Evaluating the Presence of Lycopene-Enriched Extracts from Tomato on Topical Emulsions: Physico-Chemical Characterization and Sensory Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Chemicals
2.2. Chemical Analysis of Tomato Waste
2.3. Supercritical CO2 Extraction of Tomato Waste
2.4. Fatty Acid Analysis of Extracted Oil
2.5. Preparation and Characterization of Microemulsions
2.6. Preparation and Characterization of Macroemulsions
2.7. Sensorial Analysis of Formulations
2.8. Identification of Volatile Compounds through GC-MS
3. Results and Discussion
3.1. Proximate Composition of Tomato Waste
3.2. Supercritical CO2 Extraction of LEE from Tomato Waste
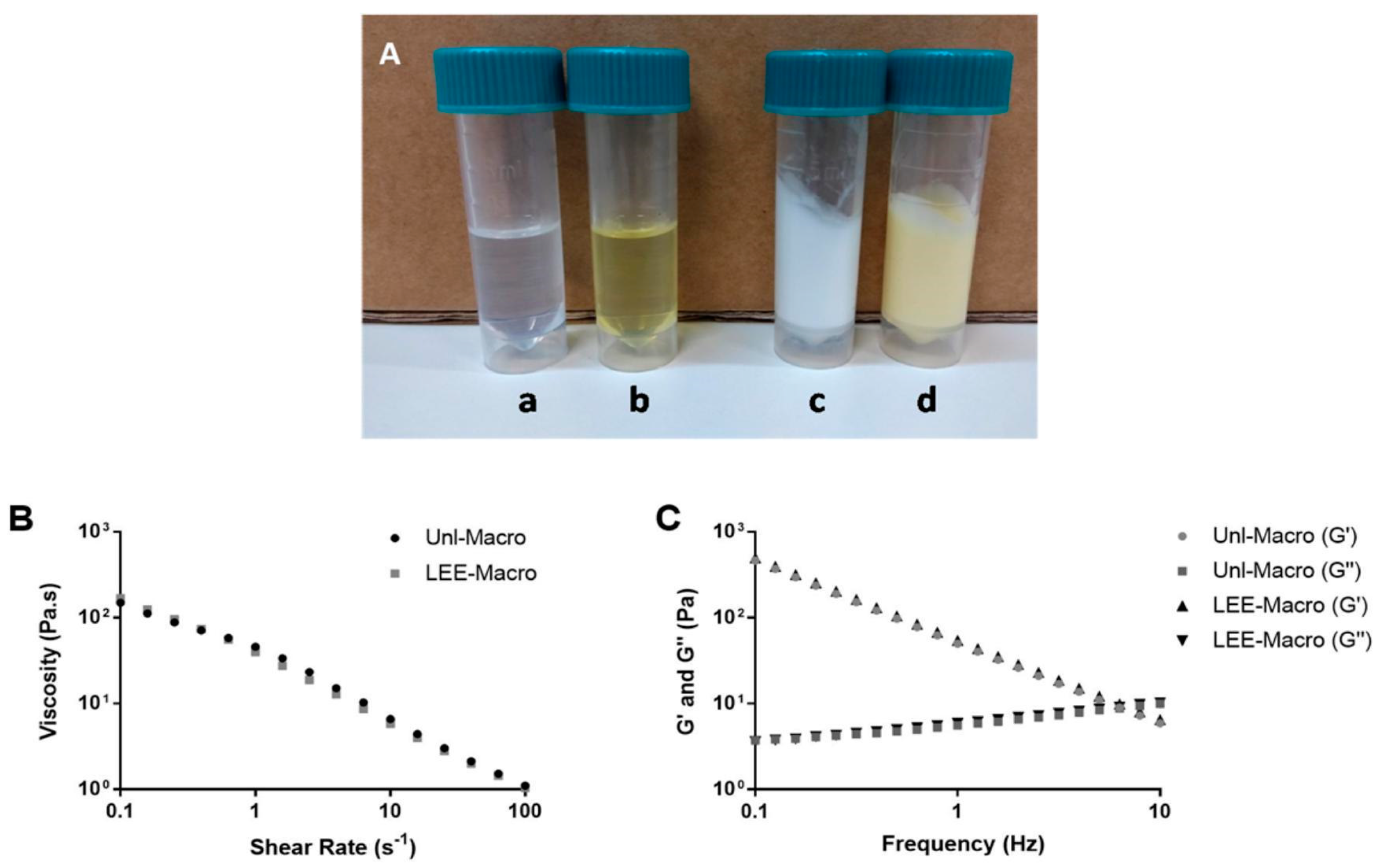
3.3. Physical Characterization of Formulations
3.4. Sensorial Analysis of Formulations
3.4.1. Odor Analysis of Formulations
3.4.2. Color Analysis of Formulations
3.5. Identification of Volatile Compounds in the Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ascenso, A.; Pinho, S.; Eleutério, C.; Praça, F.G.; Bentley, M.V.L.B.; Oliveira, H.; Santos, C.; Silva, O.; Simões, S. Lycopene from Tomatoes: Vesicular Nanocarrier Formulations for Dermal Delivery. J. Agric. Food Chem. 2013, 61, 7284–7293. [Google Scholar]
- Ascenso, A.; Pedrosa, T.; Pinho, S.; Pinho, F.; De Oliveira, J.M.; Marques, H.C.; Oliveira, H.; Simões, S.; Santos, C. The Effect of Lycopene Preexposure on UV-B-Irradiated Human Keratinocytes. Oxidative Med. Cell Longev. 2016, 2016, 8214631. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Corrêa, M.A.; Chorilli, M. Sustainability, natural and organic cosmetics: Consumer, products, efficacy, toxicological and regulatory considerations. Braz. J. Pharm. Sci. 2015, 51, 17–26. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of Natural Fragrance Ingredients: History Overview and Future Trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.E.; Mitelut, A.C.; Popa, E.E.; Matei, F. Introduction to Biotech Entrepreneurship: From Idea to Business: A European Perspective; Matei, F., Zirra, D., Eds.; Springer International Publishing: Cham, Germany, 2019; pp. 141–178. [Google Scholar]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- YESto Website. Available online: https://yesto.com/ (accessed on 13 October 2020).
- Ella Baché Website. Available online: www.ellabache.com (accessed on 13 October 2020).
- Nature Republic Website. Available online: www.naturerepublic.com (accessed on 13 October 2020).
- Haeckels Website. Available online: www.haeckels.co.uk (accessed on 13 October 2020).
- Skinfood Website. Available online: www.theskinfood.us (accessed on 13 October 2020).
- Garancia Website. Available online: www.garancia-beauty.com (accessed on 13 October 2020).
- Jenkins, G.; Wainwright, L.J.; Holland, R.; Barrett, K.E.; Casey, J. Wrinkle reduction in post-menopausal women consuming a novel oral supplement: A double-blind placebo-controlled randomized study. Int. J. Cosmet. Sci. 2014, 36, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tito, A.; Carola, A.; Bimonte, M.; Barbulova, A.; Arciello, S.; De Laurentiis, F.; Monoli, I.; Hill, J.; Gibertoni, S.; Colucci, G.; et al. A tomato stem cell extract, containing antioxidant compounds and metal chelating factors, protects skin cells from heavy metal-induced damages. Int. J. Cosmet. Sci. 2011, 33, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.M.S.; Srebernich, S.M.; Vercelino, B.G.; Zampieri, B.M. Influence of the presence and type of fragrance on the sensory perception of cosmetic formulations. Braz. Arch. Biol. Technol. 2013, 56, 203–212. [Google Scholar] [CrossRef]
- Muñoz, A.M. Sensory evaluation in quality control: An overview, new developments and future opportunities. Food Qual. Prefer. 2002, 13, 329–339. [Google Scholar] [CrossRef]
- Krumbein, A.; Auerswald, H. Characterization of aroma volatiles in tomatoes by sensory analyses. Nahrung 1998, 42, 395–399. [Google Scholar] [CrossRef]
- Zhu, Y.; Sims, C.A.; Klee, H.J.; Sarnoski, P.J. Sensory and Flavor Characteristics of Tomato Juice from Garden Gem and Roma Tomatoes with Comparison to Commercial Tomato Juice. J. Food Sci. 2018, 83, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.J.; Buttery, R.G.; Teranishi, R.; Ling, L.; Scott, K.; Cantwell, M. Effect of storage and ripening on fresh tomato quality, Part I. Food Chem. 1994, 49, 225–231. [Google Scholar] [CrossRef]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and Characterization of a New Quercus Suber-Based Pickering Emulsion for Topical Application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef] [Green Version]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit Fluid 2007, 40, 218–226. [Google Scholar] [CrossRef]
- El-Tamimi, A.H.; Morad, M.M.; Raof, M.S.; Rady, A.H. Tomato Seed Oil I Fatty Acid Composition, Stability and Hydrogenation of the Oil. Fette Seifen Anstrichm. 1979, 81, 281–284. [Google Scholar] [CrossRef]
- Giannelos, P.N.; Sxizas, S.; Lois, E.; Zannikos, F.; Anastopoulos, G. Physical, chemical and fuel related properties of tomato seed oil for evaluating its direct use in diesel engines. Ind. Crops Prod. 2005, 22, 193–199. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical Fluid Extraction of Lycopene from Tomato Processing Byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef]
- Baysal, T.; Ersus, S.; Starmans, D.A.J. Supercritical CO2 Extraction of β-Carotene and Lycopene from Tomato Paste Waste. J. Agric. Food Chem. 2000, 48, 5507–5511. [Google Scholar] [CrossRef]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Gonçalves, R.; Barreto, M.; Dias, C.; Carvalho, F.; Almeida, A.J.; Ribeiro, H.M.; Marto, J. Development of Gel-in-Oil Emulsions for Khellin Topical Delivery. Pharmaceutics 2020, 12, 398. [Google Scholar] [CrossRef]
- Randall, J.P.; Seow, W.K.; Walsh, L.J. Antibacterial activity of fluoride compounds and herbal toothpastes on Streptococcus mutans: An in vitro study. Aust. Dent. J. 2015, 60, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Stovell, A.G.; Newton, B.M.; Lynch, R.J.M. Important considerations in the development of toothpaste formulations for children. Int. Dent. J. 2013, 63, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, J.; Schieberle, P. Characterization of Aroma-Active Compounds in Italian Tomatoes with Emphasis on New Odorants. J. Agric. Food Chem. 2017, 65, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Kesen, S.; Sonmezdag, A.S.; Çetiner, B.; Kola, O.; Selli, S. Characterization of the key aroma compounds in tomato pastes as affected by hot and cold break process. J. Food Meas. Charact. 2018, 12, 2461–2474. [Google Scholar] [CrossRef]
- Marković, K.; Vahčić, N.; Ganić, K.K.; Banović, M. Aroma volatiles of tomatoes and tomato products evaluated by solid-phase microextraction. Flavour Fragr. J. 2007, 22, 395–400. [Google Scholar] [CrossRef]
- Selli, S.; Kelebek, H.; Ayseli, M.T.; Tokbas, H. Characterization of the most aroma-active compounds in cherry tomato by application of the aroma extract dilution analysis. Food Chem. 2014, 165, 540–546. [Google Scholar] [CrossRef]
- Li, J.; Di, T.; Bai, J. Distribution of Volatile Compounds in Different Fruit Structures in Four Tomato Cultivars. Molecules 2019, 24, 2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Good Scent Company Information System. Available online: http://www.thegoodscentscompany.com/index.html (accessed on 9 October 2020).
- Kazeniac, S.J.; Hall, R.M. Flavor chemistry of tomato volaties. J. Food Sci. 1970, 35, 519–530. [Google Scholar] [CrossRef]
- Sieso, V.; Crouzet, J. Tomato volatile components: Effect of processing. Food Chem. 1977, 2, 241–252. [Google Scholar] [CrossRef]
- Crouzet, J.; Kanasawud, P. Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1992; pp. 54–62. [Google Scholar]
- Rios, J.J.; Fernández-García, E.; Mínguez-Mosquera, M.I.; Pérez-Gálvez, A. Description of volatile compounds generated by the degradation of carotenoids in paprika, tomato and marigold oleoresins. Food Chem. 2008, 106, 1145–1153. [Google Scholar] [CrossRef]
- Chovatiya, R.; Silverberg, J.I. Pathophysiology of Atopic Dermatitis and Psoriasis: Implications for Management in Children. Children 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S. Essential Chemistry for Aromatherapy, 2nd ed.; Clarke, S., Ed.; Churchill Livingstone: Edinburgh, Scotland, 2008; pp. 41–77. [Google Scholar]
- Lewinsohn, E.; Sitrit, Y.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just colors-Carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]




| Compound | Area of the Peak (%) | Odor Type/Description | Odor Threshold (ppb) 2 | Odor Threshold in Tomato Fruits (ppb) 3 | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LEE | LEE-ME | Unl-ME | Cal RI | Lit RI 1 | ||||||
| 5-MS Colunm | 6-Methyl-5-hepten-2-one | 34.19 | 5.6 | - | 990 | 986 | Floral, green | 50 | 50 | [35] |
| D-Limonene | 2.9 | 1.64 | 0.92 | 1028 | 1028 | Lemon, Orange | 10 | 10 | [36] | |
| 2-Isobutylthiazole | 3.14 | 0.66 | _______ | 1033 | 1020 | Tomato leafy, green, musty | 2–3.5 | 3.5 | [19,36] | |
| 2,5-Dimethyldecane | 2.11 | 1.78 | _______ | 1088 | 1086 | _______ | _______ | _______ | _______ | |
| Dodecane | 0.2 | 17.06 | 25.76 | 1200 | 1200 | Alkane | _______ | _______ | [36] | |
| Citronellyl formate | 1.12 | 0.24 | _______ | 1276 | 1275 | Fruity, floral, rose-light odor | _______ | _______ | _______ | |
| Not identified | 2.14 | 2.08 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.15 | 0.09 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.12 | 0.27 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 1.88 | 2.17 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.37 | 0.47 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.25 | 0.26 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.37 | 0.31 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.26 | 0.36 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| 1,7-Dimethyl-4-(1-methylethyl)cyclodecane | 0.17 | 0,13 | _______ | 1478 | 1485 | _______ | _______ | _______ | _______ | |
| Not identified | 0.33 | 0.3 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Not identified | 0.04 | 0.5 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| Wax Colunm | D-Limonene | 1.27 | 0.21 | 0.06 | 1202 | 1198 | Lemon, Orange | 10 | 10 | [36] |
| Not identified | 2.39 | 0.3 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| 2-Isobutylthiazole | 4.9 | 0.20 | _______ | 1410 | 1406 | Tomato leafy, green, musty | 2–3.5 | 3.5 | [19,36] | |
| Not identified | 1.2 | 0.13 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| €-Citral | 2.02 | 0.06 | _______ | 1740 | 1741 | Citrus, Lemon | 32 | _______ | [35] | |
| Area of the Peak (%) | Cal RI | Lit RI 1 | Odor Type/Description | Odor Threshold (ppb) 2 | Odor Threshold in Tomato Fruits (ppb) 3 | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | LEE | LEE-Macro | Unl-Macro | |||||||
| 5-MS Colunm | Not identified | 0.14 | 0.08 | 0.05 | _______ | _______ | _______ | _______ | _______ | _______ |
| 6-Methyl-5-hepten-2-one | 33.95 | 1.59 | _______ | 990 | 986 | Floral, green | 50 | 50 | [35] | |
| D-Limonene | 2.9 | 1.86 | 1.27 | 1028 | 1028 | Lemon, Orange | 10 | 10 | [36] | |
| 2-Isobutylthiazole | 3.14 | 0.1 | _______ | 1033 | 1020 | Tomato leafy, green, musty | 2–3.5 | 3.5 | [19,36] | |
| Benzeneacetaldehyde | 2.44 | 0.09 | _______ | 1043 | 1035 | Green | 4 | _______ | [20] | |
| 2-Propyl-1-pentanol | 0.14 | 0.06 | _______ | 1059 | 1052 | _______ | _______ | _______ | _______ | |
| 2,5-Dimethyldecane | 2.11 | 0.05 | _______ | 1088 | 1086 | _______ | _______ | _______ | _______ | |
| Dodecane | 0.2 | 0.33 | 0.7 | 1200 | 1200 | Alkane | _______ | _______ | [36] | |
| 2-Phenoxyethanol * | 0.22 | 14.59 | 23.64 | 1226 | 1226 | _______ | _______ | _______ | _______ | |
| Citral | 1.2 | 0.17 | _______ | 1271 | 1270 | Citrus, Lemon | 30–32 | _______ | [35] | |
| Citronellyl formate | 1.12 | 0.12 | _______ | 1276 | 1275 | Floral | _______ | _______ | _______ | |
| Not identified | 2.14 | 0.16 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| 3-(3,3-Dimethylbutyl)cyclohexanone | 1.38 | 0.04 | _______ | 1352 | 1364 | _______ | _______ | _______ | _______ | |
| 2,4,4,6,6,8,8-Heptamethyl-2-nonene | 1.88 | 0.08 | _______ | 1362 | 1343 | _______ | _______ | _______ | _______ | |
| 2-Bromo dodecane | 0.33 | 0.02 | _______ | 1522 | 1505 | _______ | _______ | _______ | _______ | |
| Wax Colunm | Toluene | 3.43 | 1.05 | _______ | 1047 | 1044 | Sweet | _______ | _______ | [39] |
| n-Hexanal | 1.31 | 0.20 | 0.91 | 1090 | 1091 | Green, grassy | 4.5–5 | 4.5–5 | [19,36] | |
| D-Limonene | 1.27 | 1.42 | 0.47 | 1202 | 1198 | Lemon, Orange | 10 | 10 | [36] | |
| Not identified | 2.39 | 0.15 | _______ | _______ | _______ | _______ | _______ | _______ | _______ | |
| 1-Hexanol | 0.9 | 0.42 | 0.26 | 1358 | 1358 | Herbal | 1500 | _______ | [33] | |
| (Z)-3-Hexenol | 0.77 | 0.11 | _______ | 1388 | 1388 | Green, Cut grass | 70 | _______ | [33,35] | |
| 2-Isobutylthiazole | 4.9 | 0.38 | _______ | 1410 | 1406 | Tomato leafy, green, musty | 2–3.5 | 3.5 | [19,36] | |
| (E)-2-Octenal | 0.66 | 0.4 | 0.3 | 1437 | 1437 | Fatty | 3 | _______ | [33] | |
| Citronellylformate | 1.86 | 0.15 | _______ | 1629 | 1629 | Floral | _______ | _______ | _______ | |
| (E)-Citral | 2.02 | 0.18 | _______ | 1740 | 1741 | Citrus, Lemon | 32 | _______ | [35] | |
| Benzyl Alcohol | 0.25 | 0.08 | 0.06 | 1885 | 1882 | Floral | 10000 | _______ | [33,34] | |
| Phenylethyl Alcohol | 0.69 | 0.15 | 0.03 | 1917 | 1917 | Floral | 750–1100 | _______ | _______ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Marques, M.; Congiu, F.; Paiva, A.; Simões, P.; Ferreira, A.; Bronze, M.R.; Marto, J.; Ribeiro, H.M.; Simões, S. Evaluating the Presence of Lycopene-Enriched Extracts from Tomato on Topical Emulsions: Physico-Chemical Characterization and Sensory Analysis. Appl. Sci. 2021, 11, 5120. https://doi.org/10.3390/app11115120
Costa A, Marques M, Congiu F, Paiva A, Simões P, Ferreira A, Bronze MR, Marto J, Ribeiro HM, Simões S. Evaluating the Presence of Lycopene-Enriched Extracts from Tomato on Topical Emulsions: Physico-Chemical Characterization and Sensory Analysis. Applied Sciences. 2021; 11(11):5120. https://doi.org/10.3390/app11115120
Chicago/Turabian StyleCosta, Ana, Marta Marques, Franca Congiu, Alexandre Paiva, Pedro Simões, António Ferreira, Maria Rosário Bronze, Joana Marto, Helena Margarida Ribeiro, and Sandra Simões. 2021. "Evaluating the Presence of Lycopene-Enriched Extracts from Tomato on Topical Emulsions: Physico-Chemical Characterization and Sensory Analysis" Applied Sciences 11, no. 11: 5120. https://doi.org/10.3390/app11115120
APA StyleCosta, A., Marques, M., Congiu, F., Paiva, A., Simões, P., Ferreira, A., Bronze, M. R., Marto, J., Ribeiro, H. M., & Simões, S. (2021). Evaluating the Presence of Lycopene-Enriched Extracts from Tomato on Topical Emulsions: Physico-Chemical Characterization and Sensory Analysis. Applied Sciences, 11(11), 5120. https://doi.org/10.3390/app11115120










