiety enhanced the aphicidal potential significantly in novel chemical entities of Eβf 1,2,3-thiadiazole 120 and 121 while the methyl substitution led to lower aphicidal activity of scaffold 122 [133].
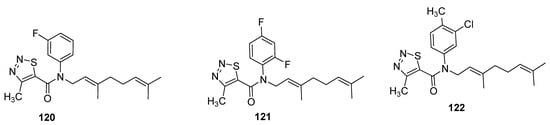
Figure 19.
Insecticide Eβf 1,2,3-thiadiazole carboxamides 120–112.
Pyrazole oxime derivatives, as a new Fenpyroximate analogue bearing a thiadiazole moiety, were synthesized by Dai et al. and then their insecticidal, acaricidal and cytotoxic activities were examined. The best insecticidal properties possessed oximes 123 and 124 (Figure 20) against Aphis craccivora at 100 μg/mL with 90% of the mortality rate. The structure–activity relationship study revealed that due to the insertion of F- and Me-groups at p-position of phenoxy moiety attached to the pyrazole oxime 123 and 124 displayed good insecticidal activities but slightly lower than commercial drug imidacloprid [134].
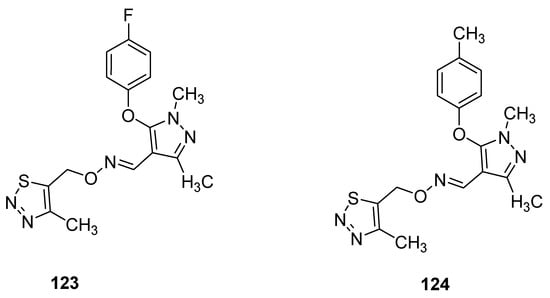
Figure 20.
Ethers of pyrazole oximes 123 and 124 with insecticidal properties.
Another interesting example of insecticidal compounds are hybrids of substituted triazole and 1,2,3-thiadizole scaffold that were investigated for their activity against Aphis laburni and TMV by Li et al. [135]. Preliminary bioassays indicated compounds 125 and 126 (Figure 21) as lead compounds by depicting enhanced activity against Aphis laburni at 100mg/mL (mortality ≥ 95%) while the derivative 127 represent the least active compound (due to CF3 substituted triazole ring) that displayed only 25.25% mortality. The structure–activity study indicated that scaffolds with aromatic moieties attached to the carbonyl carbon of triazole ring have higher insecticidal activity than scaffolds with alkyl moieties [135].
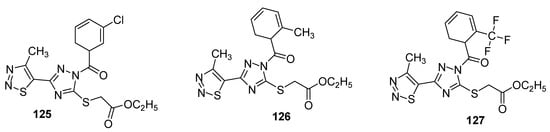
Figure 21.
Substituted 4-methyl-1,2,3-thiadiazole derivatives with insecticidal properties against Aphis laburni.
3.4. Amoebicidal Agents
A parasitic disease caused by a protozoa Entamoeba histolytica is known as amoebiasis. E. histolytica has infected 40 to 50 million people worldwide and led to the development of amoebic colitis or extraintestinal abscess that has resulted in 100,000 deaths annually [136,137,138]. The tissue amoebiasis is treated by different drugs such as metronidazole, chloroquine, tinidazole, dehydroemetine and nitazoxanide, while the diloxanide furoate and iodoquinoline drugs are used to treat luminal infections (Figure 22) [139,140,141,142,143].
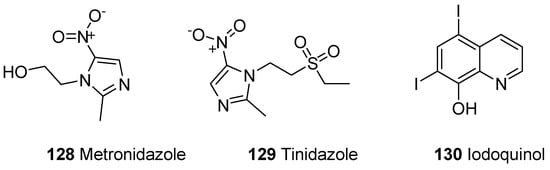
Figure 22.
Amoebicidal commercial dugs 128–130.
Hayat et al. reported the synthetic route to achieve substituted 1,2,3-thiadiazole scaffolds from the cyclization of quinoline-based hydrazone. All substituted 1,2,3-thiadiazole hybrids and quinoline-based hydrazones were evaluated for antiamoebic activity against HM1 and IMSS strains of E. histolytica. The scaffold 4-bromo phenyl-1,2,3-thiadiazole (131, Figure 23) and furan based 1,2,3-thiadiazole (132, Figure 23) exhibited potent antiamoebic activity (IC50 = 0.24 µM and IC50 = 0.23 µM, respectively) when compared with metronidazole (IC50 = 1.80 µM). The parent intermediate quinoline-based hydrazone displayed better antiamoebic potential than the targeted derivatives of 1,2,3-thiadizoles. The SAR study revealed that the significant loss of antiamoebic potential in scaffolds 131 and 132 was due to the absence of quinoline acetohydrazone moiety in final thiadiazoles [87].
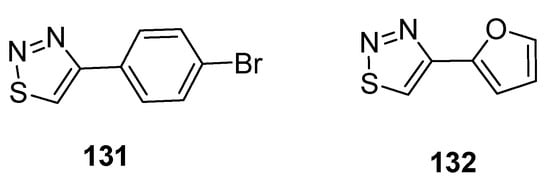
Figure 23.
The most active antiamoebic 1,2,3-thiadiazoles 131 and 132.
3.5. Plant Activator Agents
Plant activators are synthetic or natural chemicals entities that protect plants from different pathogens. Among them, pyrimidin-type plant activator 2 (PPA2 133; Figure 24) significantly enhanced plant defense system against bacterial infections unlikely traditional commercially available plant activators (INA 134, BABA 135, BHT 7; Figure 24). Plant activators not only showed their therapeutic potential against wide variety of pathogens but also increased growth of plants, rate of photosynthesis and overall yield of crops [144,145,146,147,148].
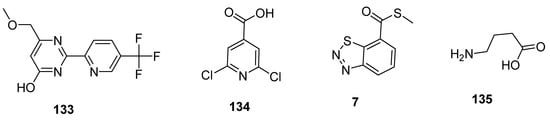
Figure 24.
Structures of commercially available plant activators 133–135.
Du et al. reported a synthetic pathway for the preparation of carboxylate derivatives of thiadiazoles as potential plant activators against seven plant diseases [90]. The 1,2,3-thiadiazole derivatives 136 and 137 (Figure 25) proved to be good plant activators and displayed excellent inhibition activities against diseases such as M. melonis, (90% for 136 and 69% for 137), C. cassiicola (77% for 136 and 52% for 137), P. syringae pv. Lachrymans (42% for 136 and 42% for 137) and P. infestans (81% for 136 and 67% for 137) when results were compared with commercial BTH plant activator. The structural motifs 136 and 137 displayed significantly better therapeutic efficacy than BHT commercial drug, but the scaffold 136 had better plant activating potency than scaffold 137 [88].
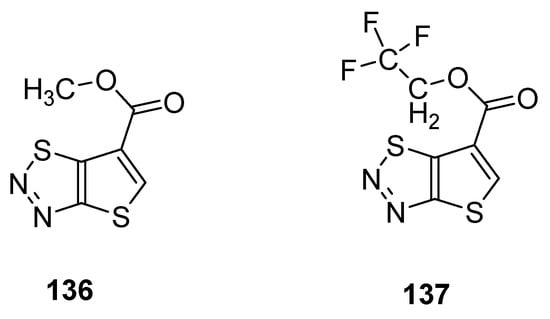
Figure 25.
Potential new plant activator with thieno [2,3-d]-1,2,3-thiadiazoles 136 and 137.
3.6. Fungicidal Agents
The chemical agents that have the potential to control and eradicate fungal infections are termed as fungicides or antifungal drugs [149]. The commercial antifungal agents are chlorothalonil (65) which is a non-systemic fungicide [150,151], fluconazole (66) [152] and ketoconazole (67) (Figure 26) [153].
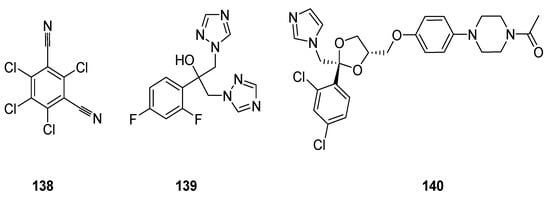
Figure 26.
Structures of commercial antifungal drugs 138–140.
As the first example, it is worth bring up oxadiazoles containing thiadiazole derivatives [154]. In this group, two oxadiazole derivatives 141 and 142 (Figure 27) displayed the best antifungal activity against Puccinia triticina at a concentration of 500 μg/mL. Both structures showed a remarkable high growth inhibition activity (98 and 83%, respectively) which was slighter lower than the standard drug chlorothalonil with 100% fungal growth inhibition activity. The remarkable high and excellent antifungal activity of scaffold 141 could lead to the development of novel oxadiazole-based 1,2,3-thiadiazole fungicides [154].
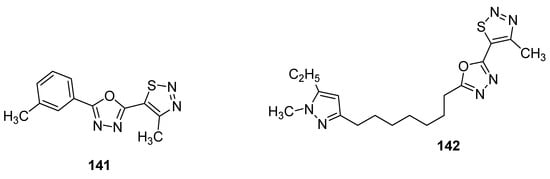
Figure 27.
Novel potent 1,2,3-thiadiazole fungicides 141 and 142.
Another example of compounds with good antifungal activities are new structures designed by Sun et al., which are 1,2,4-triazole derivatives substituted with a 1,2,3-thiadiazole ring [82]. One of the synthesized compounds 143 (Figure 28), exhibited remarkably high fungicidal 93.19% against Corynespora cassiicola, while reference compounds iprodione, validamycin and topsin-M possessed 78.20, 53.52 and 78.75% antifungal activity, respectively. The authors declared that compound containing phenyl group on triazole ring 143 exhibited higher activity, however, the results were different in the case of Pseudoperonospora cubensis as compound 144 containing cyclopropyl group displayed higher activity than that of 143. The most active compound with regard to Pseudoperonospora cubensis was 144, which inhibited the growth of this pathogen in 81.62%. However, SAR analysis did not clear the results against pseudomonas syringae pv. Lachrymans. Both the scaffolds showed excellent and the highest antifungal potential than all three commercial reference drugs that will lead to the development of promising antifungal agents [82].
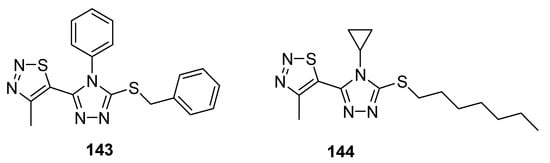
Figure 28.
Antifungal activity of triazole moiety containing 1,2,3-thiadiazolew 143 and 144.
Less antifungal activity was observed in the derivative 145 (Figure 29) reported by Tan et al. [155]. Its inhibition rates to Phytophthora infestans (Mont) de Bary, Alternaria solani, Gibberella sanbinetti, Physalospora piricola Nose, Botrytis cinerea, Phytophthora capsici Leonian, Cercospora arachidicola, Rhizoctonia solanii and Fusarium oxysporum reached 32.3, 17.4, 32.3, 25.8, 43.5%, 36.1, 15.8, 30.3 and 26.9% at 50 μg/mL respectively. The results indicated that the structural motif 145 proved to be broad-spectrum fungicide because it exhibited significant antifungal activity against different fungal strains [155].
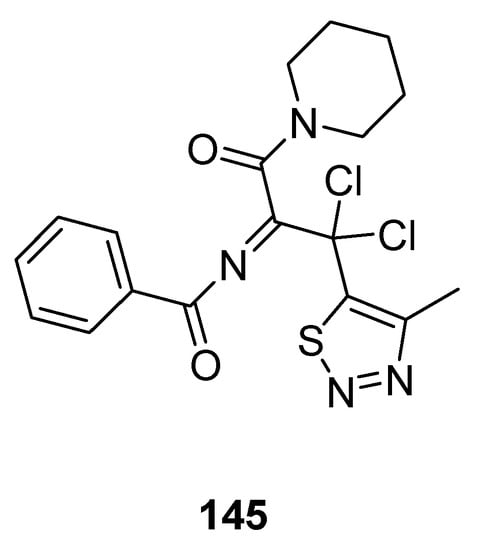
Figure 29.
Piperidine based 1,2,3-Thiadiazole 145 with moderate antifungal activity.
Another group reported the fungicidal properties of 1,2,3-thiadiazole derivatives against Alternaria solani (AS), Botrytis cinerea (BC), Cercospora arachidicola (CA), Cercospora beticola (CB), Colletotrichum lagenarium (CL), Fusarium oxysporum (FO), Gibberella zeae (GZ), Macrophoma kuwatsukai (MK), Phytophthora infestans(Mont) de Bary (PI), Physalospora piricola (PP), Pellicularia sasakii (Shirai) (PS), Puccinia triticina Eriks (PT) and Rhizoctonia solani Kuhn (RS) [156]. The most active occurred to be structure 146 (Figure 30) showed potent fungicidal inhibitory activity 84.8, 83.9, 83.3, 78.9, 75.0, 71.4, 70.7, 66.7, 64.7 and 63.6% against PS, PP, RS, PT, FO, CB, PI, AS, CL and CA, respectively. On the other hand, the propyl containing fused 1,2,4-triazolo[1,3,4]thiadiazole 147 (Figure 30) exhibited the best fungicidal inhibition activity 93.0% for FO, 84.9% for BC, 77.8% for PS, 75.8% for PI, 75.0% for GZ, 62.1% for CA, 50.0% for CL, 40.5% for PP, 34.4% for AS and 33.3% for CB. The results of the antifungal study showed that fused 1,2,4-triazolo[1,3,4]thiadiazole led to the development of wide-spectrum antifungal agents [156].
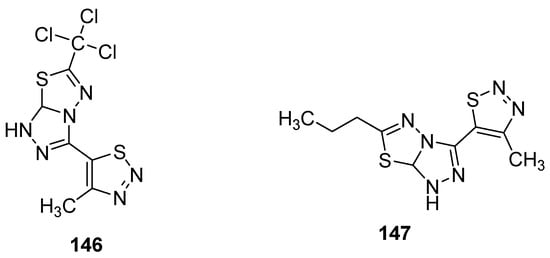
Figure 30.
1,2,3-thiadiazoles with broad antifungal activity.
Wang et al. reported a synthetic approach to new series of organotin 4-methyl-1,2,3-thiadiazole-5-carboxylates and benzo[1,2,3]thiadiazole-7-carboxylates [157] and antifungal properties against P. piricola and Gibberella zeae. The triethyltin-based 1,2,3-thiadiazole carboxylate analogue 148 (Figure 31) displayed antifungal efficacy EC50 = 0.12 μg/mL and 0.16 μg/mL against P. piricola, and Gibberella zeae, respectively. The overall results of the present study indicated the antifungal effectiveness of organotin-based benzo-1,2,3-thiadiazole or 1,2,3-thiadiazoles analogues proved to be broad-spectrum fungicides [157].
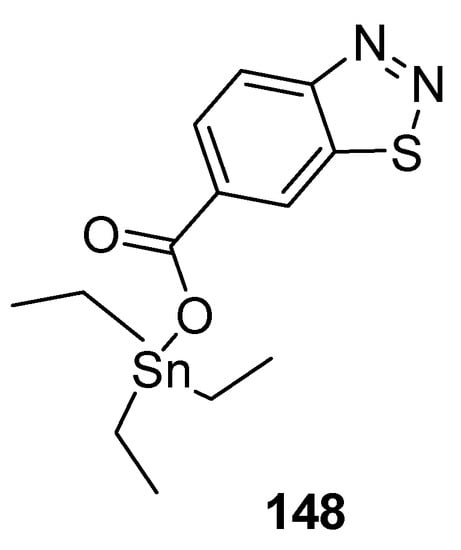
Figure 31.
Antifungal benzo[1,2,3]thiadiazole-7-carboxylate derivative 148.
Antifungal properties have also been determined for carboxamide derivatives of thiadiazoles obtained by Zuo et al. utilized one-pot four-component Ugi reaction strategy under green synthetic conditions [158]. All the synthesized analogues were investigated against eleven fungal strains to develop novel fungicidal candidates. The 1,2,3-thiadiazole containing carboxamide moiety 149 (Figure 32) displayed broad-spectrum fungicidal inhibition activities against CA 71%, AS 100%, GZ 62%, PP 85%, PG 14%, PS, 74%, CL 95%, PI 88% and RS 97%. Similarly good antifungal activity was observed for scaffold 150 that exhibited fungicidal inhibition against various fungal strains such as: CB (Cercospora beticola) 67%, CA 79%, AS 44%, GZ 34%, PP 53%, PG 57%, PS 16%, CL 43%, RS 51% and PI 57%. The scaffold 149 displayed maximum fungicidal potential 100, 97 and 95% against fungal strains AS, RS and CL, respectively, while the zero percent antifungal activity was shown against CB and FO fungal strains, just as scaffold 150 displayed the best antifungal potential 67% against CB fungal strains and 0% against FO [158].
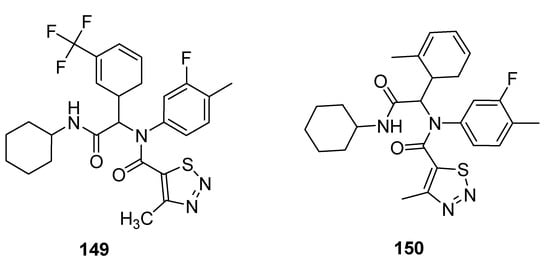
Figure 32.
The most active 4-methyl-1,2,3-thiadiazole-5-carboxamides.
4. Conclusions
The review has uncovered 1,2,3-thiadiazole is a unique heterocyclic structural motif and privileged template in the field of medicinal chemistry, specifically its antiviral profile, to attract the researchers/scientists to plan and create novel, target-based and advanced 1,2,3-thiadiazole derivatives. A broad pharmaceutical profile and biological properties such as fungicidal, antiviral, insecticidal, amoebicidal, anticancer and plant activators, etc. are shown along with a structure–activity relationship that will prompt potential pharmaceutical agents. In this review, we have tried to outline and summarize different synthetic approaches and strategies along with detailed modifications in substituents (structure–activity relationship). This review represents fruitful matrix that will help the researchers to develop lead compounds in different biological domains.
Author Contributions
Conceptualization: A.I.; resources: S.U., A.A., N.J., A.F.Z. and H.K.; writing—original draft preparation: A.I. and A.F.Z.; writing—review and editing: K.K.-M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Authors are thankful to the Department of Chemistry, GCU Faisalabad for its literature survey facilities. There was no funding support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Irfan, A.; Batool, F.; Ahmad, S.; Ullah, R.; Sultan, A.; Sattar, R.; Nisar, B.; Rubab, L. Recent trends in the synthesis of 1,2,3-thiadiazoles. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1098–1115. [Google Scholar] [CrossRef]
- Singh, A.K.; Parthsarthy, R.; Kshitiz, J.; Mishra, G. Synthesis, characterization and antibacterial activity of 1,3,4-thiadiazole derivatives. Int. J. Sci. Innov. Discov. 2011, 3, 353–361. [Google Scholar]
- Siddiqui, N.; Ahuja, P.; Ahsan, W.; Pandeya, S.N.; Alam, S.M. Thiadiazoles: Progress report on biological activities. J. Chem. Pharm. Res. 2009, 1, 19–30. [Google Scholar]
- Jalhan, S.; Jindal, A.; Gupta, A.; Hemraj, H. Synthesis, biological activities and chemistry of thiadiazole derivatives and schiff bases. Asian J. Pharm. Clin. Res. 2012, 3, 199–208. [Google Scholar]
- Srivastava, S.; Prasad, K.R.; Saini, R. Thiadiazole: A brief review. WJPPS 2014, 3, 1198–1212. [Google Scholar]
- Paulrasu, K.; Duraikannu, A.; Palrasu, M.; Shanmugasundaram, A.; Kuppusamy, M.; Thirunavukkarasu, B. Synthesis of 4-methyl-N’-(3-alkyl-2r,6cdiarylpiperidin-4-ylidene)-1,2,3-thiadiazole-5-carbohydrazides with antioxidant, antitumor and antimicrobial activities. Org. Biomol. Chem. 2014, 12, 911–921. [Google Scholar] [CrossRef]
- Kanakaraju, S.; Suresh, L. Design, synthesis, in vitro antimicrobial and cytotoxic evaluation of novel 1,2,3-selena/thiadiazolyltetrazole derivatives. RSC Adv. 2015, 5, 29325–29334. [Google Scholar] [CrossRef]
- Dogan, H.N.; Duran, A.; Rollas, S.; Sener, G.; Uysal, M.K.; Gulen, D. Synthesis of new 2,5-disubstituted-1,3,4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorg. Med. Chem. 2002, 10, 2893–2898. [Google Scholar] [CrossRef]
- Hussain, S.; Sharma, J.; Amir, M. Synthesis and Antimicrobial activities of 1,2,4-triazole and 1,3,4-thiadiazole derivatives of 5-amino-2-hydroxybenzoic acid. E J. Chem. 2008, 5, 963–968. [Google Scholar] [CrossRef]
- Demirbas, A.; Sahin, D.; Demirbas, N.; Karaoglu, A.S. Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 2896–2903. [Google Scholar] [CrossRef]
- Farshori, N.N.; Banday, M.R.; Ahmad, A.; Khan, A.U.; Rauf, A. Synthesis, characterization and in vitro antimicrobial activities of 5-alkenyl/hydroxyalkenyl-2-phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg. Med. Chem. Lett. 2010, 20, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Almajan, G.L.; Innocenti, A.; Puccetti, L.; Manole, G.; Barbuceanu, S.; Saramet, I.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the cytosolic and tumor-associated carbonic anhydrase isozymes I, II, and IX with a series of 1,3,4-thiadiazole- and 1,2,4-triazole-thiols. Bioorg. Med. Chem. Lett. 2005, 15, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.K.; Abdel-Hafez, A.A.; El-Koussi, N.A.; Mahfouz, N.M.; Innocenti, A.; Supuran, C.T. Design, synthesis, and docking studies of new 1,3,4-thiadiazole-2-thione derivatives with carbonic anhydrase inhibitory activity. Bioorg. Med. Chem. 2007, 15, 6975–6984. [Google Scholar] [CrossRef]
- Dudutien, V.; Baranauskien, L.; Matulis, D. Benzimidazo[1,2-c][1,2,3]thiadiazole-7-sulfonamides as inhibitors of carbonic anhydrase. Bioorg. Med. Chem. Lett. 2007, 17, 3335–3338. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Sattari, S.; Bineshmarvasti, M.; Daneshtalab, M.; Shafiee, A. Synthesis and in vitro antifungal and cytotoxicity evaluation of substituted 4,5-dihydronaphtho[1,2-d][1,2,3]thia(orselena)diazoles. Il Farmaco 2003, 58, 63–68. [Google Scholar] [CrossRef]
- Chu, C.H.; Hui, X.P.; Xu, P.F.; Zhang, Z.Y.; Li, Z.C.; Liao, R.A. Synthesis and antifungal activities of ω-(5-arylamino-1,3,4-thiadiazol-2-thio-)-ω-(1H-1,2,4-triazol-1-yl)acetophenones. Indian J. Chem. 2002, 41B, 2436–2438. [Google Scholar] [CrossRef]
- Moawad, E.B.; Yousif, M.Y.; Metwally, M.A. Synthesis of certain heteroaryl-fused pyrimidines and pyridines and selena- and thia-diazoles with naphthyl substituent as potential antifungal agents. Pharmazie 1989, 44, 820–822. [Google Scholar] [CrossRef]
- Yadav, L.S.; Zaidi, M.G.H.; Singh, B.N. Synthesis and fungicidal evaluation of some 1,3,4-thiadiazoles against P. oryzae and R. solani. Asian J. Chem. 2003, 15, 1805–1807. [Google Scholar]
- Zou, X.-J.; Lai, L.H.; Jin, G.Y.; Zhang, Z.X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food. Chem. 2002, 50, 3757–3760. [Google Scholar] [CrossRef]
- Balasankar, T.; Gopalakrishnan, M.; Nagarajan, S. Synthesis and antibacterial activity of some 5-(4-biphenylyl)-7-aryl[3,4-d] [1,2,3]-benzothiadiazoles. Eur. J. Med. Chem. 2005, 40, 728–731. [Google Scholar] [CrossRef]
- Padmavathi, V.; Mahesh, K.; Mohan, N.V.A.; Mohan, N.; Padmaja, A. Synthesis and bioassay of oxazolyl/thiazolyl selenadiazoles, thiadiazoles and diazaphospholes. Chem. Pharm. Bull. 2009, 57, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.S.; Ahmad, A.A.; Mawlood, S.I.; Ali, N.O. Synthesis of some series of 2-amino-1,3,4-thiadiazole derivatives with their pathogenic bacterial activity. J. Raparin Univ. 2017, 4, 63–78. [Google Scholar]
- Othman, A.A.; Kihel, M.; Amara, S. 1,3,4-Oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole derivatives as potential antibacterial agents. Arab. J. Chem. 2014, 12, 1660–1675. [Google Scholar] [CrossRef]
- .Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Berecka-Rycerz, A.; Malm, A.; Gumieniczek, A.; Wujec, M. Novel derivatives of 4-methyl-1,2,3-thiadiazole-5-carboxylic acid hydrazide: Synthesis, lipophilicity, and in vitro antimicrobial activity screening. Appl. Sci. 2021, 11, 1180. [Google Scholar] [CrossRef]
- Camoutsis, C.; Geronikaki, A.; Ciric, A.; Sokovic, M.; Zoumpoulakis, P.; Zervou, M. Sulfonamide-1,2,4-thiadiazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity and conformational studies. Chem. Pharm. Bull. 2010, 58, 160–167. [Google Scholar] [CrossRef]
- Yasuda, M.; Kusajima, M.; Nakajima, M.; Akutsu, K.; Kudo, T.; Yoshida, S.; Nakashita, H. Thiadiazole carboxylic acid moiety of tiadinil, SV-03, induces systemic acquired resistance in tobacco without salicylic acid accumulation. J. Pest. Sci. 2006, 31, 329–334. [Google Scholar] [CrossRef]
- Xu, Y.F.; Zhao, Z.J.; Qian, X.H.; Qian, Z.; Tian, W.; Zhong, J. Novel, unnatural benzo-1,2,3-thiadiazole-7-carboxylate elicitors of taxoid biosynthesis. J. Agric. Food Chem. 2006, 54, 8793–8798. [Google Scholar] [CrossRef] [PubMed]
- Nombela, G.; Pascual, S.; Aviles, M.; Guillard, E.; Muñizet, M. Benzothiadiazole induces local resistance to Bemisia tabaci (Hemiptera:Aleyrodidae) in tomato plants. J. Econ. Entomol. 2005, 98, 2266–2271. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Fan, Z.; Wu, Q.; Zhang, Y.; Mi, N.; Wang, S.; Zhang, Z.; Song, H.; Liu, F. Synthesis and insecticidal activity of N-tert- 314 butyl-N,N0-diacylhydrazines containing 1,2,3-thiadiazoles. J. Agric. Food Chem. 2011, 59, 628–634. [Google Scholar] [CrossRef]
- Bloom, J.D.; DiGrandi, M.J.; Dushin, R.G.; Curran, K.J.; Ross, A.A.; Norton, E.B.; Terefenko, E.; Jones, T.R.; Feld, B.; Lang, S.A. Thiourea inhibitors of herpes viruses. Part 1: Bis-(aryl)thiourea inhibitors of CMV. Bioorg. Med. Chem. Lett. 2003, 13, 2929–2932. [Google Scholar] [CrossRef]
- Bloom, J.D.; Dushin, R.G.; Curran, K.J.; Donahue, F.; Norton, E.B.; Terefenko, E.; Jones, T.R.; Ross, A.A.; Feld, B.; Lang, S.A.; et al. Thiourea inhibitors of herpes viruses. Part 2: N-Benzyl-N0-arylthiourea inhibitors of CMV. Bioorg. Med. Chem. Lett. 2004, 14, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Pannecouque, C.; Szafarowicz, B.; Volkova, N.; Bakulev, V.; Dehaen, W.; Mely, Y.; Daelemans, D. N,N′-Bis(1,2,3-thiadiazol-5-yl)benzene-1,2-diamine targets the HIV-1 retroviral nucleocapsid zinc fingers. Antimicrob. Agents Chemother. 2010, 54, 1461–1468. [Google Scholar] [CrossRef][Green Version]
- Tatar, E.; Kucukguzel, S.G.; Karakus, S.; Clercq, E.D.; Andrei, G.; Snoeck, R.; Pannecouque, C.; Okullu, S.O.; Ünübol, N.; Kocagöz, T.; et al. Synthesis and biological evaluation of some new 1,3,4-thiadiazole and 1,2,4-triazole derivatives from L-methionine as antituberculosis and antiviral agents. Marmara Pharm. J. 2015, 19, 88–102. [Google Scholar] [CrossRef]
- Azaam, M.M.; Kenawy, E.; El-Din, A.S.B.; Khamis, A.A.; El-Magdd, M.A. Antioxidant and Anticancer Activities of α-Aminophosphonates Containing Thiadiazole. J. Saudi Chem. Soc. 2018, 22, 34–41. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, S.; Vishwakarma, P.; Sharma, M.; Saxena, K.K.; Kumar, A. Synthesis and antipsychotic and anticonvulsant activity of some new substituted oxa/thiadiazolyl azetidinonyl/thiazolidinonyl carbazoles. Eur. J. Med. Chem. 2010, 45, 2777–2783. [Google Scholar] [CrossRef]
- Sddiqui, N.; Ahuja, P.; Ahsan, W.; Pandeya, S.N.; Alam, S.M. Synthesis of 3-arylamino-4-aryl-5-(N-arylthiocarbonylimino)-4,5-dihydro-1,2,4-thiadiazoles as anticonvulsant agents. Indian J. Heterocycl. Chem. 2004, 14, 159–160. [Google Scholar]
- Siddiqui, N.; Arshad, M.F.; Khan, S.A.; Ahsan, W. Synthesis, anticonvulsant and neurotoxicity screening of 1-(substituted phenyl)-3-[(5-substituted phenyl)-1,3,4-thiadiazole-2-yl]-2-thioxodihydro pyrimidine-4,6 (1H, 5H)-diones. J. Pharm. Res. 2008, 7, 122–125. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ahsan, W. Synthesis, anticonvulsant and toxicity screening of thiazolyl-thiadiazole derivatives. Med. Chem. Res. 2011, 20, 261–268. [Google Scholar] [CrossRef]
- Mullick, P.; Khan, S.A.; Verma, S.; Alam, O. Thiadiazole derivatives as potential anticonvulsant agents. B Korean Chem. Soc. 2011, 32, 1011–1016. [Google Scholar] [CrossRef]
- Chapleo, C.B.; Myers, M.; Myers, P.L.; Saville, J.F.; Smith, A.C.B.; Stillings, M.R.; Tulloch, J.F.; Walter, D.S.; Welbourn, A.P. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 1.Hydrazines. J. Med. Chem. 1986, 29, 2273–2280. [Google Scholar] [CrossRef]
- Rajak, H.; Behera, C.K.; Pawar, R.S.; Singour, P.K.; Kharya, M.D. Synthesis and anticonvulsant evaluation of some novel 2,5-disubstituted 1,3,4-thiadiazoles: Pharmacophore model studies. Acta Pol. Pharm. 2010, 67, 503–510. [Google Scholar]
- Samel, A.B.; Pai, N.R. Synthesis of novel aryloxypropanoylthiadiazoles as potential antihypertensive agents. Chin. Chem. Soc. TAIP 2010, 57, 1327–1330. [Google Scholar] [CrossRef]
- Turner, S.; Myers, M.; Gadie, B.; Nelson, A.J.; Pape, R.; Saville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive thiadiazoles 1. Synthesis of some 2-aryl-5-hydrazino-1,3,4- thiadiazole with vasodilator activity. J. Med. Chem. 1988, 31, 902–906. [Google Scholar] [CrossRef]
- Wei, M.-X.; Feng, L.; Li, X.-Q.; Zhou, X.-Z.; Shao, Z.-H. Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur. J. Med. Chem. 2009, 44, 3340–3344. [Google Scholar] [CrossRef]
- Tripathy, R.; Ghose, A.; Singh, J.; Bacon, E.R.; Angeles, T.S.; Yang, S.X.; Albom, M.S.; Aimone, L.D.; Herman, J.L.; Mallamo, J.P. 1,2,3-Thiadiazole substituted pyrazolones as potent KDR/VEGFR-2 kinase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1793–1798. [Google Scholar] [CrossRef]
- Ibrahim, D.A. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur. J. Med. Chem. 2009, 44, 776–2781. [Google Scholar] [CrossRef]
- Padmavathi, V.; Reddy, G.S.; Padmaja, A.; Kondaiah, P.P.; Shazia, A. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 2009, 44, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Karakuü, S.; Çoruh, U.; Barlas-Durgun, B.; Vázquez-López, E.M.; Özbaü-Turan, S.; Akbuùa, J.; Rollas, S. Synthesis and cytotoxic activity of some 1,2,4-triazoline-3-thione and 2,5-disubstituted-1,3,4-thiadiazole derivatives. Marmara Pharm. J. 2010, 14, 84–90. [Google Scholar] [CrossRef]
- Holla, B.S.; Poorjary, K.N.; Rao, B.S.; Shivananda, M.K. New bis-aminomercaptotriazoles and bis-triazolothiadiazoles as possible anti-cancer agents. Eur. J. Med. Chem. 2002, 37, 511–517. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Mishra, P. Synthesis, antimicrobial and anticancer activities of 5-(4-substituted phenyl)-1,3,4-thiadiazole-2-amines. Rasayan J. Chem. 2017, 10, 254–262. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Ranise, A.; Filippelli, W.; Rinaldi, B.; Capuano, A.; Falcone, G. New 1,3,4-thiadiazole derivatives endowed with analgesic and anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 1698–1705. [Google Scholar] [CrossRef]
- Rostom, S.A.F.; El-Ashmawy, I.M.; el Razik, H.A.A.; Badr, M.H.; Ashour, H.M.A. Design and synthesis of some thiazolyl and thiadiazolyl derivatives of antipyrine as potential non-acidic anti-inflammatory, analgesic and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 882–895. [Google Scholar] [CrossRef]
- Labanauskas, L.; Kalcas, V.; Udrenaite, E.; Gaidelis, P.; Brukstus, A.; Dauksas, A. Synthesis of 3-(3,4-dimethoxyphenyl)-1H-1,2,4-triazole-5-thiol and 2-amino-5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazole derivatives exhibiting anti-inflammatory activity. Pharmazie 2001, 56, 617–619. [Google Scholar] [CrossRef]
- Kadi, A.A.; El-Brolossy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef]
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and anti-inflammatory activi¬ties of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5006–5011. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Ashour, H.M.A.; Ghany, Y.S.A.; Bekhit, A.E.A.; Baraka, A. Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.D.; Gawad, H.A.; Rageb, E.A.; Ellithey, M.; Mohamed, H.A. Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg. Med. Chem. 2006, 14, 3672–3680. [Google Scholar] [CrossRef]
- Du, Q.S.; Zhu, W.P.; Zhao, Z.J.; Qian, X.H.; Xu, Y.F. Novel benzo-1,2,3-thiadiazole-7-carboxylate derivatives as plant activators and the development of their agricultural applications. J. Agric. Food Chem. 2012, 60, 346–353. [Google Scholar] [CrossRef]
- Stanetty, P.; Kremslehner, M.; Veollenkle, H. A new type of plant activator: Synthesis of thieno[2,3-d][1,2,3]-thiadiazole-6-carboxylic acid derivatives via Hurd-Mori cyclization. J. Chem. Soc. Perkin Trans. 1998, 1, 853–856. [Google Scholar] [CrossRef]
- Pattan, S.R.; Kekare, P.; Dighe, N.S.; Nirmal, S.A.; Musmade, D.S.; Parjane, S.K.; Daithankar, A.V. Synthesis and biological evaluation of some 1,3,4-thiadiazoles. J. Chem. Pharm. Res. 2009, 1, 191–198. [Google Scholar]
- Ram, V.J.; Goel, A.; Kandpal, M. Tetraazaacenaphthene, tetraazaphenalene and 1,3,4-thiadiazole derivatives as potential leishmanicides. Bioorg. Med. Chem. Lett. 1997, 7, 651–656. [Google Scholar] [CrossRef]
- Silva, E.F.; Canto-Cavalheiro, M.M.; Braz, V.R.; Cysne-Finkelstein, L.; Leon, L.L.; Echevarria, A. Synthesis, and biological evaluation of new 1,3,4-thiadiazolium-2-phenylaminenderivatives against Leishmania amazonensis promastigotes and amastigotes. Eur. J. Med. Chem. 2002, 37, 979–984. [Google Scholar] [CrossRef]
- Behrouzi-Fardmoghadam, M.; Poorrajab, F.; Ardestani, S.K.; Emami, S.; Shafiee, A.; Foroumadi, A. Synthesis and in vitro anti-leishmanial activity of 1-[5-(5-nitrofuran-2-yl)- 1,3,4-thiadiazol-2-yl]- and 1-[5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-yl]-4-aroyl piperazines. Bioorg. Med. Chem. 2008, 16, 4509–4515. [Google Scholar] [CrossRef]
- Foroumadi, A.; Emami, S.; Pournourmohammadi, S.; Kharazmi, A.; Shafiee, A. Synthesis and in vitro leishmanicidal activity of 2-(1-methyl-5-nitro-1H- imidazol-2-yl)-5-substituted-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2005, 40, 1346–1350. [Google Scholar] [CrossRef]
- Oruc, E.E.; Rollas, S.; Kandermirli, S.; Shvets, N.; Dimoglo, A.S. 1,3,4-thiadiazole derivatives. Synthesis, structure elucidation, and structure-antituberculosis activity relationship investigation. J. Med. Chem. 2004, 47, 6760–6767. [Google Scholar] [CrossRef]
- Karakus, S.; Rollas, S. Synthesis and antituberculosis activity of new N-phenyl-N′-[4-(5-alkyl/arylamino-1,3,4-thiadiazole-2-yl)phenyl]thioureas. Farmaco 2002, 57, 577–581. [Google Scholar] [CrossRef]
- Solak, N.; Rollas, S. Synthesis and antituberculosis activity of 2-(aryl/alkylamino)-5-(4-aminophenyl)-1,3,4-thiadiazoles and their Schiff bases. ARKIVOC 2006, 12, 173–181. [Google Scholar] [CrossRef]
- Patole, J.; Shingnapurkar, D.; Padhye, S.; Ratledge, C. Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Bioorg. Med. Chem. Lett. 2006, 16, 1514–1517. [Google Scholar] [CrossRef]
- Foroumadi, A.; Kargar, Z.; Sakhteman, A.; Sharifzadeh, Z.; Mohammadi, R.F.; Kazemi, M.; Shafiee, A. Synthesis and antimycobacterial activity of some alkyl [5-(nitroaryl)-1,3,4-thiadiazol-2-ylthio]propionates. Bioorg. Med. Chem. Lett. 2006, 16, 1164–1167. [Google Scholar] [CrossRef]
- Tahghighi, A.; Babalouei, F. Thiadiazoles: The appropriate pharmacological scaffolds with leishmanicidal and antimalarial activities: A review. Iran. J. Basic Med. Sci. 2017, 20, 613–622. [Google Scholar] [CrossRef]
- Gudala, S.; Ambati, S.R.; Patel, J.L.; Vedula, R.R.; Penta, S. An Efficient Synthesis of Pyrazolyl-1,2,3-thiadiazoles viaHurd-Mori Reaction. J. Heterocycl. Chem. 2019, 56, 2163–2169. [Google Scholar] [CrossRef]
- Jain, K.A.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and its Derivatives: A Review on Recent Progress in Biological Activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrase inhibitors. Curr. Med. Chem. Immunol. Endocrinol. Metab. Agents 2001, 1, 61–97. [Google Scholar] [CrossRef]
- Georgeta, S.; Oana, S.; Eugenia, S.; Santa, B. 2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des. Devel. Ther. 2018, 12, 1545–1566. [Google Scholar] [CrossRef]
- Iizawa, Y.; Okonogi, K.; Hayashi, R.; Iwahi, T.; Yamazaki, T.; Imada, A. Therapeutic effect of cefozopran (SCE-2787), a new parenteral cephalosporin, against experimental infections in mice. Antimicrob. Agents Chemother. 1993, 37, 100–105. [Google Scholar] [CrossRef]
- Sharma, B.; Verma, A.; Prajapati, S.; Sharma, K.U. Synthetic methods, chemistry, and the anticonvulsant activity of thiadiazoles. Int. J. Med. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Kucuk, H.B.; Salt, Z.B.; Kara, E.M.; Mehan, A.S.; Yusufoglu, A.S. Synthesis of novel 1,2,3-thiadizoles and 1,2,3-selenadiazoles as new antimicrobial agents. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194. [Google Scholar] [CrossRef]
- Ngoc, T.D. Synthesis and characterization of some new 1,2,3-thiadiazole and 1,2,3-selenadiazole triterpene derivatives from allobetulone and 2-oxoallobetulin. Synth. Commun. 2020, 50, 1665–1671. [Google Scholar] [CrossRef]
- Kumar, A.; Muthyala, K.M.; Choudhary, S.; Tiwari, R.K.; Parang, K. Ionic Liquid as Soluble Support for Synthesis of 1,2,3-Thiadiazoles and 1,2,3-Selenadiazoles. J. Org. Chem. 2012, 77, 9391–9396. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Yu, J.T.; Cheng, J. TBAI-Catalyzed Reaction between N-Tosylhydrazones and Sulfur: A Procedure toward 1,2,3-Thiadiazole. J. Org. Chem. 2016, 81, 271–275. [Google Scholar] [CrossRef]
- Sun, N.-B.; Fu, J.Q.; Weng, J.-Q.; Jin, J.-Z.; Tan, C.-X.; Liu, X.-H. Microwave Assisted Synthesis, Antifungal Activity and DFTTheoretical Study of Some Novel 1,2,4-Triazole Derivatives Containing the 1,2,3-Thiadiazole Moiety. Molecules 2013, 18, 12725–12739. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Mi, N.; Fan, Z.; Zuo, X.; Zhang, H.; Wang, H.; Yang, Z. 5-Methyl-1,2,3-thiadiazoles Synthesized via Ugi Reaction and Their Fungicidal and Antiviral Activities. J. Agric. Food Chem. 2010, 58, 7846–7855. [Google Scholar] [CrossRef]
- Wang, S.-X.; Fang, Z.; Fan, Z.J.; Wang, D.; Li, Y.-D.; Li, X.-T.; Hua, X.-W.; Huang, Y.; Kalinina, A.T.; Bakulev, A.V.; et al. Synthesis of tetrazole containing 1,2,3-thiadiazole derivatives via U-4CR and their anti-TMV activity. Chin. Chem. Lett. 2013, 24, 889–892. [Google Scholar] [CrossRef]
- Wang, C.; Geng, X.; Zhao, P.; Zhou, Y.; Wu, Y.D.; Cui, Y.F.; Wu, A.X. I2/CuCl2-promoted one-pot three-component synthesis of aliphatic or aromatic substituted 1,2,3-thiadiazoles. Chem. Commun. 2019, 55, 8134–8137. [Google Scholar] [CrossRef]
- Dong, W.-L.; Liu, Z.-X.; Liu, X.-H.; Li, Z.-M.; Zhao, W.-G. Synthesis and antiviral activity of new acrylamide derivatives containing1,2,3-thiadiazole as inhibitors of hepatitis B virus replication. Eur. J. Med. Chem. 2010, 45, 1919–1926. [Google Scholar] [CrossRef]
- Hayat, F.; Salahuddin, A.; Zargan, J.; Azam, A. Synthesis, characterization, antiamoebic activity and cytotoxicity of novel 2-(quinolin-8-yloxy) acetohydrazones and their cyclized products (1,2,3-thiadiazole and 1,2,3-selenadiazole derivatives). Eur. J. Med. Chem. 2010, 45, 6127–6134. [Google Scholar] [CrossRef]
- Du, Q.-S.; Shi, Y.-X.; Li, P.-F.; Zhao, Z.-J.; Zhu, W.-P.; Qian, X.-H.; Li, B.-J.; Xu, Y.-F. Novel plant activators with thieno[2,3-d]-1,2,3-thiadiazole-6-carboxylate scaffold: Synthesis and bioactivity. Chin. Chem. Lett. 2013, 24, 967–969. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, Y.; Li, A.; Liu, K.; Li, L.; Yang, T.; Zhou, C. One-Pot Synthesis of 5-Acyl-1,2,3-Thiadiazoles from Enaminones, Tosylhydrazine and Elemental Sulfur under Transition Metal-Free Conditions. J. Org. Chem. 2019, 84, 16262–16267. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, B.; Liu, Q.; Mo, F. Addition of Diazo Compounds ipso-C-H Bond to Carbon Disulfide: Synthesis of 1,2,3-Thiadiazoles under Mild Conditions. J. Org. Chem. 2018, 83, 4275–4278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Lu, L.; Zhang, S.; Bao, W.; Huang, S.; Rao, Y. Perylenequinonoid-Catalyzed [4 + 1] and [4 + 2] Annulations of Azoalkenes: Photocatalytic Access to 1,2,3-Thiadiazole/1,4,5,6-Tetrahydropyridazine Derivatives. J. Org. Chem. 2019, 84, 7711–7721. [Google Scholar] [CrossRef]
- Teplyakov, S.F.; Vasileva, G.T.; Petrov, L.M.; Androsov, A.D. A New Synthesis of Benzo[b]thiophene-2-thiolates and Their Derivatives via Base-Promoted Transformation of 4-(2-Mercaptophenyl)-1,2,3-thiadiazoles. Org. Lett. 2013, 15, 4038–4041. [Google Scholar] [CrossRef]
- Fan, W.; Li, Q.; Li, Y.; Sun, H.; Jiang, B.; Li, G. I2/O2-Enabled N-S Bond Formation to Access Functionalized 1,2,3-Thiadiazoles. Org. Lett. 2016, 18, 1258–1261. [Google Scholar] [CrossRef]
- Belyaev, A.N.; Beryozkina, V.T.; Bakulev, A.V. Synthesis of N-heteroarylamidines of 1,2,3-thiadiazole-4-carboxylic acid from 2-cyanothioacetamides and 5-azido-1-methyl-4-nitroimidazole. Chem. Heterocycl. Compd. 2016, 52, 206–208. [Google Scholar] [CrossRef]
- Liu, B.-B.; Bai, H.-W.; Liu, H.; Wang, S.-Y.; Ji, S.-J. A cascade trisulfur radical anion (S3•-) addition/electron detosylation process for the synthesis of 1,2,3-thiadiazoles and isothiazoles. J. Org. Chem. 2018, 83, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.; Chawla, A.; Sharma, P.; Arora, S. Novel drug delivery approaches on antiviral and antiretroviral agents. J. Adv. Pharm. Technol. Res. 2012, 3, 147–159. [Google Scholar] [CrossRef]
- Rossignol, J.F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antivir. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Raymund, R.; Razonable, M.D. Antiviral Drugs for Viruses Other Than Human Immunodeficiency Virus. Mayo Clin. Proc. 2011, 86, 1009–1026. [Google Scholar] [CrossRef]
- Nystrom, K.; Waldenstrom, J.; Tang, K.-W.; Lagging, M. Ribavirin: Pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019, 14, 153–160. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.; Witkowski, J.T.; Robins, R.K. Broad-spectrum antiviral activity of virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. J. Sci. 1972, 177, 705–706. [Google Scholar] [CrossRef]
- Rakhmanina, N.Y.; Vanda, V.J.N. Efavirenz in the therapy of HIV infection. Expert Opin. Drug Metab. Toxicol. 2009, 6, 95–103. [Google Scholar] [CrossRef]
- Larru, B.; Eby, J.; Lowenthal, E.D. Antiretroviral treatment in HIV-1 infected pediatric patients: Focus on efavirenz. Pediatric Health Med. Ther. 2014, 2014, 29–42. [Google Scholar] [CrossRef][Green Version]
- Kryst, J.; Kawalec, P.; Pilc, A. Efavirenz-Based Regimens in Antiretroviral-Naive HIV-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0124279. [Google Scholar] [CrossRef]
- Leneva, I.A.; Russell, R.J.; Boriskin, Y.S.; Hay, A.J. Characteristics of arbidol-resistant mutants of influenza virus: Implications for the mechanism of anti-influenza action of arbidol. Antivir. Res. 2009, 81, 132–140. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, H.; Lu, L.; Liu, Y.; Luo, F.; Hou, W.; Yang, Z. Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol. Sin. 2013, 34, 1075–1083. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, X.; Cao, Y.; Wang, Y.; Pannecouque, C.; Clercq, E.D. 1,2,3-thiadiazole thioacetanilides as a novel class of potent HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5368–5371. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, X.; Li, Z.; Fang, Z.; Li, Z.; Wang, D.; Pannecouque, C.; Clercq, E.D. Novel 1,2,3-thiadiazole derivatives As HIV-1 NNRTIs with improved potency: Synthesis and preliminary SAR studies. Bioorg. Med. Chem. Lett. 2009, 17, 5920–5927. [Google Scholar] [CrossRef]
- Mao, W.T.; Zhao, H.; Fan, Z.J.; Ji, X.T.; Hua, X.W.; Kalinina, T.; Yury, Y.M.; Vasiliy, A.B. Synthesis and bioactivity of N-tert-butyl-N′-acyl-5-methy l-1,2,3-thiadiazole-4-carbohydrazides. Chin. Chem. Lett. 2012, 23, 1233–1236. [Google Scholar] [CrossRef]
- Xu, W.-M.; Li, S.-Z.; He, M.; Yang, S.; Li, X.-Y.; Li, P. Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 2013, 23, 5821–5824. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, X.Y.; Li, Z.Y.; Fang, Z.-J.; Pannecouque, C.; Clercqet, E.D. Synthesis and biological evaluation of a new series of 2-{[4-(3,4-dichlorophenyl)-1,2,3-thiadiazol-5-yl] sulfanyl}acetanilides as HIV-1 inhibitors. Chem. Biodivers. 2010, 7, 1717–1727. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.J.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Naqvi, S.A.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Pattabiraman, V.R.; Bode, J.W. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- He, Z.C.; Ling, G.L.; Yi, Z.Y.; Fei, Z.F.; Zhou, W.G.; Lei, J.; Xia, G.R. Review on supermolecules as chemical drugs. Sci. China Ser. B 2009, 52, 415–458. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Gangadhara, S.; Bertelli, G. Long-term efficacy and safety of anastrozole for adjuvant treatment of early breast cancer in postmenopausal women. Ther. Clin. Risk Manag. 2009, 5, 291–300. [Google Scholar] [CrossRef]
- Barros-Oliveira, M.C.; Costa-Silva, D.R.; Andrade, D.B.; Borges, U.S.; Tavares, C.B.; Borges, R.S.; Silva, J.M.; Silva, B.B. Use of anastrozole in the chemoprevention and treatment of breast cancer: A literature review. Rev. Assoc. Med. Bras. 2017, 63, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva, F.P. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez, B.T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Al-Malky, S.H.; Damanhouri, Z.A.; Aama, J.Y.A.; Qahtani, A.A.A.; Ramadan, W.S.; AlKreathy, H.M.; Harthi, S.E.A.; Osman, A.M.M. Diltiazem potentiation of doxorubicin cytotoxicity and cellular uptake in human breast cancer cells. Breast Cancer Manag. 2019, 8, BMT31. [Google Scholar] [CrossRef]
- Wu, M.; Sun, Q.; Yang, C.; Chen, D.; Ding, J.; Chen, Y.; Lin, L.; Xie, Y. Synthesis and activity of Combretastatin A-4 analogues: 1,2,3-thiadiazoles as potent antitumor agents. Bioorg. Med. Chem. Lett. 2007, 17, 869–873. [Google Scholar] [CrossRef]
- Hosny, M.A.; El-Sayed, T.H.; El-Sawi, E.A. A New Type of Synthesis of 1,2,3-thiadiazole and 1,2,3-diazaphosphole derivatives Via-Hurd-Mori cyclization. J. Chem. 2012, 9, 1276–1287. [Google Scholar] [CrossRef]
- Cikotiene, I.; Kazlauskas, E.; Matuliene, J.; Michailoviene, V.; Torresan, J.; Jachno, J.; Matuliset, D. 5-Aryl-4-(5-substituted-2,4-dihydroxyphenyl)-1,2,3-thiadiazoles as inhibitors of Hsp90 chaperone. Bioorg. Med. Chem. Lett. 2009, 19, 1089–1092. [Google Scholar] [CrossRef]
- Cui, H.W.; Peng, S.; Gu, X.Z.; Chen, H.; He, Y.; Gao, W.; Lv, F.; Wang, J.-H.; Wang, Y.; Xie, J.; et al. Synthesis and biological evaluation of D-ring fused 1,2,3-thiadiazole dehydro epiandro sterone derivatives as antitumor agents. Eur. J. Med. Chem. 2016, 111, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A short history of insecticides. J. Plant Prot. Res. 2015, 55, 221–226. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Jiang, D.; Cheng, J.; Shao, X.; Li, Z. Azobenzene Modified Imidacloprid Derivatives as Photoswitchable Insecticides: Steering Molecular Activity in a Controllable Manner. Sci. Rep. 2015, 5, 13962. [Google Scholar] [CrossRef]
- Carlson, G.R. Tebufenozide: A Novel Caterpillar Control Agent with Unusually High Target Selectivity. Green Chemical Syntheses and Processes. ACS Symp. Ser. 2000, 767, 8–17. [Google Scholar] [CrossRef]
- Zhang, A.; Kayser, H.; Maeinfisch, P. Insect nicotinic acetylcholine receptor: Conserved neonicotinoid specificity of imidacloprid binding site. J. Neurochem. 2002, 75, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, A.; Kumar, A. Accidental human poisoning with a neonicotinoid insecticide, imidacloprid: A rare case report from rural India with a brief review of literature. Egypt. J. Forensic Sci. 2013, 3, 123–126. [Google Scholar] [CrossRef]
- Mundhe, S.A.; Birajdar, S.V.; Chavan, S.S.; Pawar, N.R. Imidacloprid Poisoning: An Emerging Cause of Potentially Fatal Poisoning. Indian J. Crit. Care Med. 2017, 21, 786–788. [Google Scholar] [CrossRef]
- Ausborn, J.; Wolf, H.; Mader, W.; Kayser, H. The insecticide pymetrozine selectively affects chordotonal mechanoreceptors. J. Exp. Biol. 2005, 28, 4451–4466. [Google Scholar] [CrossRef]
- Zhang, J.P.; Qin, Y.G.; Dong, Y.W.; Song, D.-L.; Duan, H.-X.; Yang, X.-L. Synthesis and biological activities of (E)-B-farnesene analogues 4 containing 1,2,3-thiadiazole. Chin. Chem. Lett. 2016, 28, 372–376. [Google Scholar] [CrossRef]
- Dai, H.; Ge, S.; Li, G.; Chen, J.; Shi, Y.; Ye, L.; Ling, Y. Synthesis and bioactivities of novel pyrazole oxime derivatives containing a 1,2,3-thiadiazole moiety. Bioorg. Med. Chem. Lett. 2016, 28, 4504–4507. [Google Scholar] [CrossRef]
- Li, Y.-D.; Mao, W.-T.; Fan, Z.-J.; Li, J.-J.; Fang, Z.; Ji, X.-T.; Hua, X.-W.; Zong, G.-N.; Li, F.-Y.; Liu, C.-L.; et al. Synthesis and biological evaluation of novel 1,2,4-triazole containing 1,2,3-thiadiazole derivatives. Chin. Chem. Lett. 2013, 24, 1134–1136. [Google Scholar] [CrossRef]
- Bercu, T.E.; Petri, W.A.; Behm, J.W. Amebic colitis: New insights into pathogenesis and treatment. Curr. Gastroenterol. Rep. 2007, 9, 429–433. [Google Scholar] [CrossRef]
- Choudhuri, G.; Rangan, M. Amebic infection in humans. Indian J. Gastroenterol. 2012, 31, 153–162. [Google Scholar] [CrossRef]
- Shirley, D.T.; Farr, L.; Watanabe, K.; Moonah, S. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect. Dis. 2018, 5, ofy161. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.; Hotez, P.; Junghanss, T.; Kang, G.; Lalloo, D.; White, N. Manson’s Tropical Diseases; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; pp. 664–671. ISBN 9780702053061. [Google Scholar]
- Lofmark, S.; Edlund, C.; Nord, C.E. Metronidazole Is Still the Drug of Choice for Treatment of Anaerobic Infections. Clin. Infect. Dis. 2010, 50, S16–S23. [Google Scholar] [CrossRef]
- Steinitz, H. The Treatment of Chronic Amoebic Infection with Metronidazole (Flagyl). Digestion 1972, 6, 75–82. [Google Scholar] [CrossRef]
- Gonzales, M.L.M.; Dans, L.F.; Martinez, E.G. Antiamoebic drugs for treating amoebic colitis. Cochrane Database Syst. Rev. 2009, 2, CD006085. [Google Scholar] [CrossRef]
- Ghaskadbi, S.; Vaidya, V.G. In vivo antimutagenic effect of ascorbic acid against mutagenicity of the common antiamebic drug diiodohydroxyquinoline. Mutat. Res. 1989, 222, 219–222. [Google Scholar] [CrossRef]
- Aranega-Bou, P.O.; Leyva, M.; Finiti, I.; Garcia-Agustin, P.; Gonzalez-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef]
- Li, J.; Long, T.; Sun, T.-J.; Lu, Y.; Yin, J.; Yang, Y.-B.; Dai, G.Y.; Zhu, X.Y.; Yao, N. Pyrimidin-Like Plant Activator Stimulates Plant Disease Resistance and Promotes the Synthesis of Primary Metabolites. Int. J. Mol. Sci. 2020, 21, 2705. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, F.R.; Resendeb, M.L.V.; Limac, J.P.M.S.; Silveirac, J.A.G.; Oliveira, J.T.A. Activities of antioxidant enzymes and photosynthetic responses in tomato pretreated by plant activators and inoculated by Xanthomonas vesicatoria. Int. J. Plant Pathol. 2006, 68, 198–208. [Google Scholar]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; Dam, N.M.V. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Agarwal, M. Toxicity of fungicides. In Veterinary Toxicology: Basic and Clinical Principles, 2nd ed.; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherland, 2012; pp. 653–670. [Google Scholar]
- Ramudu, A.C.; Mohiddin, G.J.; Srinivasulu, M.; Madakka, M.; Rangaswamy, V. Impact of Fungicides Chlorothalonil and Propiconazole on Microbial Activities in Groundnut (Arachis hypogaea L.) Soils. ISRN Microbiol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Bacmaga, T.M.; Wyszkowska, J.; Kucharski, J. The influence of chlorothalonil on the activity of soil microorganisms and enzymes. Ecotoxicology 2018, 27, 1188–1202. [Google Scholar] [CrossRef]
- Martin, M.V. The use of fluconazole and itraconazole in the treatment of Candida albicans infections: A review. J. Antimicrob. 1999, 44, 429–437. [Google Scholar] [CrossRef]
- Greer, D.; Jolly, H.W. Topical ketoconazole treatment of cutaneous candidiasis. J. Am. Acad. Dermatol. 1988, 18, 748–750. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, Z.; Zhang, H.; Liu, X.; Bao, L.; Ma, L.; Zuo, X.; Zheng, Q.; Mi, N. Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance. J. Agric. Food Chem. 2009, 57, 4279–4286. [Google Scholar] [CrossRef]
- Tan, C.-X.; Weng, J.-Q.; Liu, Z.-X.; Liu, X.-H.; Zhao, W.-G. Synthesis, Crystal Structure, and Fungicidal Activity of a Novel 1,2,3-Thiadiazole Compound. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 990–996. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, Z.; Zhang, H.; Mi, N.; Wang, H.; Cai, F.; Zuo, X.; Zheng, Q.; Song, H. Synthesis, crystal structure, and biological activity of 4-methyl-1,2,3-thiadiazole-containing 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. J. Agric. Food Chem. 2010, 58, 2630–2636. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Guo, Y.-Z.; Zhang, J.; Ma, L.; Song, H.-B.; Fan, Z.-J. Synthesis and biological activity of organotin 4-Methyl-1,2,3-thiadiazole-5-carboxylates and benzo[1,2,3]thiadiazole-7-carboxylates. J. Agric. Food Chem. 2010, 58, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Mi, N.; Fan, Z.; Zheng, Q.; Zhang, H.; Wang, H.; Yang, Z. Synthesis of 4-methyl-1,2,3-thiadiazole derivatives via Ugi reaction and their biological Activities. J. Agric. Food Chem. 2010, 58, 2755–2762. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).