An Overview on Atomization and Its Drug Delivery and Biomedical Applications
Abstract
1. Introduction
1.1. Stagnant Drop Formation
1.2. Dynamic Drop Formation
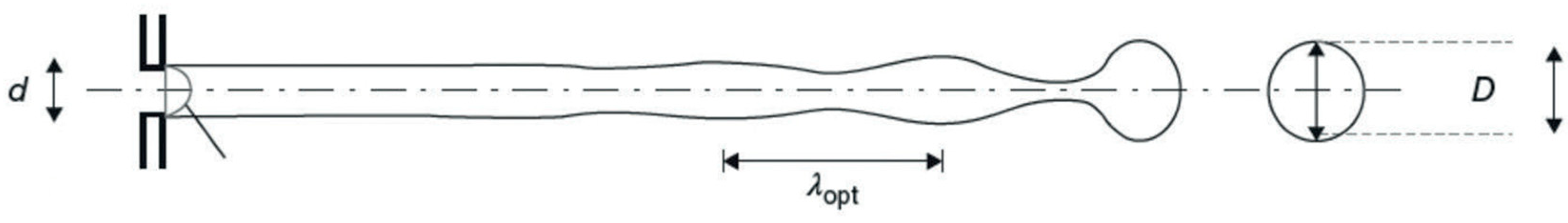
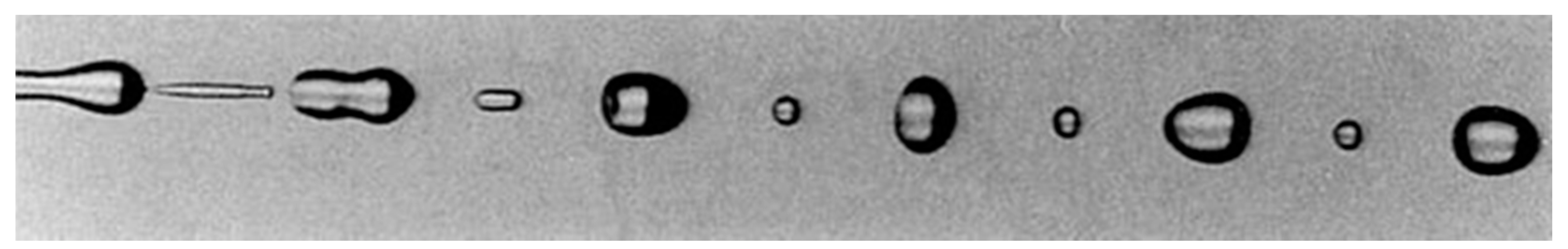
- The liquid is allowed to flow through a nozzle to accelerate its velocity to a required level causing the liquid to lengthen into jets or sheets;
- Liquid surface is initiated with external or internal disturbances like pressure, airflow, waves, etc.;
- Short liquid ligaments are shaped on the surface as a result of these disturbances;
- The surface tension of the liquid causes the ligaments to break into small droplets;
- These droplets will be further broken down into fine and uniform drops as they travel across the gassy channel.
1.3. General Theory
1.3.1. Primary Atomization
1.3.2. Secondary Atomization
2. Kinetics of Liquid Jets and Sheets
2.1. Instability of Liquid Jets
2.2. Mechanism of Liquid Sheet Instability
3. Atomization Processes
4. Drop Size and Distribution in Spray
5. Mathematical Formulations
5.1. Disruption of Liquid Jet
- µL—viscosity of the liquid
- ρL—density of liquid
- σ—surface tension
- Oh—Ohnesorge number
- We—Weber Number = ρU2d/σ, U is the jet velocity
- Re—Reynolds Number = ρUd/µ
5.2. Disruption of the Liquid Sheet
6. Drug Delivery Applications
7. Electrospray Atomization and Its Characteristics
Working Principle and Components of Electrospray
8. Biomedical Applications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lapple, C.E.; Henry, J.P.; Blake, D.E. Atomization—A Survey and Critique of the Literature; Special Report; Department of the Army: Edgewood Arsenal, MD, USA, 1967; pp. 11–14. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/821314.pdf (accessed on 6 March 2021).
- Graco, Atomization Concept and Theory, 1–18. Available online: http://wwwd.graco.com/training/concept_and_theory/Atomization%20v2.pdf (accessed on 6 March 2021).
- Zare, M.; Zare, M.; Butler, J.A.; Ramakrishna, S. Nanoscience-Led Antimicribial Surface Engineering to Prevent Infections. ACS Appl. Nano. Mater. 2021, 4, 4269–4283. [Google Scholar] [CrossRef]
- Lee, J.G.; Cho, H.J.; Huh, N.; Ko, C.; Lee, W.C.; Jang, Y.H.; Lee, B.S.; Kang, I.S.; Choi, J.W. Electrohydrodynamic (EHD) Dispensing of Nanoliter DNA Droplets for Microarrays. Biosens. Bioelectron. 2006, 21, 2240–2247. [Google Scholar] [CrossRef]
- Wu, Y.; Clark, R.L. Controllable Porous Polymer Particles Generated by Electrospraying. J. Colloid Interface Sci. 2007, 310, 529–535. [Google Scholar] [CrossRef]
- Leeuwenburg, S.; Wolke, J.; Schoonman, J.; Jansen, J. Electrostatic Spray Deposition (ESD) of Calcium Phosphate Coatings. J. Biomed. Mater. Res. A 2003, 66, 330–334. [Google Scholar] [CrossRef]
- Al-Jumaily, A.M.; Meshkinzar, A. On the Development of Focused Ultrasound Liquid Atomizers. Adv. Acoust. Vib. 2017, 1–10. [Google Scholar] [CrossRef]
- Topp, M.N. Ultrasonic Atomization—A Photographic Study of Themechanism of Disintegration. J. Aerosol. Sci. 1973, 4, 17–25. [Google Scholar] [CrossRef]
- Avvaru, B.; Patil, M.N.; Gogate, P.R.; Pandit, A.B. Ultrasonic atomization: Effect of liquid phase properties. Ultrasonics 2006, 44, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Marek, M.; Robert, O.; Marek, S. Comparison of the Viscous Liquids Spraying by the Oig and the Oil Configurations of an Effervescent Atomizer at Low Inlet Pressures. J. Mech. Eng. 2016, 66, 53–64. [Google Scholar] [CrossRef]
- Benjamin, M.A.; Jensen, R.J.; Arienti, M. Review of atomization: Current knowledge and future requirements for propulsion combustors. At. Sprays 2010, 20, 485–512. [Google Scholar] [CrossRef]
- Rayleigh, L. On the Instability of Jets. Proc. Lond. Math. Soc. 1878, 10, 4–13. [Google Scholar] [CrossRef]
- Marmottant, P.; Villermaux, E. On spray formation. J. Fluid Mech. 2004, 498, 73–111. [Google Scholar] [CrossRef]
- Faeth, G.M. Liquid Atomization in Multiphase Flows: A Review. In Proceedings of the 30th AIAA Fluid Dynamics Conference, Norfolk, VA, USA, 28 June–1 July 1999; pp. 1–20. [Google Scholar] [CrossRef]
- Santos, D.; Mauricio, A.C.; Sencadas, V.; Santos, J.D.; Fernandez, M.H.; Gomes, P.S. Spray Dryin—An Overview. In Biomaterials—Physics and Chemistry—New Edition; IntechOpen: London, UK, 2017; Chapter 2; pp. 9–35. [Google Scholar] [CrossRef]
- Gelfand, B. Droplet breakup phenomena in flows with velocity lag. Prog. Energy Combust. Sci. 1996, 22, 201–265. [Google Scholar] [CrossRef]
- Kennedy, J.B.; Roberts, J. Rain Ingestion in a Gas Turbine Engine. In Proceedings of the 4th ILASS Meeting, Hartford, CT, USA, 21–23 May 1990. [Google Scholar]
- Reitz, R.D.; Bracco, F.V. Mechanism of Breakup of Round Liquid Jets. In Encyclopedia of Fluid Mechanics; Gulf Publications: Houston, TX, USA, 1986; Volume 3, pp. 223–249. [Google Scholar]
- Liu, Z.; Reitz, R. An analysis of the distortion and breakup mechanisms of high-speed liquid drops. Int. J. Multiph. Flow 1997, 23, 631–650. [Google Scholar] [CrossRef]
- Nicholls, J.A.; Ranger, A.A. Aerodynamic shattering of liquid drops. AIAA J. 1969, 7, 285–290. [Google Scholar] [CrossRef]
- Reinecke, W.G.; Waldman, G.D. A Study of Drop Breakup Behind Strong Shocks with Applications to Flight. AVCO Report AVSD-0110–70–77. 1970. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/871218.pdf (accessed on 12 March 2021).
- Liu, Z.; Hwang, S.S.; Reitz, R.D. Breakup mechanisms and drag coefficients of high-speed vaporizing liquid drops. At. Sprays 1996, 6, 353–376. [Google Scholar] [CrossRef]
- Godavarthi, V.; Dhivyaraja, K.; Sujith, R.I.; Panchagnula, M.V. Analysis and classification of droplet characteristics from atomizers using multifractal analysis. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Lefebvre, A.H.; McDonell, V.G. Atomization and Sprays; CRC Press: Boca Raton, FL, USA, 2017; Available online: https://www.routledge.com/Atomization-and-Sprays/Lefebvre-McDonell/p/book/9781498736251 (accessed on 14 March 2021).
- Kooij, S.; Astefanei, A.; Corthals, G.L.; Bonn, D. Size distributions of droplets produced by ultrasonic nebulizers. Sci. Rep. 2019, 9, 6128. [Google Scholar] [CrossRef]
- Lefebvre, A.H. Airblast atomization. Prog. Energy Combust. Sci. 1980, 6, 233–261. [Google Scholar] [CrossRef]
- Faeth, G.; Hsiang, L.-P.; Wu, P.-K. Structure and breakup properties of sprays. Int. J. Multiph. Flow 1995, 21, 99–127. [Google Scholar] [CrossRef]
- Desjardins, O.; McCaslin, J.; Owkes, M.; Brady, P. Direct numerical and large-eddy simulation of primary atomization in complex geometries. At. Sprays 2013, 23, 1001–1048. [Google Scholar] [CrossRef]
- Ramamurthi, K.; Tharakan, T.J. Flow Transition in Swirled Liquid Sheets. AIAA J. 1998, 36, 420–427. [Google Scholar] [CrossRef]
- Ashgriz, N. Handbook of Atomization and Sprays—Theory and Applications; Springer: Toronto, ON, Canada, 2011; Chapters 1 & 3; Available online: https://www.springer.com/gp/book/9781441972637 (accessed on 5 March 2021).
- McCarthy, M.; Molloy, N. Review of stability of liquid jets and the influence of nozzle design. Chem. Eng. J. 1974, 7, 1–20. [Google Scholar] [CrossRef]
- Bazilevskii, A.V.; Rozhkov, A.N. Dynamics of capillary breakup of elastic jets. Fluid Dyn. 2014, 49, 827–843. [Google Scholar] [CrossRef]
- Mashayek, F.; Ashgriz, N. Instability of liquid coatings on cylindrical surfaces. Phys. Fluids 1995, 7, 2143–2153. [Google Scholar] [CrossRef]
- Donnelly, R.J.; Glaberson, W. Experiments on the Capillary Instability of a Jet. Proc. R. Soc. Lond. A 1966, 209, 547–556. [Google Scholar] [CrossRef]
- Goedde, E.F.; Yuen, M.C. Experiments on liquid jet instability. J. Fluid Mech. 1970, 40, 495–511. [Google Scholar] [CrossRef]
- Vassallo, P.; Ashgriz, N. Satellite formation and merging in liquid jet breakup. Proc. R. Soc. Lond. Ser. A 1991, 433, 269–286. [Google Scholar]
- Weber, C. To the disintegration of a jet of liquid. ZAMM J. Appl. Math. Mech. 1931, 11, 136–154. [Google Scholar] [CrossRef]
- Bogy, D.B. Drop Formation in a Circular Liquid Jet. Annu. Rev. Fluid Mech. 1979, 11, 207–228. [Google Scholar] [CrossRef]
- Sirignano, W.; Mehring, C. Review of theory of distortion and disintegration of liquid streams. Prog. Energy Combust. Sci. 2000, 26, 609–655. [Google Scholar] [CrossRef]
- Michell, J.H. On the Theory of Free Stream Lines. Philos. Trans. R. Soc. A. 1890, 181, 389–431. Available online: www.jstor.org/stable/90572 (accessed on 15 March 2021).
- Hagerty, W.W.; Shea, J.F. A Study of the Stability of Plane Fluid Sheets. J. Appl. Mech. 1955, 22, 509–514. [Google Scholar]
- Rangel, R.H.; Sirignano, W.A. The Linear and Nonlinear Shear Instability of a Fluid Sheet. Phys. Fluids 1999, 3, 2392–2400. [Google Scholar] [CrossRef]
- Dombrowski, N.; Fraser, R.P. A Photographic Investigation into the Disintegration of Liquid Sheets. Phil. Trans. 1954, 247, 101–130. [Google Scholar] [CrossRef]
- Dombrowski, N.; Hasson, D.; Ward, D. Some aspects of liquid flow through fan spray nozzles. Chem. Eng. Sci. 1960, 12, 35–50. [Google Scholar] [CrossRef]
- Dombrowski, N.; Hooper, P. The effect of ambient density on drop formation in sprays. Chem. Eng. Sci. 1962, 17, 291–305. [Google Scholar] [CrossRef]
- Senecal, P.; Schmidt, D.; Nouar, I.; Rutland, C.; Reitz, R.; Corradini, M. Modeling high-speed viscous liquid sheet atomization. Int. J. Multiph. Flow 1999, 25, 1073–1097. [Google Scholar] [CrossRef]
- Vasilyev, A.Y.; Domrina, E.S.; Kaufman, S.V.; Maiorova, A.I. Classification of Atomization Devices. J. Phys. Conf. Ser. 2019, 1359, 012131. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, S.P. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Hoboken, NJ, USA, 2015; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118863985 (accessed on 16 March 2021).
- Barbosa-Canovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C. Handbook of Drying for Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2017; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118930526 (accessed on 8 March 2021).
- Kooij, S.; Sijs, R.; Denn, M.M.; Villermaux, E.; Bonn, D. What Determines the Drop Size in Sprays? Phys. Rev. X 2018, 8, 031019. [Google Scholar] [CrossRef]
- Lake, J.R. The effect of drop size and velocity on the performance of agricultural sprays. Pestic. Sci. 1977, 8, 515–520. [Google Scholar] [CrossRef]
- Kublik, H.; Vidgren, M. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [CrossRef]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Le Brun, P.P.H.; de Boer, A.H.; Heijerman, H.G.M.; Frijlink, H.W. A Review of the Technical Aspects of Drug Nebulization. Pharm. World Sci. 2000, 22, 75–81. [Google Scholar] [CrossRef]
- Squire, H.B. Investigation of the instability of a moving liquid film. Br. J. Appl. Phys. 1953, 4, 167–169. [Google Scholar] [CrossRef]
- Chen, B.; Gao, D.; Li, Y.; Chen, C.; Yuan, X.; Wang, Z.; Sun, P. Investigation of the droplet characteristics and size distribution during the collaborative atomization process of a twin-fluid nozzle. Int. J. Adv. Manuf. Technol. 2020, 107, 1625–1639. [Google Scholar] [CrossRef]
- Dalmoro, A.; Barba, A.A.; d’Amore, M. Analysis of Size Correlations for Microdroplets Produced by Ultrasonic Atomization. Sci. World J. 2013, 1–8. [Google Scholar] [CrossRef]
- Poozesh, S.; Akafuah, N.K.; Campbell, H.R.; Bashiri, F.; Saito, K. Experimental and Mathematical Tools to Predict Droplet Size and Velocity Distribution for a Two-Fluid Nozzle. Fluids 2020, 5, 231. [Google Scholar] [CrossRef]
- Pauletti, G.M.; Gangwar, S.; Knipp, G.T.; Nerurkar, M.M.; Okumu, F.W.; Tamura, K.; Siahaan, T.J.; Borchardt, R.T. Structural requirements for intestinal absorption of peptide drugs. J. Control. Release 1996, 41, 3–17. [Google Scholar] [CrossRef]
- Ciach, T.; Diaz, L.; Ijssel, E.V.D.; Marijnissen, J.C.M. Application of Electro Hydro Dynamic Atomisation in the Production of Engineered Drug Particles. Optim. Aerosol Drug Deliv. 2003, 189–204. [Google Scholar] [CrossRef]
- Adjei, L.; Gupta, P. Inhalation Delivery of Therapeutic Peptides and Proteins. Pharm. Res. 1997, 15, 301–313. [Google Scholar]
- Banker, G.S.; Rodhes, C.T. Modern Pharmaceutics; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Gomez, A. The electrospray and its application to targeted drug inhalation. Respir. Care 2002, 47, 1419–1431. [Google Scholar] [PubMed]
- Tobyn, M.; Staniforth, J.N.; Morton, D.; Harmer, Q.; Newton, M.E. Active and Intelligent Inhaler Device Development. Int. J. Pharm. 2004, 277, 31–37. [Google Scholar] [CrossRef]
- Shrewsbury, S.B.; Cook, R.O.; Taylor, G.; Edwards, C.; Ramadan, N.M. Safety and Pharmacokinetics of Dihydroergotamine Mesylate Administered Via a Novel (TempoTM) Inhaler. Headache 2007, 48, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.; Yang, H.; Wilson, T.; Blasetti, K.; Di Ninno, V.; Schnell, G.; Wong, J.P. Aerosol delivery of liposome-encapsulated ciprofloxacin: Aerosol characterization and efficacy against Francisella tularensis infection in mice. Antimicrob. Agents Chemother. 1997, 41, 1288–1292. [Google Scholar] [CrossRef]
- Hettiarachchi, K.; Talu, E.; Longo, M.L.; Dayton, P.A.; Lee, A.P. On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging. Lab Chip 2007, 7, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Heyder, J. Deposition of Inhaled Particles in the Human Respiratory Tract and Consequences for Regional Targeting in Respiratory Drug Delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Davoodi, P.; Feng, F.; Xu, Q.; Yan, W.-C.; Tong, Y.W.; Srinivasan, M.; Sharma, V.K.; Wang, C.-H. Coaxial electrohydrodynamic atomization: Microparticles for drug delivery applications. J. Control. Release 2015, 205, 70–82. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.; Woodruff, M. Electrospraying of polymers with therapeutic molecules: State of the art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Lee, Y.H.; Mei, F.; Bai, M.Y.; Zhao, S.; Chen, D.R. Release Profile Characteristics of Biodegradable-polymer-coated Drug Par-ticles Fabricated by Dual-Capillary Electrospray. J. Control Release 2010, 145, 58–65. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Bai, M.-Y.; Chen, D.-R. Multidrug encapsulation by coaxial tri-capillary electrospray. Colloids Surf. B 2011, 82, 104–110. [Google Scholar] [CrossRef]
- Nie, H.; Dong, Z.; Arifin, D.Y.; Hu, Y.; Wang, C.-H. Core/shell microspheres via coaxial electrohydrodynamic atomization for sequential and parallel release of drugs. J. Biomed. Mater. Res. Part A 2010, 95, 709–716. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Jiang, P.; Hong, T. Coaxial Electrohydrodynamic Atomization for the Production of Drug-loaded Micro/Nanoparticles. Micromachines 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Jitendra, M.A.; Dutta, S.; Jitendra, M.A. Application of Electrospray Atomization (EA): A Review Article. Chem. Sci. Rev. Lett. 2018, 7, 745–750. Available online: https://chesci.com/wp-content/uploads/2018/09/V7i27_6_CS172049051_Samit_745-750.pdf (accessed on 9 March 2021).
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Raza, Z.A.; Nazir, A. Current applications of electrospun polymeric nanofibers in cancer therapy. Mater. Sci. Eng. C 2019, 97, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.Z.; Chai, L.C.; Ang, B.C.; Ong, B.H. Enhancing the Antibacterial Performance of Titanium Dioxide Nanofibers by Coating with Silver Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 5743–5751. [Google Scholar] [CrossRef]
- Deitzel, J.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Gilbert, W. Gilbert’s Book on Magnets, De Magnete, Londini, Anno MDC. 1600. Available online: https://www.sciencephoto.com/media/765107/view/gilbert-s-book-on-magnets-1600 (accessed on 18 March 2021).
- Taylor, G. The Force Exerted by an Electric Field on a Long Cylindrical Condutor. Proc. Royal Soc. A 1966, 291, 145–158. [Google Scholar] [CrossRef]
- Grayson, M.A. John Bennett Fenn: A Curious Road to the Prize. J. Am. Soc. Mass Spectrom. 2011, 22, 1301–1308. [Google Scholar] [CrossRef][Green Version]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic Atomization: A Two-decade Effort to Produce and Process Micro-/Nanoparticulate Materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef]
- Xie, J.; Lim, L.K.; Phua, Y.; Hua, J.; Wang, C.-H. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Colloid Interface Sci. 2006, 302, 103–112. [Google Scholar] [CrossRef]
- Xie, J.; Ng, W.J.; Lee, L.Y.; Wang, C.H. Encapsulation of Protein Drugs in Biodegradable Microparticles by Coaxial Electrospray. J. Colloid Interface Sci. 2008, 317, 469–476. [Google Scholar] [CrossRef]
- Hernandez, J.A.T.; Chavez, P.I.T.; Wong, B.R.; Chu, A.R.; Jatomea, M.P.; Urbina, C.G.B.; Vazquez, N.A.R.; Felix, F.R. Micro and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Ré, M.-I. Formulating Drug Delivery Systems by Spray Drying. Dry. Technol. 2006, 24, 433–446. [Google Scholar] [CrossRef]
- Peltonen, L.; Valo, H.; Kolakovic, R.; Laaksonen, T.; Hirvonen, J. Electrospraying, Spary Drying and Related Techniques for Production and Formulation of Drug Nanoparticles. Expert Opin. Drug Deliv. 2010, 7, 705–719. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur. J. Pharm. Sci. 2005, 24, 67–75. [Google Scholar] [CrossRef]
- Hammond, P.T. Building biomedical materials layer-by-layer. Mater. Today 2012, 15, 196–206. [Google Scholar] [CrossRef]
- Wang, J.; Jansen, J.A.; Yang, F. Electrospraying: Possibilities and Challenges of Engineering Carriers for Biomedical Applications—A Mini Review. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Gorty, A.; Barringer, S. Electrohydrodynamic spraying of chocolate. J. Food Process. Preserv. 2011, 35, 542–549. [Google Scholar] [CrossRef]
- Marthina, K.; Barringer, S.A. Confectionery Coating with an Electrohydrodynamic (EHD) System. J. Food Sci. 2011, 77, E26–E31. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lee, T.; Wang, C.-H. Fabrication of monodispersed Taxol-loaded particles using electrohydrodynamic atomization. J. Control. Release 2005, 102, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Naraharisetti, P.K.; Ong, B.Y.S.; Xie, J.W.; Lee, T.K.Y.; Wang, C.H.; Sahinidis, N.V. In vivo Performance of Implantable Bio-degradable Preparations Delivering Paclitaxel and Etanidazole for the Treatment of Glioma. Biomaterials 2007, 28, 886–894. [Google Scholar] [CrossRef]
- Gasperini, L.; Maniglio, D.; Migliaresi, C. Microencapsulation of cells in alginate through an electrohydrodynamic process. J. Bioact. Compat. Polym. 2013, 28, 413–425. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C.-H. Electrospray in the dripping mode for cell microencapsulation. J. Colloid Interface Sci. 2007, 312, 247–255. [Google Scholar] [CrossRef]
- Matsushima, Y.; Yamazaki, T.; Maeda, K.; Suzuki, T. Fabrication of SnO2 Particle-Layers using the Electrospray Method and Gas Sensing Properties for H2. J. Electroceram. 2004, 13, 765–770. [Google Scholar] [CrossRef]
- Cusano, A.; Consales, M.; Pisco, M.; Pilla, P.; Cutolo, A.; Buosciolo, A.; Viter, R.; Smyntyna, V.; Giordano, M. Optochemical sensor for water monitoring based on SnO2 particle layer deposited onto optical fibers by the electrospray pyrolysis method. Appl. Phys. Lett. 2006, 89, 111103. [Google Scholar] [CrossRef]
- Foroutan, F.; Jokerst, J.V.; Gambhir, S.S.; Vermesh, O.; Kim, H.-W.; Knowles, J.C. Sol–Gel Synthesis and Electrospraying of Biodegradable (P2O5)55–(CaO)30–(Na2O)15 Glass Nanospheres as a Transient Contrast Agent for Ultrasound Stem Cell Imaging. ACS Nano 2015, 9, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yao, Z.-C.; Ding, Q.; Choi, J.; Ahmad, Z.; Chang, M.-W.; Li, J.-S. Tri-Needle Coaxial Electrospray Engineering of Magnetic Polymer Yolk–Shell Particles Possessing Dual-Imaging Modality, Multiagent Compartments, and Trigger Release Potential. ACS Appl. Mater. Interfaces 2017, 9, 21485–21495. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, F.; Ge, P.; Mao, Y.; Wang, L. Anti-acute thrombogenic surface using coaxial electrospraying coating for vascular graft application. Mater. Lett. 2017, 205, 15–19. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Varshosaz, J.; Mhaisalkar, P.S.; Ramakrishna, S. Electrosprayed Montelukast/poly (lac-tic-co-glycolic acid) Particle Based Coating: A New Therapeutic Approach Towards the Prevention of Instent Restenosis. Acta Biomater. 2016, 42, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Zhang, X.H.; Xu, M.M.; Liu, F.; Ma, Q.; Cai, Q.; Deng, X. PLGA/PDLLA Core-Shell Submicron Spheres Se-quential Release System: Preparation, Characterization and Promotion of Bone Regeneration In vitro and In vivo. Chem. Eng. J. 2015, 273, 490–501. [Google Scholar] [CrossRef]
- Venkatesan, Y.C.; Kumar, T.S.S.; Raj, D.K.; Kumary, T.V. Osteogenic apatite particles by sol-gel assisted electrospraying. J. Biomed. Mater. Res. Part B 2018, 106, 1941–1954. [Google Scholar] [CrossRef]
- Steipel, R.T.; Gallovic, M.D.; Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Electrospray for generation of drug delivery and vaccine particles applied in vitro and in vivo. Mater. Sci. Eng. C 2019, 105, 110070. [Google Scholar] [CrossRef]
- Suksamran, T.; Ngawhirunpat, T.; Rojanarata, T.; Sajomsang, W.; Pitaksuteepong, T.; Opanasopit, P. Methylated N-(4-N,N-dimethylaminocinnamyl) chitosan-coated electrospray OVA-loaded microparticles for oral vaccination. Int. J. Pharm. 2013, 448, 19–27. [Google Scholar] [CrossRef]
- Doavi, T.; Mousavi, S.L.; Kamali, M.; Amani, J.; Ramandi, M.F. Chitosan-Based Intranasal Vaccine against Escherichia coli O157:H7. Iran. Biomed. J. 2016, 20, 97–108. [Google Scholar]
| Breakup Systems | Weber Number Range | Description | Ref |
|---|---|---|---|
| Bag breakup | 12–80 | The region at which there is no movement in a droplet will be puffed out into a narrow void (bag) which is anchored at the middle; holes will be developed in this void that shrink and split into small drops | [17,18] |
| Stretching-thinning breakup | 80–300 | The droplets are flattened and stretched and the middle of these stretched droplets are strained into thin stream by the force exerted on liquid by the air; this stream split into thin ligaments which breaks into small drops | [19,20] |
| Catastrophic breakup | >300 | Same as stretching-thinning system; the edges of the droplets are strained into thin liquid stream by the force of high velocity air; also causes surface instabilities and then breaks up into fine jet of drops | [21,22] |
| Types | Energy Source | Mechanism | Class of Spray | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Hydraulic | Fluid pressure | Pressure exerted on the fluid drives it through the nozzle to generate fluid sheets with high velocity and leading its disruption to fragments and then to droplets by friction between liquid and air | Non-uniform, rough | Economical, utilize small rooms for drying | Not relevent for viscous fluids, wide range of droplet size dispersion |
| Pneumatic | Air pressure | Fluid at low velocity passing through the nozzle is surrounded by high velocity flow of air, boosting friction between two medium causing disruption of fluid sheet | Heterogenous, average roughness | Uniform droplets, applicable for viscous fluids, superior productivity | Expensive, ensuing instability |
| Rotary | Centrifugal force | Atomizer has a spinning disc at the center to which the fluid is introduced through the nozzle; centrifugal force takes the fluid to the margin of the disc and flip off the boundary setting up ligaments that then breaks into droplets | Heterogenous | Uniform droplets, no clogging of atomizer, superior productivity | Expensive, not relevant for viscous fluids, requires larger rooms for drying |
| Electrostatic | Electric charge | An electric field applied between atomizer and workpiece to make it conductive; fluid passed through the electric field and the repulsive force disrupt the fluid into droplets and is gathered at the workpiece | Finer, homogenous | Fine & uniform droplets, no clogging of atomizer | Varying film thickness due to diverse electrostatic excitation in the core & shell of the system |
| Ultrasonic | Electromechanical device | Fluid is passed through a vibrating electromechanical device causing the disruption of the fluid into droplets | Very fine and homogenous | Control spray size by altering the vibrational frequency | Not relevant for viscous fluids, restriction in scaling up of the system |
| Types | Applications | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Electro hydrodynamic atomization | Bodywide delivery of hypoglycemic agents | Can control the size of the drops | Poor yield, usage of electric field | [65] |
| Dry powder nebulizer | Delivery of hypochloride salt of apomorphine | Transferable, stable, free of chemical substances to produce pressurized air | Larger drop size yield, restricted for powdered medicines, accumulation in lungs | [66] |
| Pressurized metered dose inhalers | Delivery of Flovent, Migranal | Transferable, manageable | Uses chemicals to produce pressurized air, accumulation in upper lung | [67] |
| Liquid atomization | Delivery of Cetraxal, beclometasone dipropionate, Rubex | Drug processing is not essential | Comparatively larger drops, can degrade the drug, bulky | [68] |
| Techniques | Merits | Challenges | Ref |
|---|---|---|---|
| Electrospray | One step process; confined particle size that is micro and nanoparticles; polymers with higher molecular weights can be employed; surfactant free; utilizes less solvents; drugs that are slightly soluble in water can be processed | Low yield; sometimes requires cross-linking factors; advancing the technique for bulk manufacturing is not possible | [89] |
| Spray drying | Develops inorganic polymeric microparticles; scale-up production | Uses higher temperature gases as transporter; denatures heat sensitive substances | [90,91] |
| Nanoprecipitation | Easy procedure; nanoparticles generated by desorption; efficiently encloses hydrophobic drugs; surfactant free | Utilizes substantial amount of solvent; drug loaded in the particles are low | [92] |
| Emulsion solvent vaporization | Adaptable technique; generates diverse biomolecular particles | Only uses low molar mass polymers; not free of surfactants and solvents; not a single step technique; wide range of particle size | [89] |
| Sheet by sheet fabrication | Accurate; multi-tiered particles; uniform sheet thickness; regulated drug delivery | Tiresome and lengthy process; advancing the technique for bulk manufacturing is not possible | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohandas, A.; Luo, H.; Ramakrishna, S. An Overview on Atomization and Its Drug Delivery and Biomedical Applications. Appl. Sci. 2021, 11, 5173. https://doi.org/10.3390/app11115173
Mohandas A, Luo H, Ramakrishna S. An Overview on Atomization and Its Drug Delivery and Biomedical Applications. Applied Sciences. 2021; 11(11):5173. https://doi.org/10.3390/app11115173
Chicago/Turabian StyleMohandas, Anu, Hongrong Luo, and Seeram Ramakrishna. 2021. "An Overview on Atomization and Its Drug Delivery and Biomedical Applications" Applied Sciences 11, no. 11: 5173. https://doi.org/10.3390/app11115173
APA StyleMohandas, A., Luo, H., & Ramakrishna, S. (2021). An Overview on Atomization and Its Drug Delivery and Biomedical Applications. Applied Sciences, 11(11), 5173. https://doi.org/10.3390/app11115173







