Developing an Assisting Device to Reduce the Vibration on the Hands of Elders
Abstract
:1. Introduction
2. Research Facility on the Parkinsonian Tremor
3. Proposed Approach
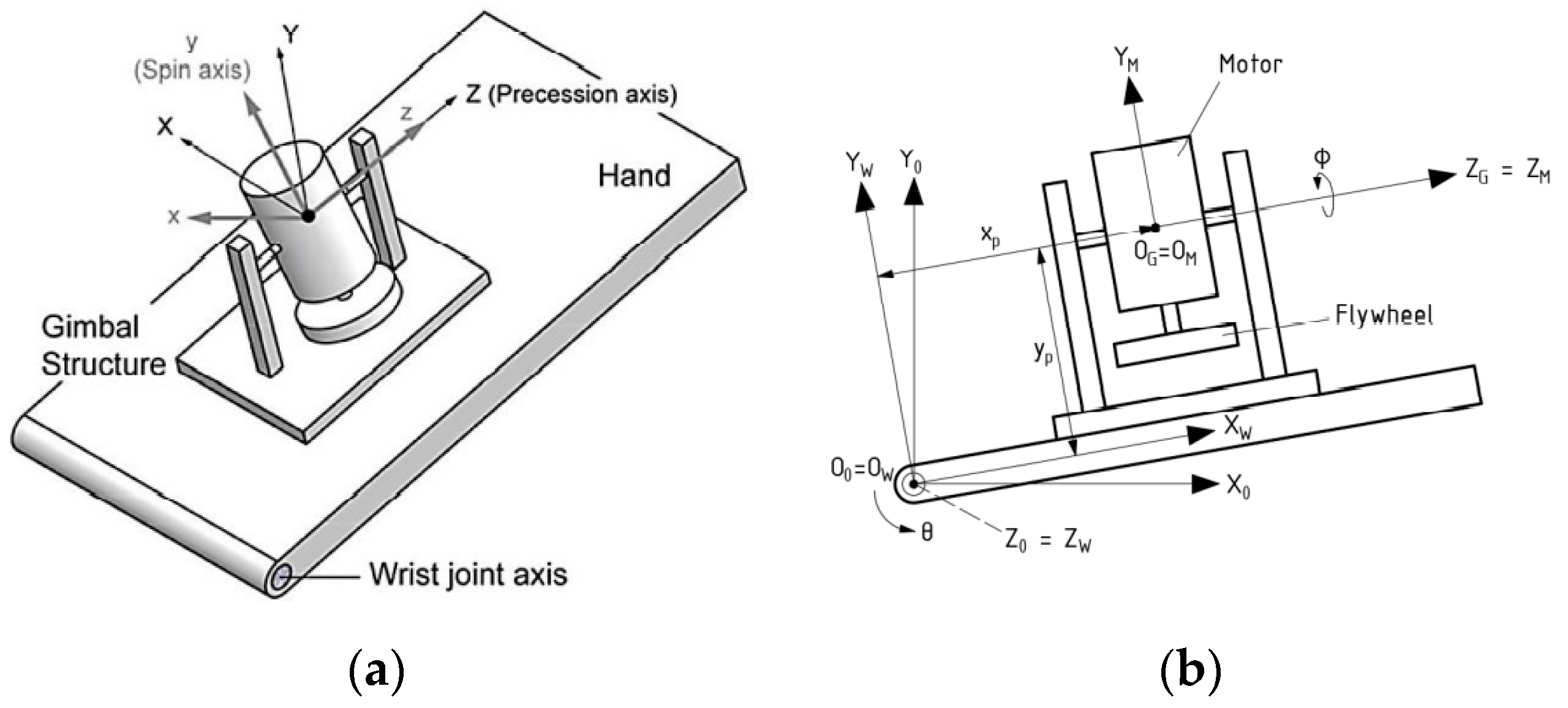
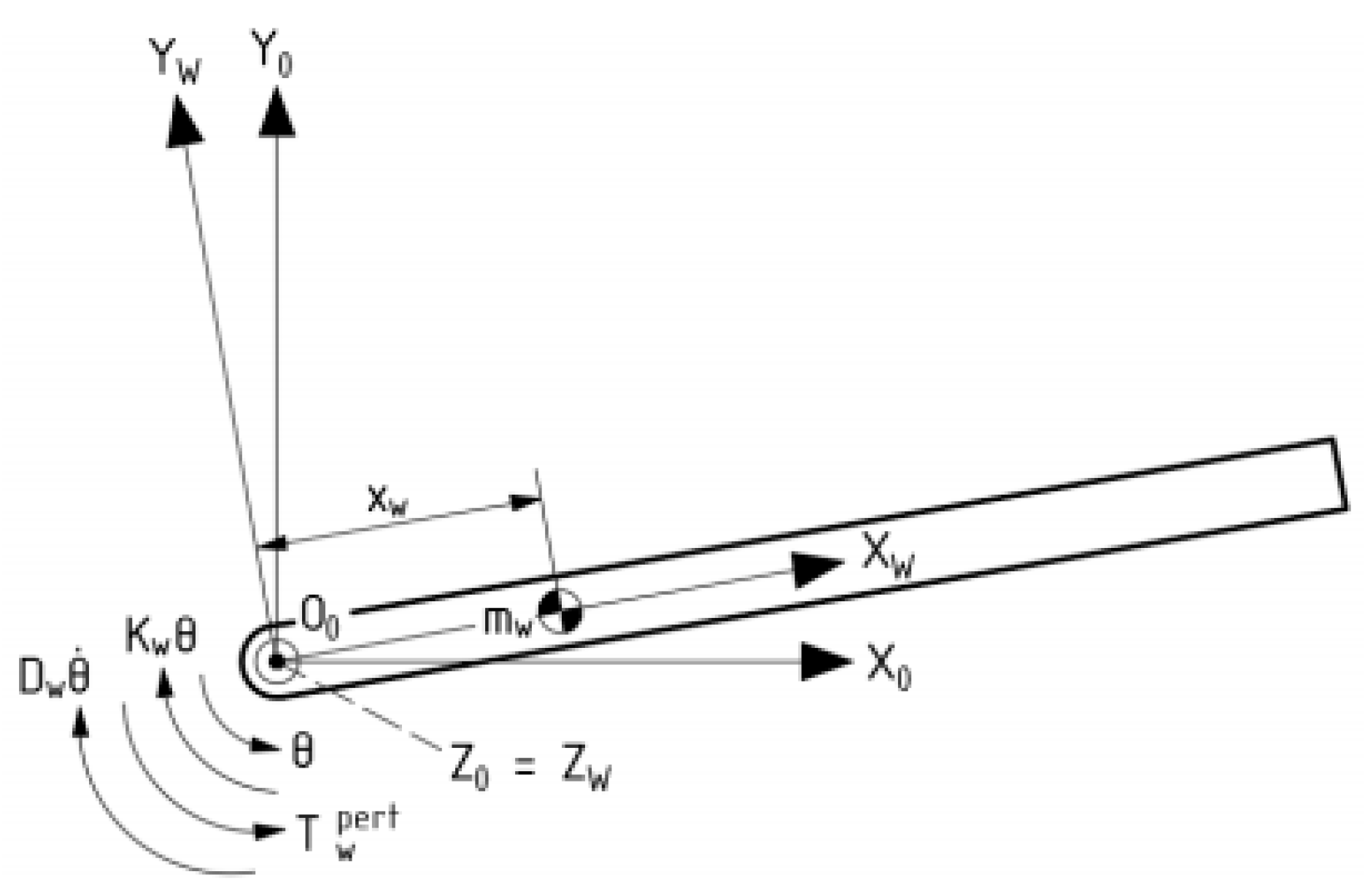
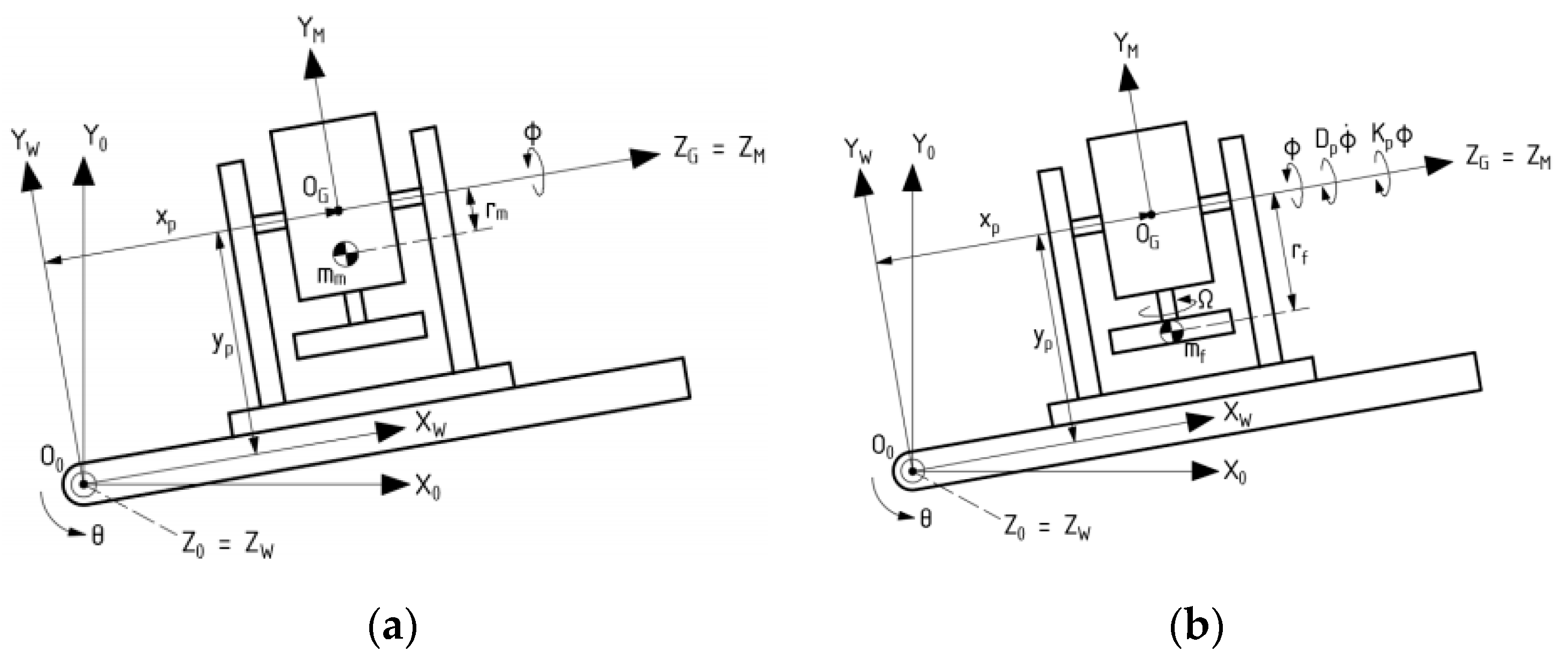
3.1. Dynamic Analysis of Gyroscopic Modeling System
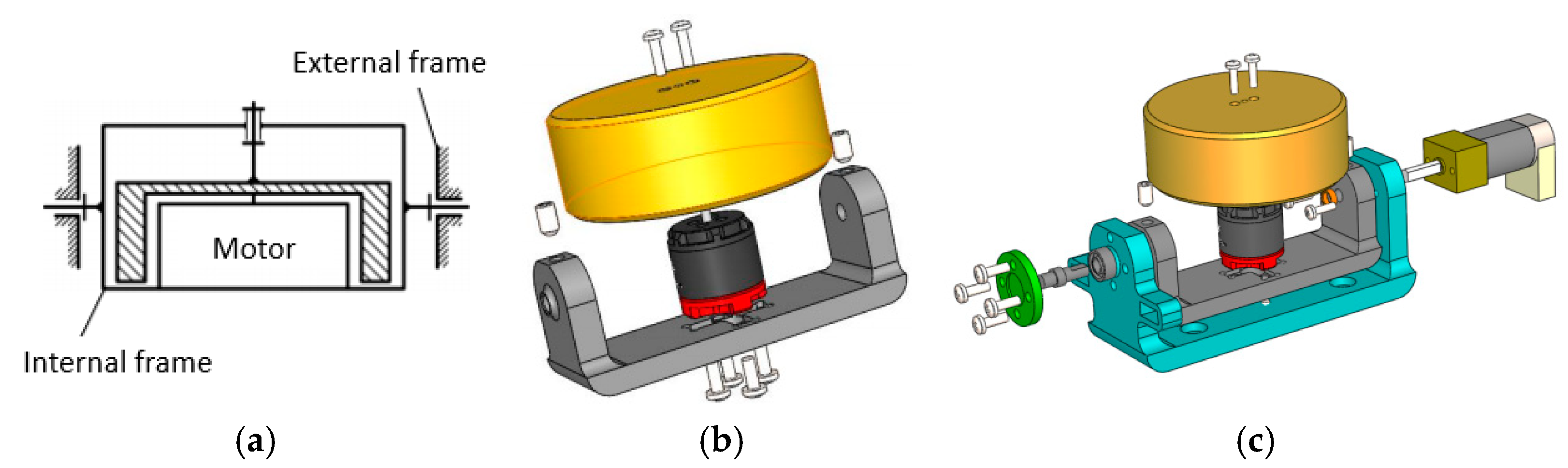
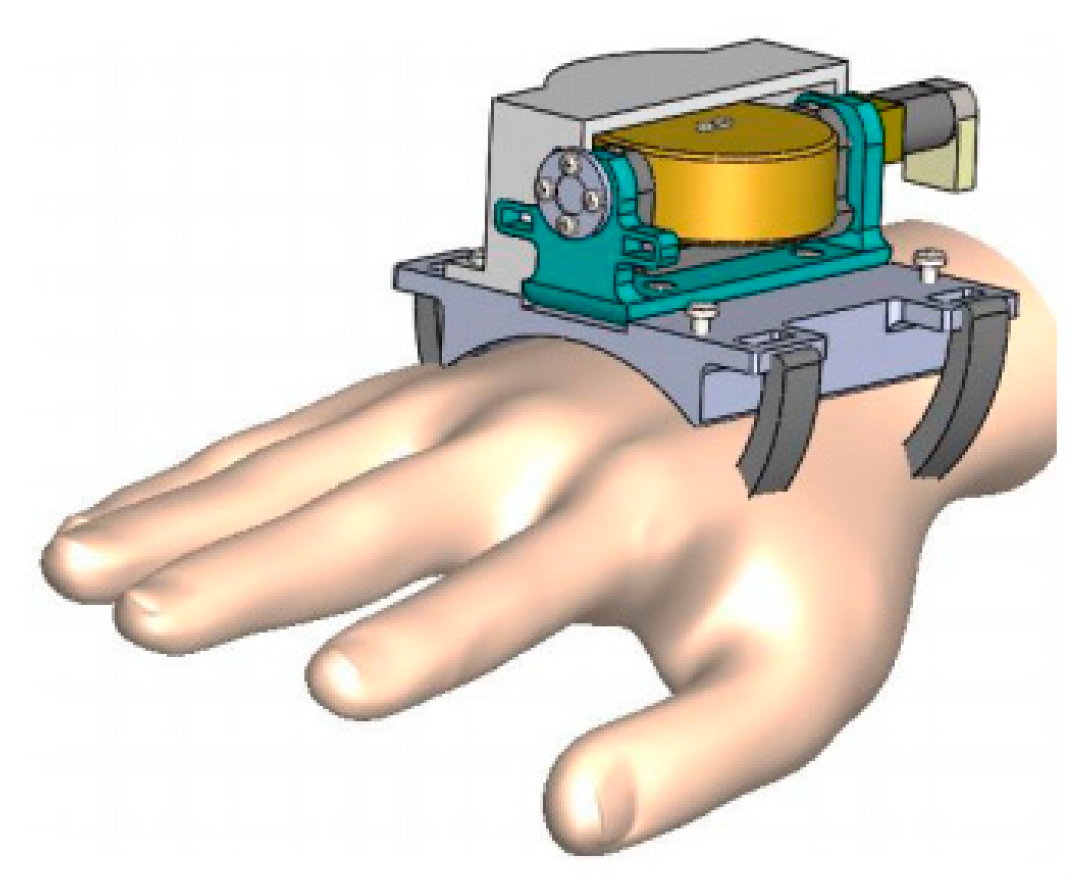
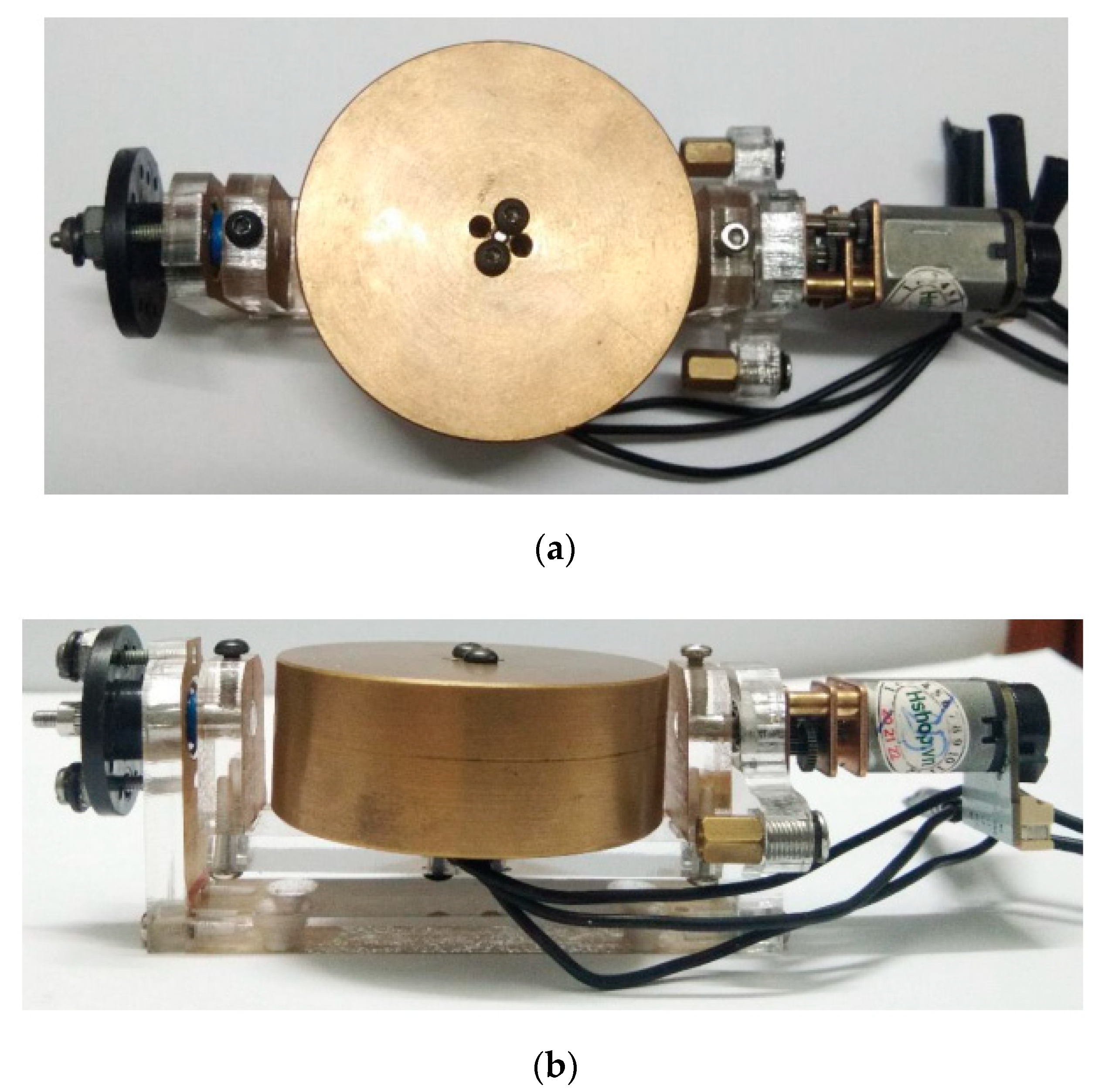
3.2. Gyroscope-Enabled Framework of Wearable Device to Suppress Vibration
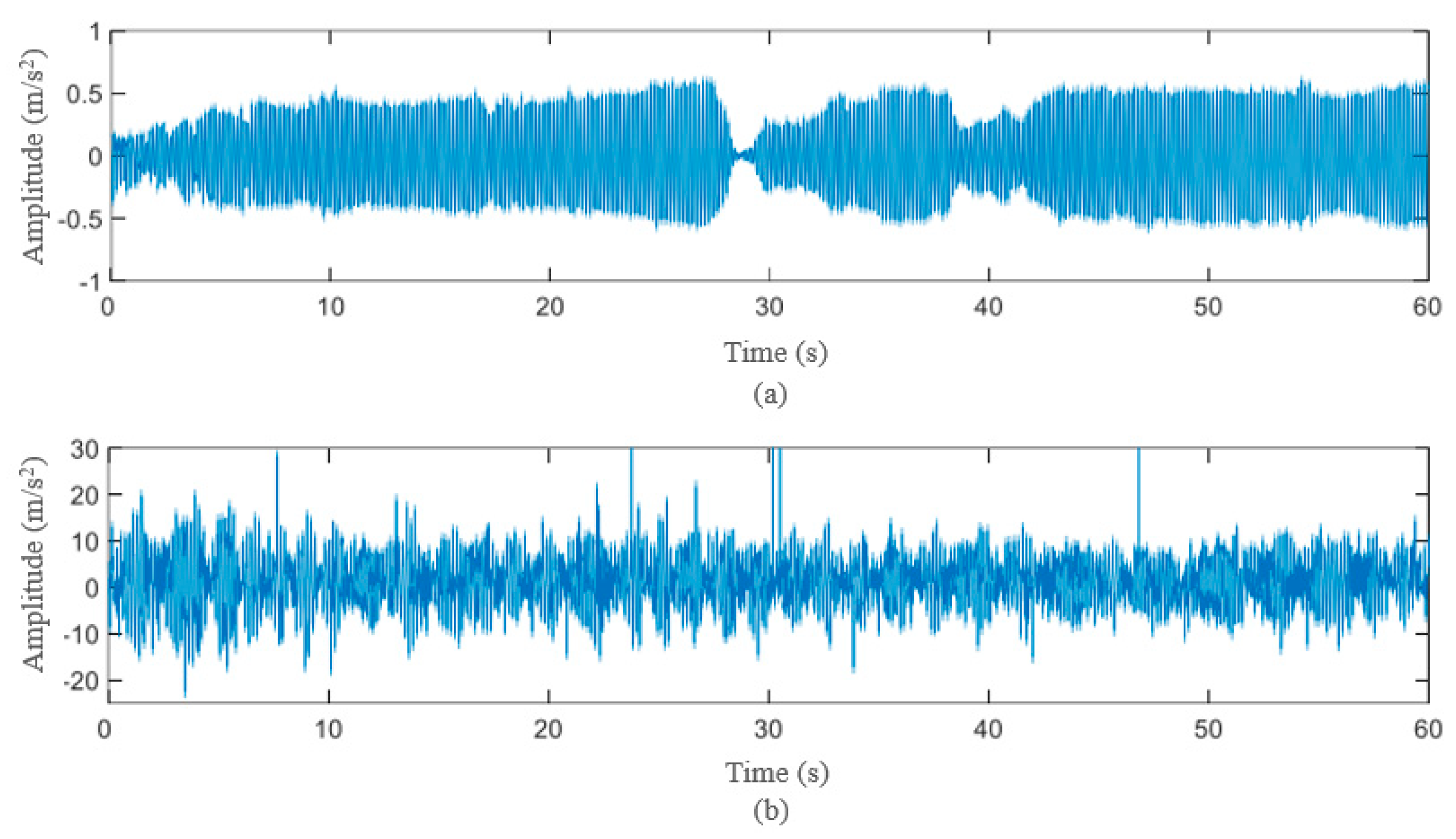
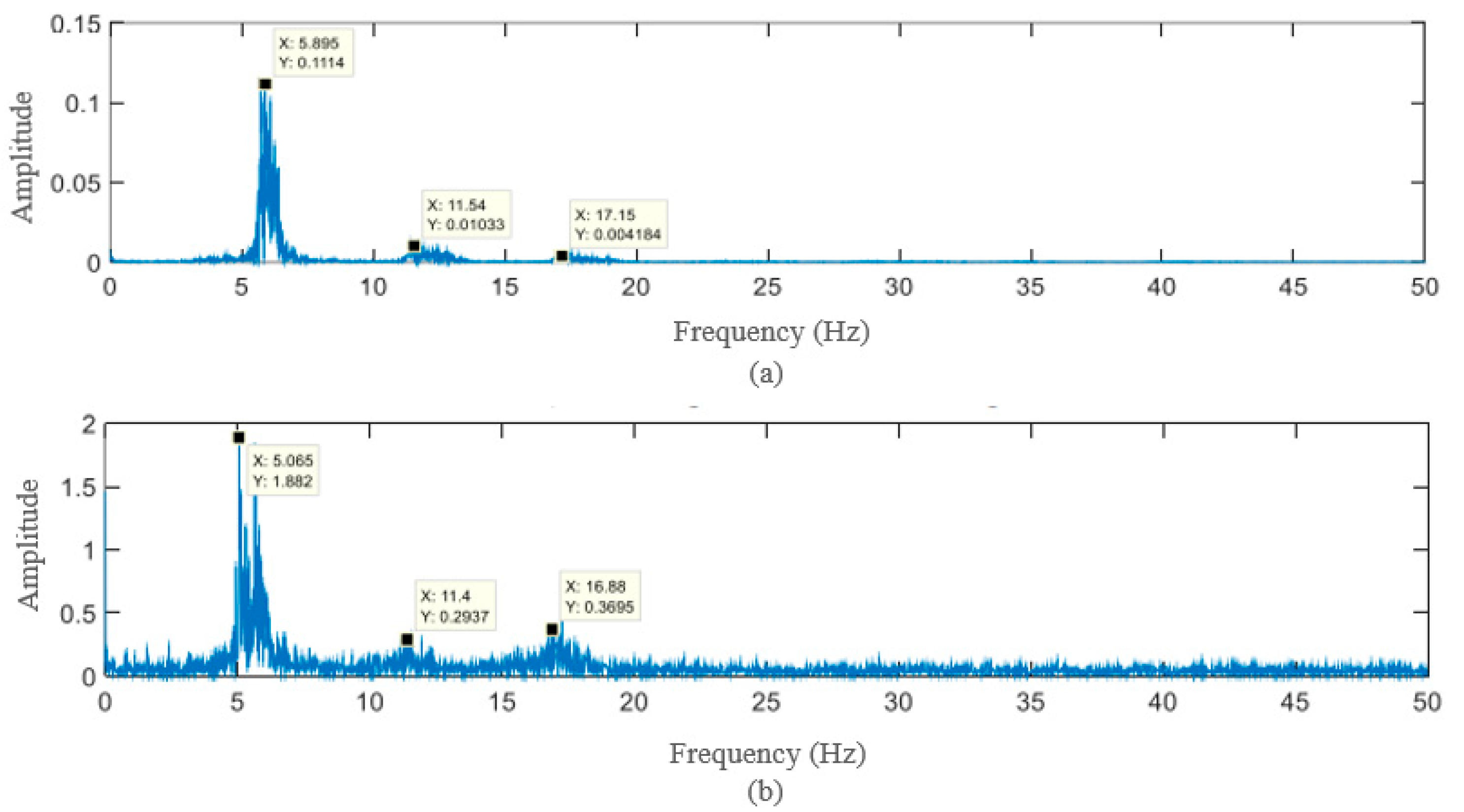
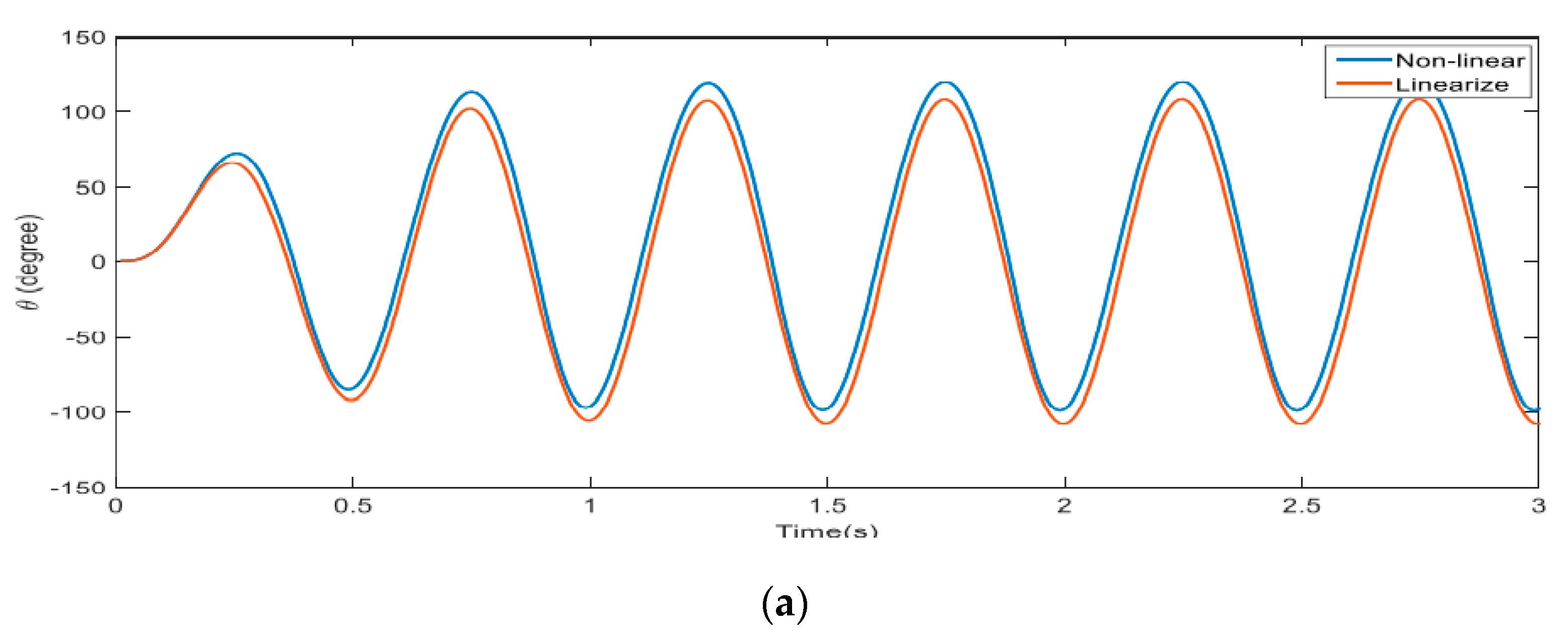
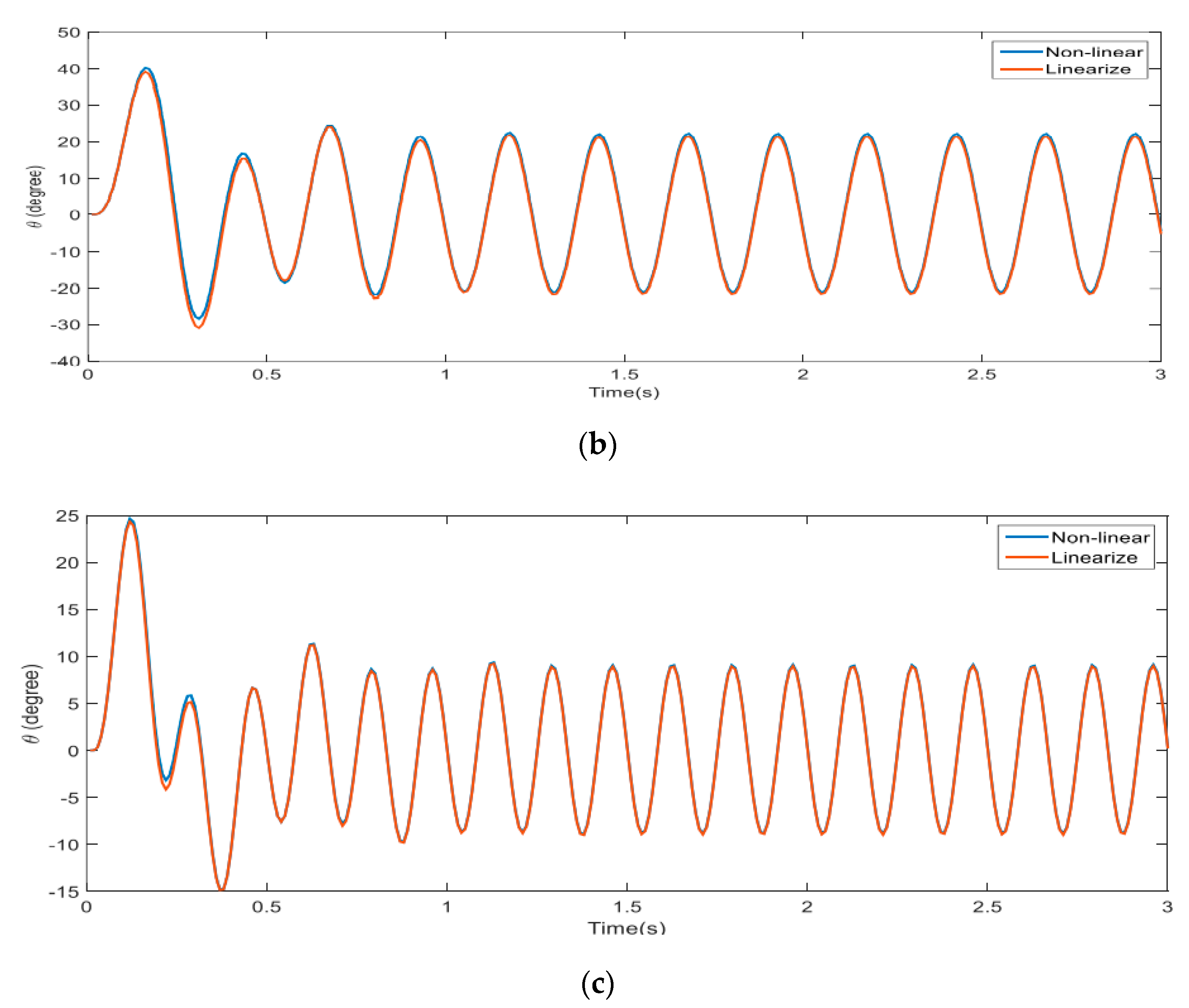
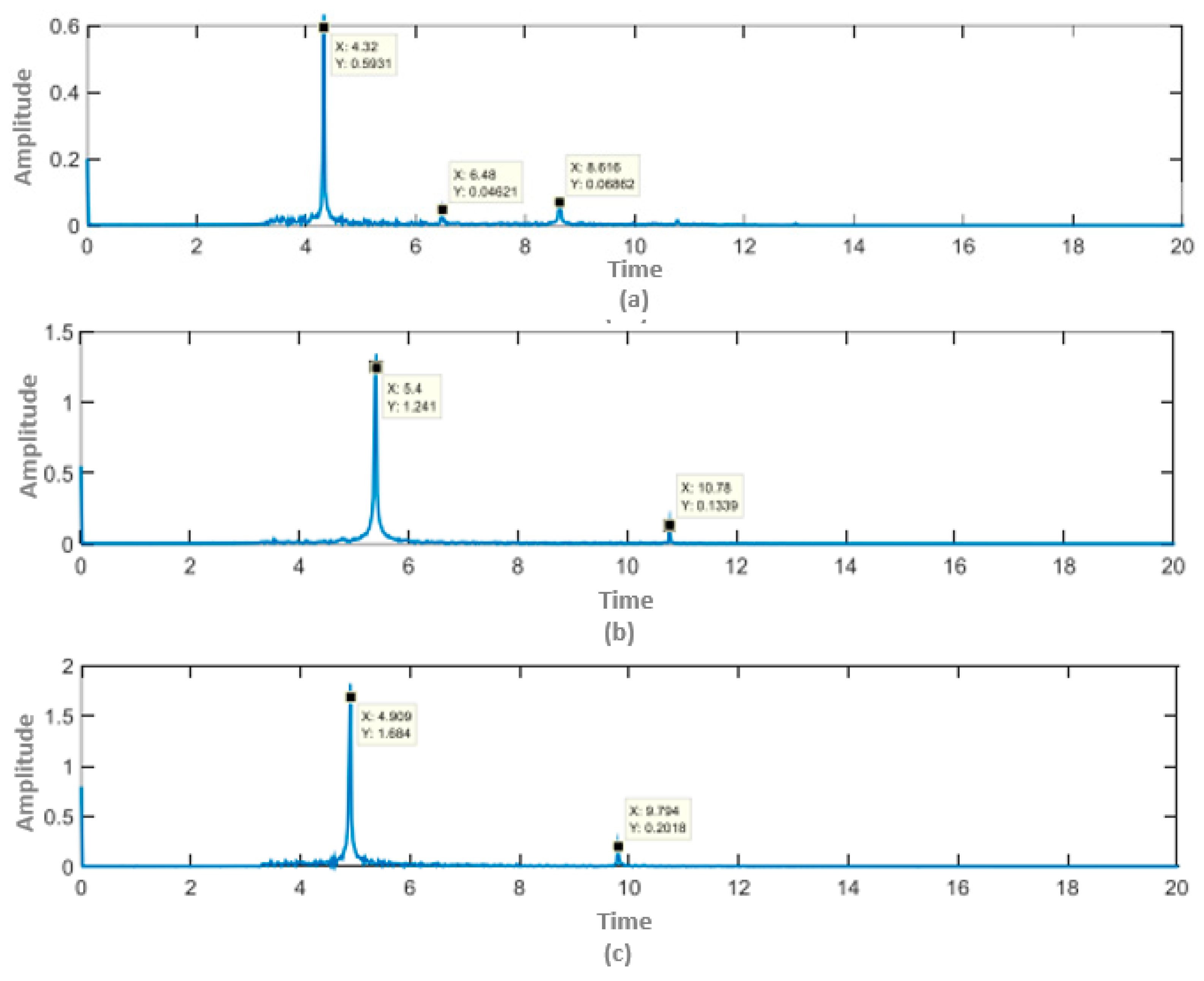
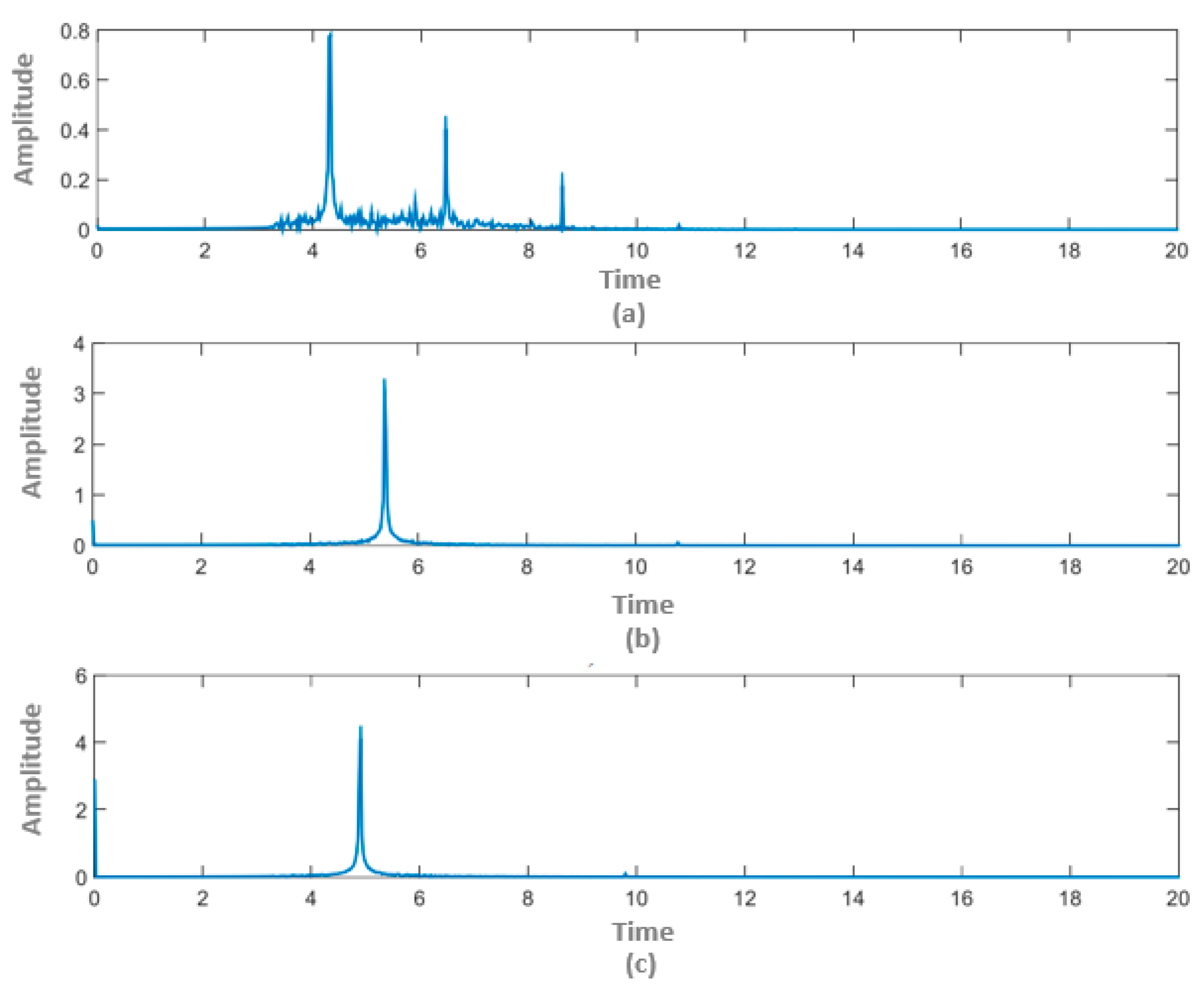
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, A.; Zheng, W.; He, Y.; Tang, W.; Wei, X.; He, R.; Huang, W.; Su, Y.; Huang, Y.; Xie, H.; et al. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat. Disord. 2018, 53, 82–88. [Google Scholar] [CrossRef]
- Liu, W.M.; Wu, R.M.; Lin, J.W.; Liu, Y.C.; Chang, C.H.; Lin, C.H. Time trends in the prevalence and incidence of Parkinson’s disease in Taiwan: A nationwide, population-based study. J. Formos. Med. Assoc. 2016, 115, 531–538. [Google Scholar] [PubMed] [Green Version]
- Moisan, F.; Kab, S.; Mohamed, F.; Canonico, M.; Le Guern, M.; Quintin, C.; Carcaillon, L.; Nicolau, J.; Duport, N.; Elbaz, A.; et al. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 952–957. [Google Scholar] [CrossRef] [Green Version]
- Gal, O.; Srp, M.; Konvalinkova, R.; Hoskovcova, M.; Capek, V.; Roth, J.; Ruzicka, E. Physiotherapy in Parkinson’s disease: Building ParkinsonNet in Czechia. Parkinson’s Dis. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.; Brobert, G.P.; Johansson, S.; Jick, S.S.; Meier, C.R. Risk of incident depression in patients with Parkinson’s disease in the UK. Eur. J. Neurol. 2011, 18, 448–453. [Google Scholar] [CrossRef]
- Benito-Leon, J.; Bermejo-Pareja, F.; Morales-Gonzalez, J.M.; Porta-Etessam, J.; Trincado, R.; Vega, S.; Louis, E.D. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 2004, 62, 734–741. [Google Scholar] [CrossRef]
- Kowal, S.L.; Dall, T.M.; Chakrabarti, R.; Storm, M.V.; Jain, A. The current and projected economic burden of Parkinson’s disease in the United States. Mov. Disord. 2013, 28, 311–318. [Google Scholar] [CrossRef]
- Tan, S.B.; Williams, A.F.; Morris, M.E. Experiences of caregivers of people with Parkinson’s disease in Singapore: A qualitative analysis. J. Clin. Nurs. 2012, 21, 2235–2246. [Google Scholar] [CrossRef]
- Winter, Y.; von Campenhausen, S.; Brozova, H.; Skoupa, J.; Reese, J.P.; Bötzel, K.; Eggert, K.; Oertel, W.H.; Dodel, R.; Ruzicka, E. Costs of Parkinson’s disease in eastern Europe: A Czech cohort study. Parkinsonism Relat. Disord. 2010, 16, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Berger, K.; Chen, H.; Leslie, D.; Mailman, R.B.; Huang, X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov. Disord. 2018, 33, 156–159. [Google Scholar] [CrossRef]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Łos, M.J.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [Green Version]
- Taheri, B.; Case, D.; Richer, E. Adaptive suppression of severe pathological tremor by torque estimation method. IEEE/ASME Trans. Mechatron. 2014, 20, 717–727. [Google Scholar] [CrossRef]
- Louis, E.D. Treatment of essential tremors: Are there issues we are overlooking? Front. Neurol. 2012, 2, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, K.E.; Pahwa, R. Pharmacotherapy of essential tremor. CNS Drugs 2008, 22, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.-H.; et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell 2017, 169, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Stoker, T.B.; Torsney, K.M.; Barker, R.A. They are emerging treatment approaches for Parkinson’s disease. Front. Neurosci. 2018, 12, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, C.S.; Tam, J.; Lozano, A.M. The changing landscape of surgery for Parkinson’s Disease. Mov. Disord. 2018, 33, 36–47. [Google Scholar] [CrossRef]
- Louis, E.D.; Faust, P.L.; Vonsattel JP, G.; Honig, L.S.; Rajput, A.; Robinson, C.A.; Pahwa, R.; Lyons, K.E.; Ross, G.; Borden, S.; et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007, 130, 3297–3307. [Google Scholar] [CrossRef] [Green Version]
- Sprenger, F.; Poewe, W. Management of motor and non-motor symptoms in Parkinson’s disease. CNS Drugs 2013, 27, 259–272. [Google Scholar] [CrossRef]
- Laffranchi, M.; Tsagarakis, N.G.; Caldwell, D.G. Analysis and development of a semiactive damper for compliant actuation systems. IEEE/ASME Trans. Mechatron. 2012, 18, 744–753. [Google Scholar] [CrossRef]
- Rahnavard, M.; Hashemi, M.; Farahmand, F.; Dizaji, A.F. Designing a hand rest tremor dynamic vibration absorber using the H 2 optimization method. J. Mech. Sci. Technol. 2014, 28, 1609–1614. [Google Scholar] [CrossRef]
- Taheri, B.; Case, D.; Richer, E. Robust controller for tremor suppression at a musculoskeletal level in human wrist. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 22, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Naish, M.D.; Jenkins, M.E.; Trejos, A.L. Design and validation of a novel mechatronic transmission system for a wearable tremor suppression device. Robot. Auton. Syst. 2017, 91, 38–48. [Google Scholar] [CrossRef]
- Dideriksen, J.L.; Laine, C.M.; Dosen, S.; Muceli, S.; Rocon, E.; Pons, J.L.; Benito-Leon, J.; Farina, D. Electrical stimulation of afferent pathways for the suppression of pathological tremor. Front. Neurosci. 2017, 11, 178. [Google Scholar] [CrossRef]
- Herrnstadt, G.; Menon, C. Admittance-based voluntary-driven motion with speed-controlled tremor rejection. IEEE/ASME Trans. Mechatron. 2016, 21, 2108–2119. [Google Scholar] [CrossRef]
- Tremor Fact Sheet|National Institute of Neurological Disorders and Stroke (nih.gov). May 2017. Web. 11 November 2018. Available online: https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/tremor-fact-sheet (accessed on 12 April 2021).
- Lenka, A.; Louis, E.D. Revisiting the clinical phenomenology of “cerebellar tremor”: Beyond the intention tremor. Cerebellum 2019, 18, 565–574. [Google Scholar] [CrossRef]
- Elble, R.J. Defining dystonic tremor. Curr. Neuropharmacol. 2013, 11, 48–52. [Google Scholar] [PubMed] [Green Version]
- Haubenberger, D.; Hallett, M. Essential tremor. N. Engl. J. Med. 2018, 378, 1802–1810. [Google Scholar] [CrossRef]
- Niemann, N.; Jankovic, J. Botulinum toxin for the treatment of hand tremors. Toxins 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.A.; Edwards, M.J.; Mir, P.; Cordivari, C.; Hooker, J.; Dickson, J.; Quinn, N.; Bhatia, K.P. Patients with adult-onset dystonic tremors resembling parkinsonian tremors have scans without evidence of dopaminergic deficit (SWEDDs). Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 2210–2215. [Google Scholar] [CrossRef]
- Anouti, A.; Koller, W.C. Tremor disorders. Diagn. Manag. West. J. Med. 1995, 162, 510. [Google Scholar]
- Lin, Y.; Ling, B.W.K.; Xu, N.; Lam, R.W.K.; Ho, C.Y.F. Effectiveness analysis of bio-electronic stimulation therapy to Parkinson’s diseases via joint singular spectrum analysis and discrete fourier transform approach. Biomed. Signal Process. Control 2020, 62, 102131. [Google Scholar] [CrossRef]
- Beuter, A.; Titcombe, M.S.; Richer, F.; Gross, C.; Guehl, D. Effect of deep brain stimulation on amplitude and frequency characteristics of rest tremor in Parkinson’s disease. Thalamus Relat. Syst. 2001, 1, 203–211. [Google Scholar] [CrossRef]
- Riviere, C.N.; Thakor, N.V. We are modeling and canceling tremors in human-machine interfaces. IEEE Eng. Med. Biol. Mag. 1996, 15, 29–36. [Google Scholar] [CrossRef]
- Veluvolu, K.C.; Tan, U.X.; Latt, W.T.; Shee, C.Y.; Ang, W.T. Bandlimited multiple Fourier linear combiner for real-time tremor compensation. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 2847–2850. [Google Scholar]
- Zhou, Y.; Jenkins, M.E.; Naish, M.D.; Trejos, A.L. Characterization of parkinsonian hand tremor and validation of a high-order tremor estimator. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.; Steinmann, P.D. Analysis of ship roll gyrostabilizer control. IFAC Proc. Vol. 2009, 42, 310–315. [Google Scholar] [CrossRef]
- Simas, H.; Di Gregorio, R. A Technique Based on Adaptive Extended Jacobians for Improving the Robustness of the Inverse Numerical Kinematics of Redundant Robots. J. Mech. Robot. 2019, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Jenkins, M.E.; Naish, M.D.; Trejos, A.L. Development of a wearable tremor suppression glove. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 640–645. [Google Scholar]

| Main Category | Child Category | Description | Author(s) | Finding(s) |
|---|---|---|---|---|
| 1. Action tremor | 1.1 Cerebellar tremor | Causes agitation at the ends of extremities and is initiated by damage to the cerebellum and its pathways to other regions of the brain. | Lenka, A. and Louis, E. D. [27] | Via the available data, the clinical phenomenology of tremors of cerebellar origin is heterogeneous, although intention tremor is the sole signature of cerebellar lesions is an over-simplification. |
| 1.2 Dystonic tremor | Occurs due to incorrect message transfer from the brain to the corresponding muscle, causing them to be overactive. | Elble, R. J. [28] | There are two forms of tremor in dystonia: dystonic tremors produced by dystonic muscle contractions and tremors associated with dystonia, which is a tremor in a body part that is not dystonic, but there is dystonia elsewhere. | |
| 1.3 Essential tremor | This is one of the most frequent movement disorders. It affects the hand and can expand to the head. The shaking frequency will often decay with age, but the severity or magnitude of the tremor might increase. | Haubenberger, D. et al. [29] | The neurologic examination shows a postural and tremor of both hands in medium (4 to 8 Hz) from a 62-year-old woman. The tremor started slowly and symmetrically and progressed gradually. | |
| 1.4 Orthostatic tremor | This is a rare disorder consisting of erratic and rapid contractions of the legs that tend to occur when the individual is standing. | Niemann, N. and Jankovic, J. [30] | Drugs commonly used to treat Parkinson’s disease may also be prescribed for individuals with primary orthostatic tremors. Surgical treatment should be reserved only for the most disabling cases. Additional treatments are required. | |
| 2. Parkinsonian tremor | 2.1 Resting tremor | Primarily affects an individual’s appendages, such as fingers, hands, or jaw, when they are at rest. In addition, it is also very apparent in isometric and task-specific situations. | Schneider, S. A. et al. [31] | Clinicians should be aware that primary adult-onset dystonia can present with an asymmetric resting arm tremor. Among patients suspected of PD, Parkinsonian tremor might be one cause. |
| 2.2 Postural tremor | Occurs when muscles are contracted, such as when maintaining part of the body at a fixed position against gravity. | Anouti, A. et al. [32], Lin, Y. et al. [33] | The energies of the tremor in seven patients before applying the bio-electronic stimulation therapy is higher than those after |
| Patient | Level of Tremor | Samples |
|---|---|---|
| g2 | Too much | 7176 |
| v4 | Too much | 6282 |
| v5 | Too much | 3400 |
| s6 | Too much | 9114 |
| s7 | Too much | 6264 |
| s8 | Too much | 6216 |
| g9 | Too much | 6354 |
| g10 | Too much | 6180 |
| g11 | Too much | 6306 |
| g12 | Too much | 6360 |
| Item | Our Approach | The Other [40] |
|---|---|---|
| Type | Wearable | Wearable |
| Location | Wrist | Finger |
| Transmission | Mechanical | Cable-enabled |
| Driving motor | DC | Brushless DC |
| Microcontroller | Tiva-C | LPC1768 |
| Sampling rate | 1 kHz | 100 Hz |
| Sensor(s) | 03 Inertial Measurement Units | 05 Inertial Measurement Units |
| Motion | Flywheel | Rotary-to-linear converter |
| Level of harmonics | 03 | 03 |
| Overall tremor amplitude reduction | 92.6% | 85% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan Van, H.; Ngo, H.Q.T. Developing an Assisting Device to Reduce the Vibration on the Hands of Elders. Appl. Sci. 2021, 11, 5026. https://doi.org/10.3390/app11115026
Phan Van H, Ngo HQT. Developing an Assisting Device to Reduce the Vibration on the Hands of Elders. Applied Sciences. 2021; 11(11):5026. https://doi.org/10.3390/app11115026
Chicago/Turabian StylePhan Van, Hieu, and Ha Quang Thinh Ngo. 2021. "Developing an Assisting Device to Reduce the Vibration on the Hands of Elders" Applied Sciences 11, no. 11: 5026. https://doi.org/10.3390/app11115026
APA StylePhan Van, H., & Ngo, H. Q. T. (2021). Developing an Assisting Device to Reduce the Vibration on the Hands of Elders. Applied Sciences, 11(11), 5026. https://doi.org/10.3390/app11115026






