Light Scattering and Absorption Complementarities to Neutron Scattering: In Situ FTIR and DLS Techniques at the High-Intensity and Extended Q-Range SANS Diffractometer KWS-2
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. In Situ FTIR
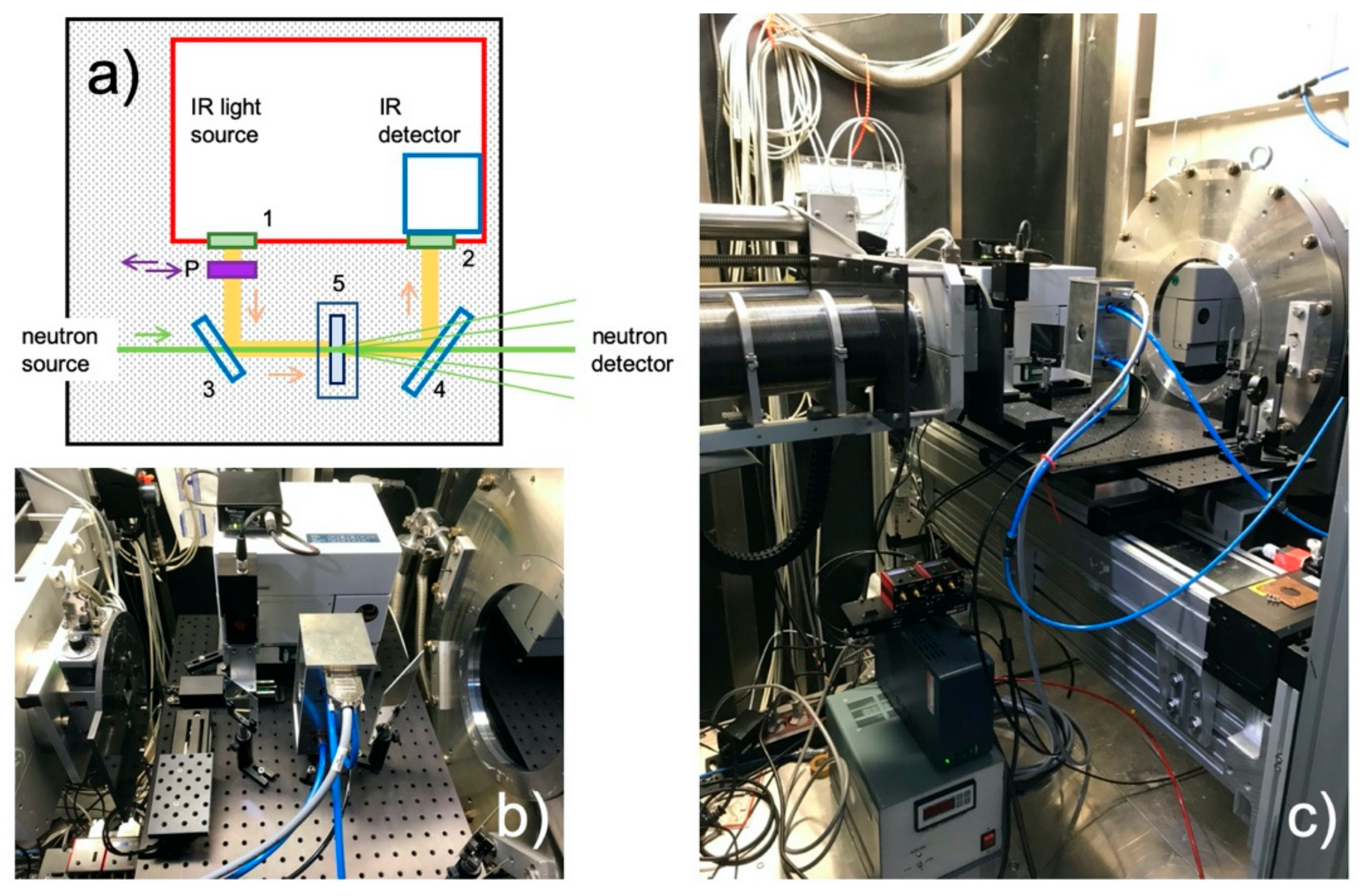
3.1.1. FTIR Sample Holder and Neutron Background
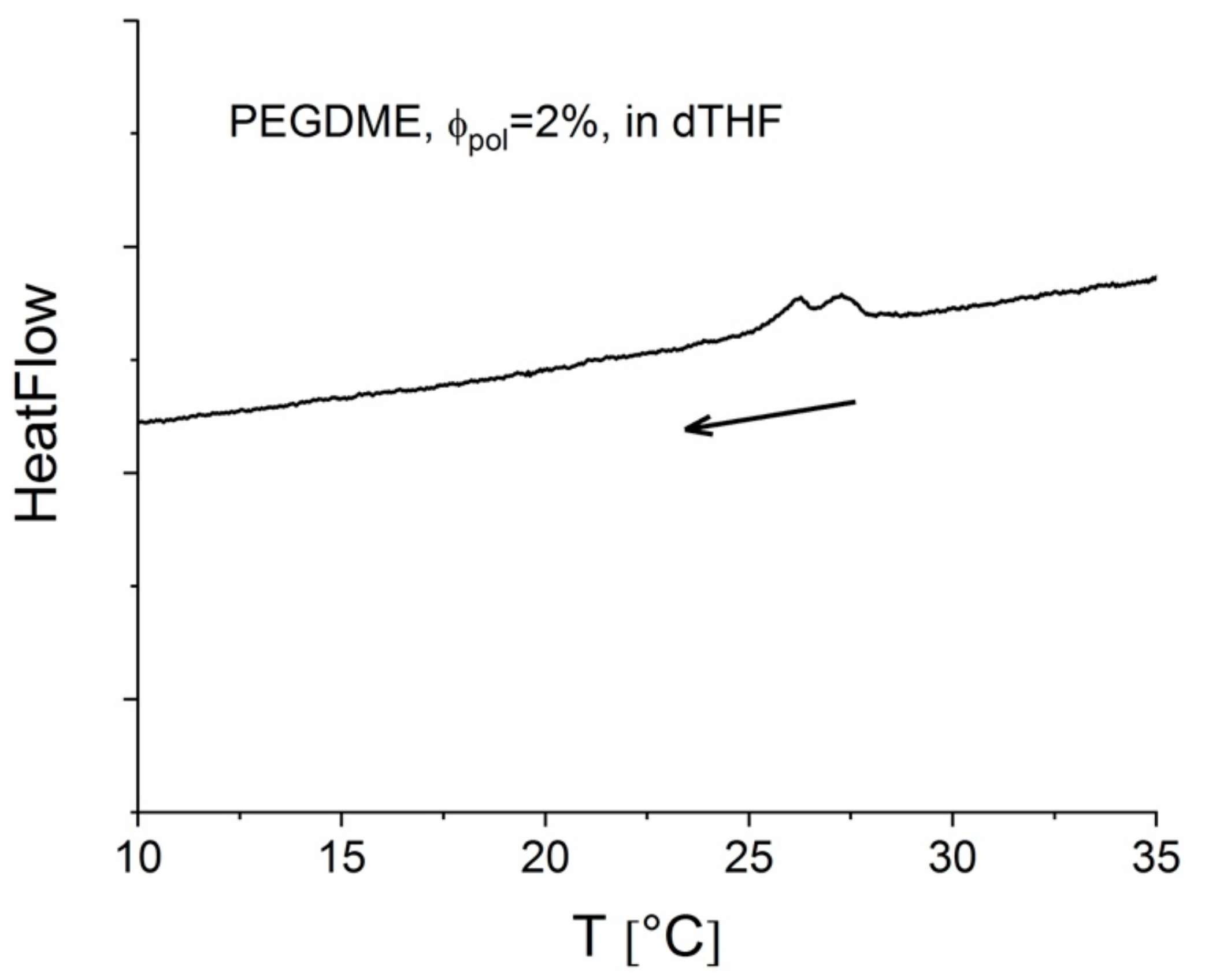
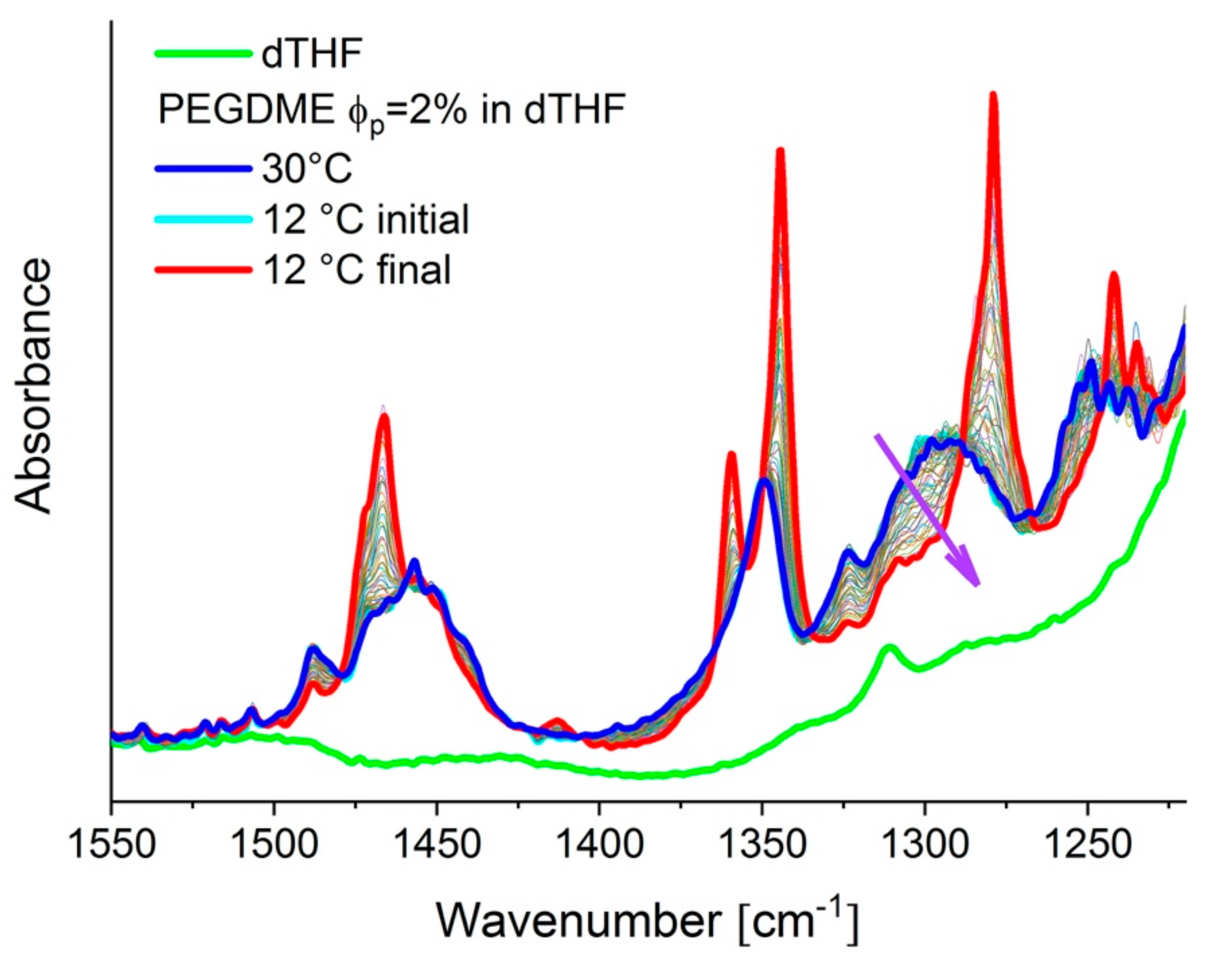
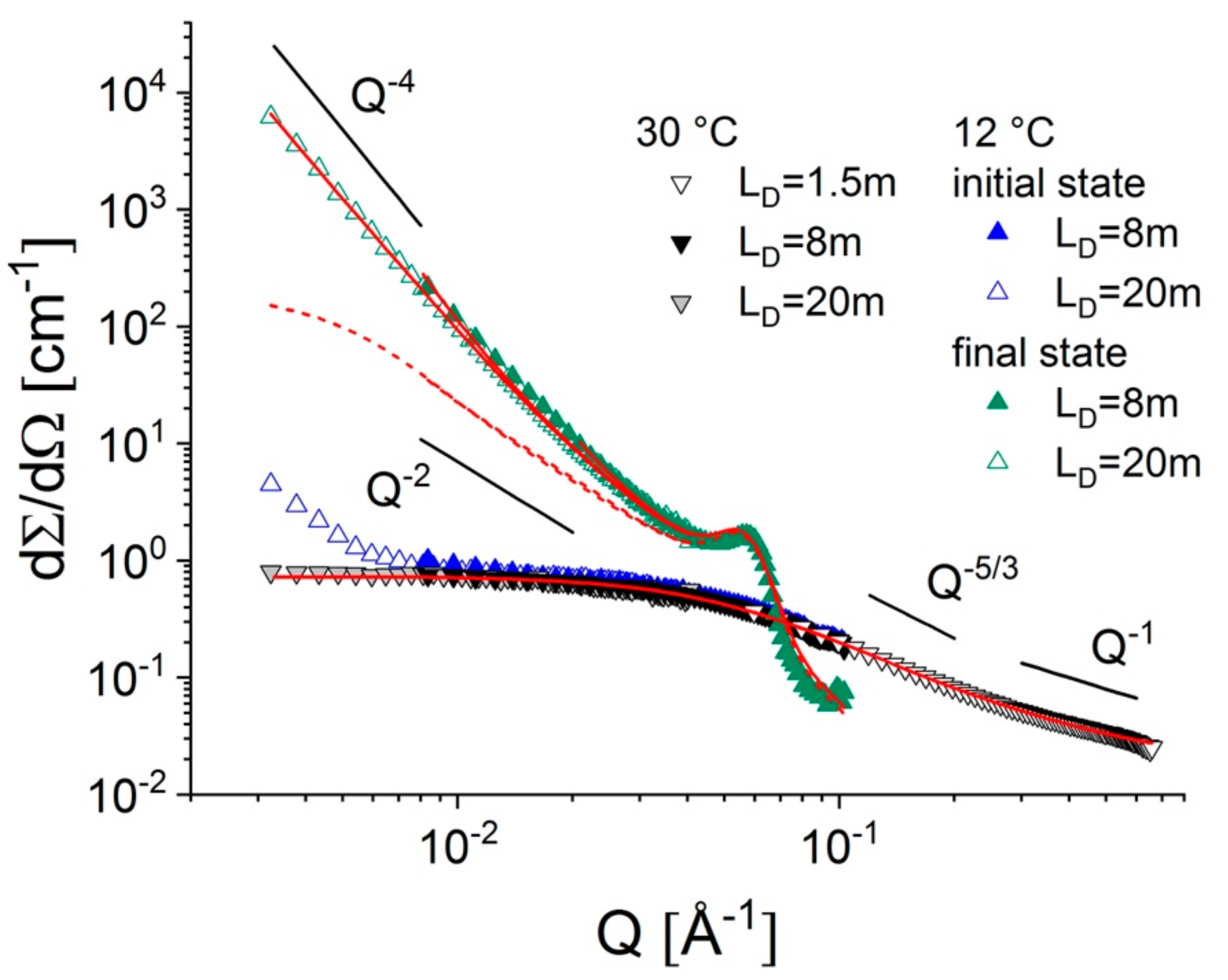
3.1.2. Simultaneous FTIR and SANS on PEGDME in Solution
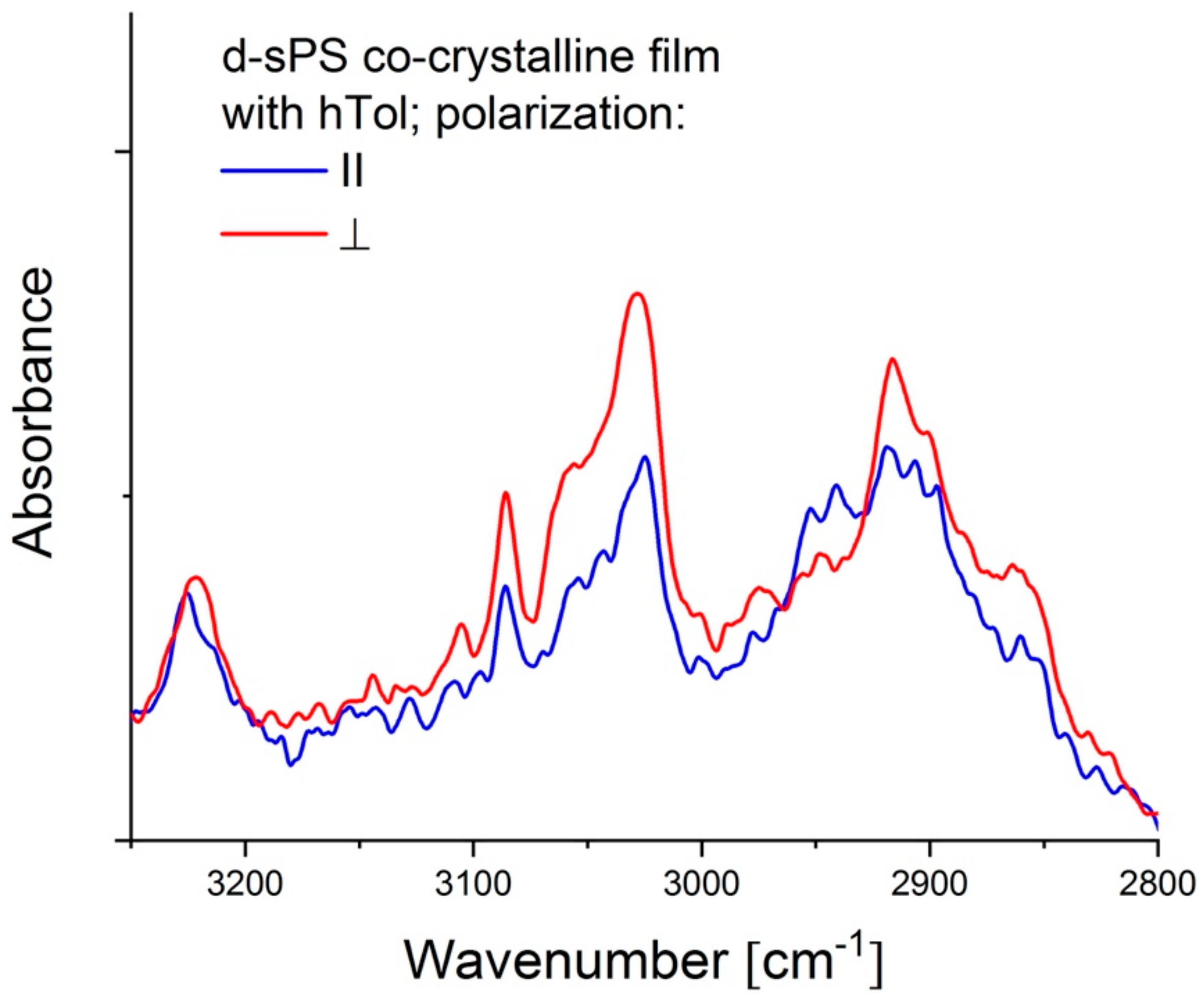
3.1.3. Polarized FTIR Measurements
3.2. In Situ Dynamic Light Scattering
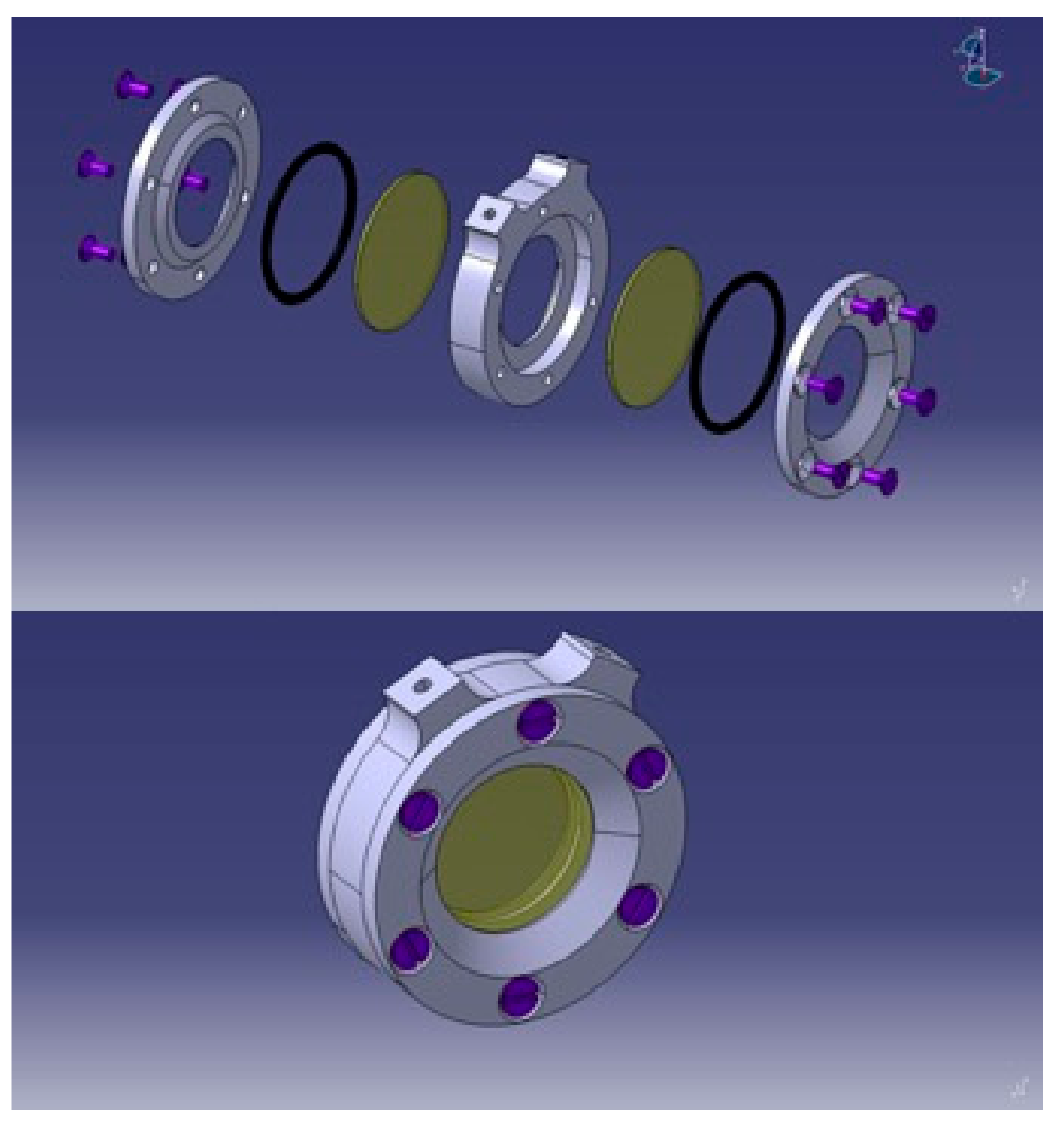
3.2.1. DLS Setup
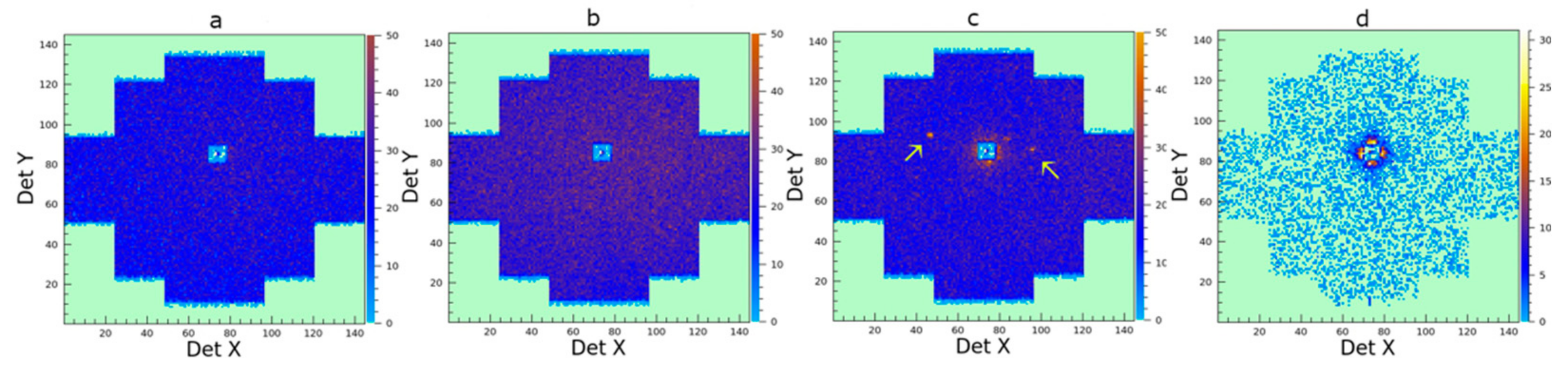
3.2.2. Proof of Concept: Investigation of Silica Nanospheres
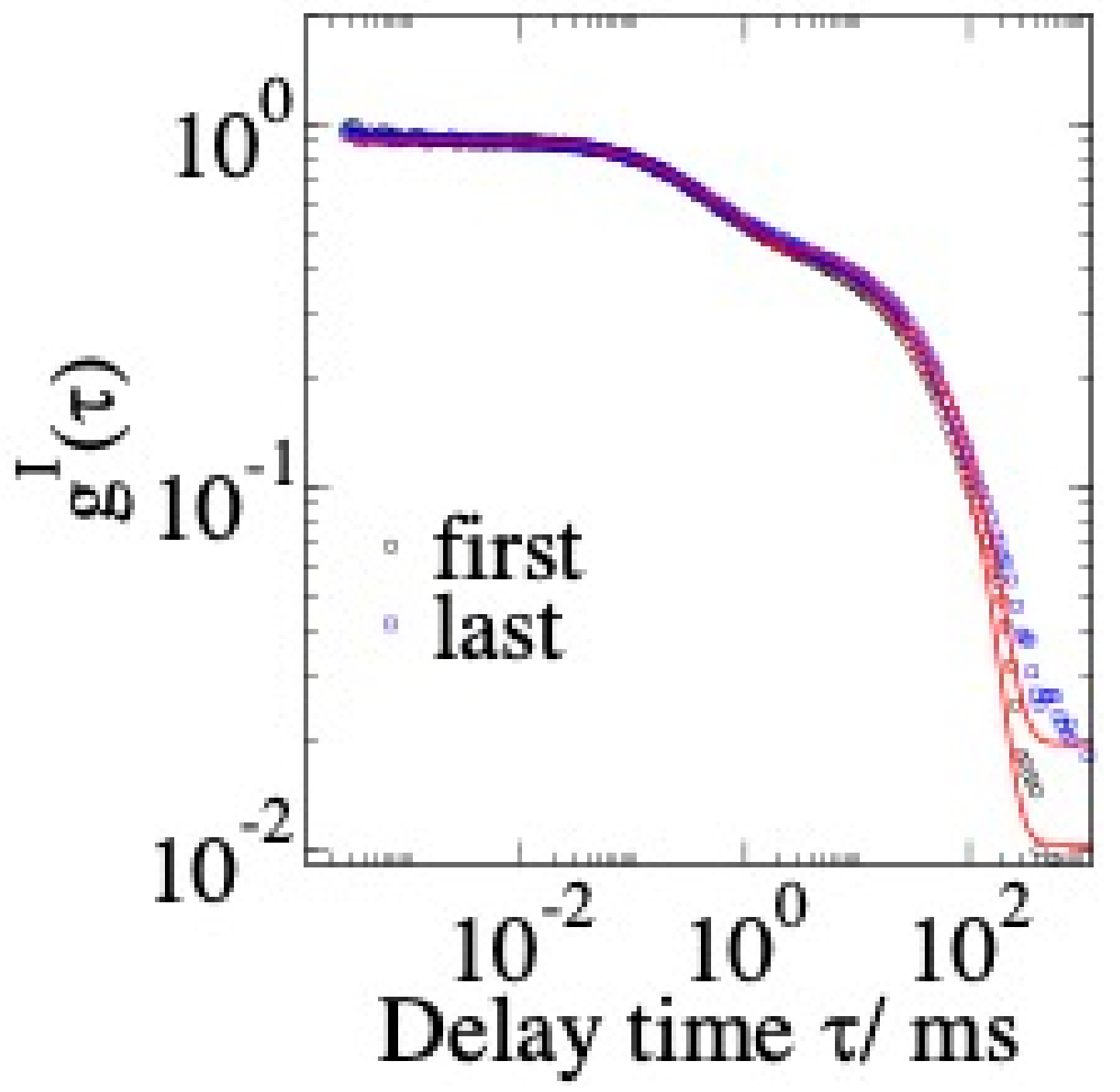
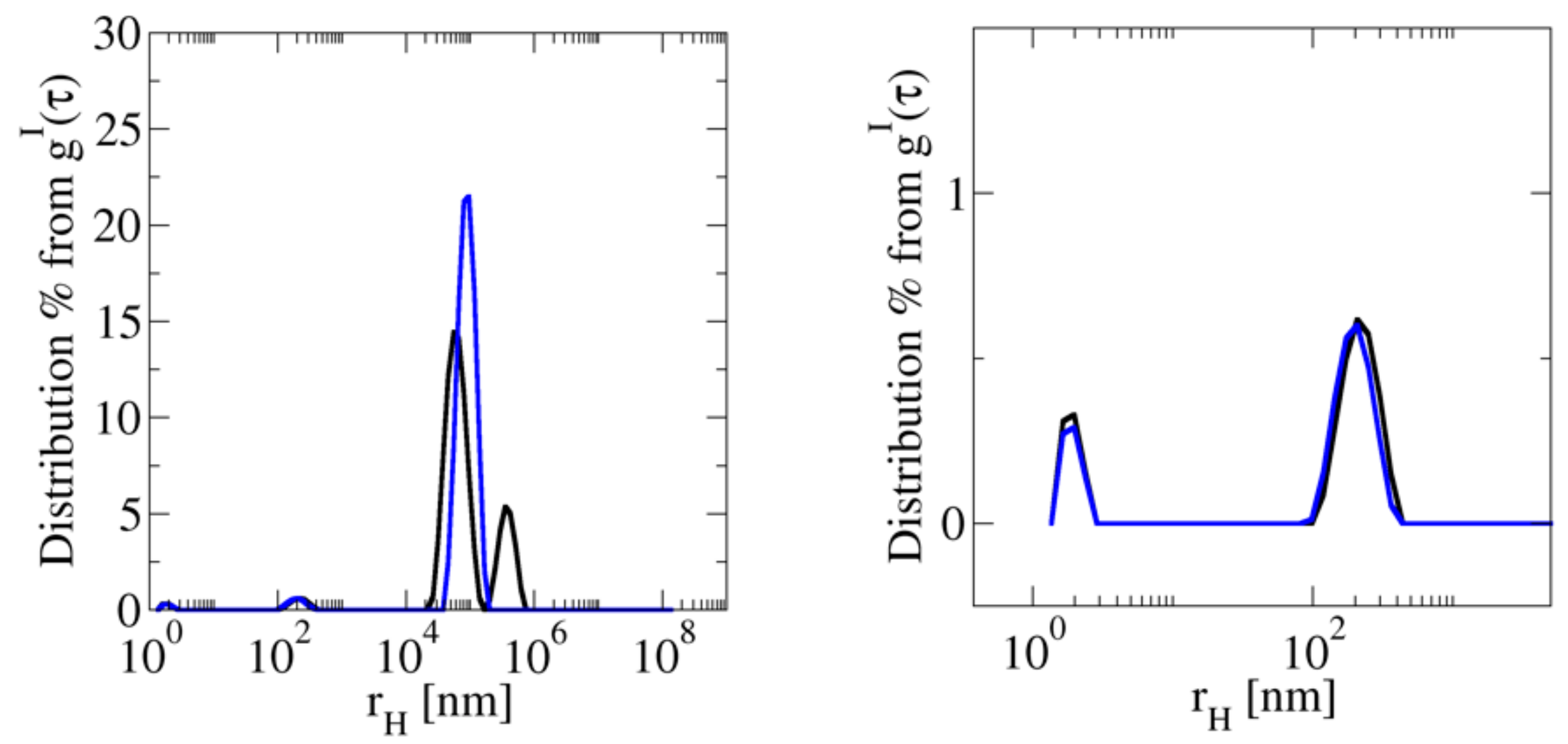
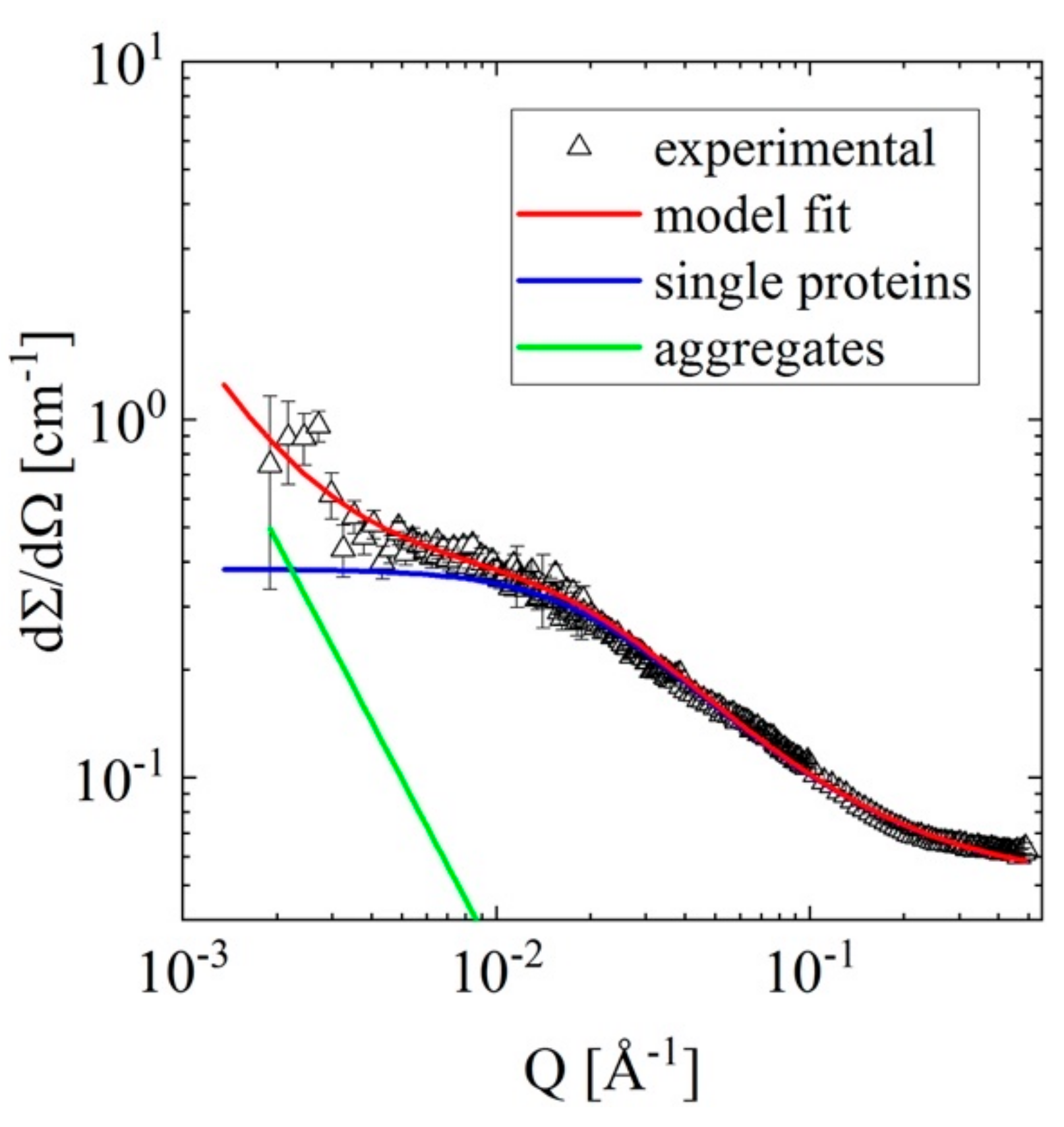
3.2.3. A Typical Sample: Apomyoglobin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radulescu, A.; Pipich, V.; Frielinghaus, H.; Appavou, M.-S. KWS-2, the high intensity/wide Q-range small-angle neutron diffractometer for soft-matter and biology at FRM II. J. Phys. Conf. Ser. 2012, 351, 012026. [Google Scholar] [CrossRef] [Green Version]
- Bras, W.; Derbyshire, G.E.; Devine, A.; Clark, S.M.; Cooke, J.; Komanschek, B.E.; Ryan, A.J. The Combination of Thermal Analysis and Time-Resolved X-ray Techniques: A Powerful Method for Materials Characterization. J. Appl. Crystallogr. 1995, 28, 26–32. [Google Scholar] [CrossRef]
- Bryant, G.K.; Gleeson, H.F.; Ryan, A.J.; Fairclough, J.P.A.; Bogg, D.; Goossens, J.G.P.; Bras, W. Raman spectroscopy combined with small angle X-ray scattering and wide angle X-ray scattering as a tool for the study of phase transitions in polymers. Rev. Sci. Instrum. 1998, 69, 2114. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, K.; Sasaki, S. Structural changes in the ordering process of polymers as studied by an organized combination of the various measurement techniques. Prog. Polym. Sci. 2003, 28, 451–519. [Google Scholar] [CrossRef]
- Hirose, R.; Yoshioka, T.; Yamamoto, H.; Reddy, K.R.; Tahara, D.; Hamada, K.; Tashiro, K. In-house simultaneous collection of small-angle X-ray scattering, wide-angle X-ray diffraction and Raman scattering data from polymeric materials. J. Appl. Crystallogr. 2014, 47, 922–930. [Google Scholar] [CrossRef]
- Mathew, E.; Mirza, A.; Menhart, N. Liquid-chromatography-coupled SAXS for accurate sizing of aggregating proteins. J. Synchrotron Radiat. 2004, 11, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Radulescu, A.; Pipich, V.; Ioffe, A. Quality assessment of neutron delivery system for small-angle neutron scattering diffractometers of the Jülich Centre for Neutron Science at the FRM II. Nucl. Instrum. Methods Phys. Res. A 2012, 689, 1–6. [Google Scholar] [CrossRef]
- Houston, J.E.; Brandl, G.; Drochner, M.; Kemmerling, G.; Engels, R.; Papagiannopoulos, A.; Sarter, M.; Stadler, A.; Radulescu, A. The high-intensity option of the SANS diffractometer KWS-2 at JCNS—Characterization and performance of the new multi-megahertz detection system. J. Appl. Crystallogr. 2018, 51, 323–336. [Google Scholar] [CrossRef]
- Jordan, A.; Jacques, M.; Merrick, C.; Devos, J.; Forsyth, T.; Porcar, L.; Martel, A. SEC-SANS: Size exclusion chromatography combined in-situ with small-angle neutron scattering. J. Appl. Crystallogr. 2016, 49, 2015–2020. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, F.; Seto, N.; Sato, S.; Radulescu, A.; Schiavone, M.M.; Allgaier, J.; Ute, K. Development of a Simultaneous SANS/FTIR Measuring System. Chem. Lett. 2015, 44, 497–499. [Google Scholar] [CrossRef] [Green Version]
- Kohlbrecher, J.; Bollhalder, A.; Vavrin, R. A high pressure cell for small angle neutron scattering up to 500MPa in combination with light scattering to investigate liquid samples. Rev. Sci. Instrum. 2007, 78, 125101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heigl, R.J.; Longo, M.; Stellbrink, J.; Radulescu, A.; Schweins, R.; Schrader, T.E. Crossover from a Linear to a Branched Growth Regime in the Crystallization of Lysozyme. Crystallogr. Growth Des. 2018, 18, 1483–1494. [Google Scholar] [CrossRef]
- Nawroth, T.; Buch, P.; Buch, K.; Langguth, P.; Schweins, R. Liposome Formation from Bile Salt–Lipid Micelles in the Digestion and Drug Delivery Model FaSSIFmod Estimated by Combined Time-Resolved Neutron and Dynamic Light Scattering. Mol. Pharm. 2011, 8, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Angelini, R.; King, S.; Franco, S.; Buratti, E.; Bomboi, F.; Mahmoudi, N.; Corvasce, F.; Scaccia, R.; Church, A.; et al. Apparatus for simultaneous dynamic light scattering–small angle neutron scattering investigations of dynamics and structure in soft matter. Rev. Sci. Instr. 2021, 92, 023907. [Google Scholar] [CrossRef]
- Radulescu, A.; Szekely, N.K.; Appavou, M.S.; Pipich, V.; Kohnke, T.; Ossovyi, V.; Staringer, S.; Schneider, G.J.; Amann, M.; Zhang-Haagen, B.; et al. Studying Soft-matter and Biological Systems over a Wide Length-scale from Nanometer and Micrometer Sizes at the Small-angle Neutron Diffractometer KWS-2. J. Vis. Exp. 2016, 118, e54639. [Google Scholar] [CrossRef] [Green Version]
- Hövelmann, C.H.; Gooßen, S.; Allgaier, J. Scale-Up Procedure for the Efficient Synthesis of Highly Pure Cyclic Poly(ethylene glycol). Macromolecules 2017, 50, 4169–4179. [Google Scholar] [CrossRef]
- Kaneko, F.; Radulescu, A.; Ute, K. Time-resolved SANS studies on guest exchange processes in cocrystals of syndiotactic polystyrene. Polymer 2013, 54, 3145–3149. [Google Scholar] [CrossRef]
- Stadler, A.M.; Koza, M.M.; Fitter, J. Determination of conformational entropy of fully and partially folded conformations of holo- and apomyoglobin. J. Phys. Chem. B 2015, 119, 72. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E. Expasy: The proteomics server for in-depth protein knowledge and analysis. Nucl. Acids Res. 2003, 31, 3784. [Google Scholar] [CrossRef] [Green Version]
- Larkin, P.J. Infrared and Raman Spectroscopy. Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kaneko, F.; Seto, N.; Sato, S.; Radulescu, A.; Schiavone, M.M.; Allgaier, J.; Ute, K. Simultaneous small-angle neutron scattering and Fourier transform infrared spectroscopic measurements on cocrystals of syndiotactic polystyrene with polyethylene glycol dimethyl ethers. J. Appl. Crystallogr. 2016, 49, 1420–1427. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, F.; Kawaguchi, T.; Radulescu, A.; Iwase, H.; Morikawa, T.; Takata, S.; Nishiura, M.; Hou, Z. A new simultaneous measurement system of wide Q-range small angle neutron scattering combined with polarized Fourier transform infrared spectroscopy. Rev. Sci. Instrum. 2019, 90, 093906. [Google Scholar] [CrossRef]
- Kaneko, F.; Radulescu, A.; Iwase, H.; Takata, S.; Nishiura, M.; Hou, Z. Application of Simultaneous Measurement System Combining Wide Q-Range Small-Angle Neutron Scattering and Polarized Fourier Transform Infrared Spectroscopy: Cocrystal of Syndiotactic Polystyrene with Methyl Benzoate. JPS Conf. Proc. 2021, 33, 011076. [Google Scholar]
- Brandl, G.; Felder, C.; Pedersen, B.; Faulhaber, E.; Lenz, A.; Krüger, J. NICOS–The Instrument Control solution at the MLZ. In Proceedings of the 10th International Workshop on Personal Computers and Particle Accelerator Controls, Karlsruhe, Germany, 14–17 October 2014. [Google Scholar]
- Sasaki, T.; Miyazaki, A.; Sugiura, S.; Okada, K. Crystallization of Poly(ethylene oxide) from Solutions of Different Solvents. Polym. J. 2002, 34, 794–800. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Sumita, I.; Tadokoro, H. Structural studies of polyethers. IX. Planar zigzag modification of Poly(ethylene oxide). J. Polym. Sci. A Polym. Phys. 1973, 11, 2113–2122. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kitagawa, K. Microstructure of poly (ethylene oxide) gels dispersed in various organic solvents. Macromol. Symp. 1997, 114, 291–296. [Google Scholar] [CrossRef]
- Yoshihara, T.; Tadokoro, H.; Murahashi, S.J. Normal Vibrations of the Polymer Molecules of Helical Conformation. IV. Polyethylene Oxide and Polyethylene-d4 Oxide. Chem. Phys. 1964, 41, 2902–2911. [Google Scholar] [CrossRef]
- Matsuura, H.; Miyazawa, T.J. Vibrational analysis of molten poly (ethylene glycol). Polym. Sci. A Polym. Phys. 1969, 7, 1735–1744. [Google Scholar] [CrossRef]
- Gines, J.M.; Arias, M.J.; Rabasco, A.M.; Novak, C.; Ruiz-Conde, A.; Sanchez-Soto, P.J. Thermal characterization of polyethylene glycols applied in the pharmaceutical thchnology using differential scanning calorimetry and hot stage microscopy. J. Thermal Anal. 1996, 46, 291–304. [Google Scholar] [CrossRef]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E.J. Molecular Conformation in Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines Their Ability To Resist Protein Adsorption. Phys. Chem. B 1998, 102, 426–435. [Google Scholar] [CrossRef]
- Miyazawa, T.; Fukushima, K.; Ideguchi, Y.J. Molecular vibrations and structure of high polymers. III. Polarized infrared spectra, normal vibrations, and helical conformation of polyethylene glycol. Chem. Phys. 1962, 37, 2764–2776. [Google Scholar] [CrossRef]
- Radulescu, A.; Fetters, L.J.; Richter, D. Polymer driven wax crystal control using partially crystalline polymeric materials. Adv. Polym. Sci. 2008, 210, 1–100. [Google Scholar]
- Ramachandran, R.; Beaucage, G.; Kulkarni, A.S.; McFaddin, D.; Merrick-Mack, J.; Galiatsatos, V. Persistence Length of Short-Chain Branched Polyethylene. Macromolecules 2008, 41, 9802–9806. [Google Scholar] [CrossRef]
- Hammouda, B. Analysis of the Beaucage model. J. Appl. Crystallogr. 2010, 43, 1474–1478. [Google Scholar] [CrossRef]
- Kaneko, F.; Radulescu, A.; Ute, K. Time-resolved small-angle neutron scattering study on guest-exchange processes in co-crystals of syndiotactic polystyrene. J. Appl. Crystallogr. 2014, 47, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Cruickshank, M.C. The Stokes-Einstein law for diffusion in solution. Proc. R. Soc. Lond. A. 1924, 106, 724–749. [Google Scholar]
- Hassan, P.; Kulshreshtha, S. Modification to the cumulant analysis of polydispersity in quasielastic light scattering data. J. Colloid Interface Sci. 2006, 300, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S. Contin: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Petrescu, A.J.; Receveur, V.; Calmettes, P.; Durand, D.; Desmadril, M.; Roux, B.; Smith, J.C. Small-angle neutron scattering by a strongly denatured protein: Analysis using random polymer theory. Biophys. J. 1997, 72, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Balacescu, L.; Schrader, T.E.; Radulescu, A.; Zolnierczuk, P.; Holderer, O.; Pasini, S.; Fitter, J.; Stadler, A.M. Transition between protein-like and polymer-like dynamic behavior: Internal friction in unfolded apomyoglobin depends on denaturing conditions. Sci. Rep. 2020, 10, 1570–1580. [Google Scholar] [CrossRef] [Green Version]
- Kok, C.M.; Rudin, A. Relationship between the hydrodynamic radius and the radius of gyration of a polymer solution. Makromol. Chem. Rapid Commun. 1981, 2, 655–659. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balacescu, L.; Brandl, G.; Kaneko, F.; Schrader, T.E.; Radulescu, A. Light Scattering and Absorption Complementarities to Neutron Scattering: In Situ FTIR and DLS Techniques at the High-Intensity and Extended Q-Range SANS Diffractometer KWS-2. Appl. Sci. 2021, 11, 5135. https://doi.org/10.3390/app11115135
Balacescu L, Brandl G, Kaneko F, Schrader TE, Radulescu A. Light Scattering and Absorption Complementarities to Neutron Scattering: In Situ FTIR and DLS Techniques at the High-Intensity and Extended Q-Range SANS Diffractometer KWS-2. Applied Sciences. 2021; 11(11):5135. https://doi.org/10.3390/app11115135
Chicago/Turabian StyleBalacescu, Livia, Georg Brandl, Fumitoshi Kaneko, Tobias Erich Schrader, and Aurel Radulescu. 2021. "Light Scattering and Absorption Complementarities to Neutron Scattering: In Situ FTIR and DLS Techniques at the High-Intensity and Extended Q-Range SANS Diffractometer KWS-2" Applied Sciences 11, no. 11: 5135. https://doi.org/10.3390/app11115135
APA StyleBalacescu, L., Brandl, G., Kaneko, F., Schrader, T. E., & Radulescu, A. (2021). Light Scattering and Absorption Complementarities to Neutron Scattering: In Situ FTIR and DLS Techniques at the High-Intensity and Extended Q-Range SANS Diffractometer KWS-2. Applied Sciences, 11(11), 5135. https://doi.org/10.3390/app11115135





