LED Lighting and High-Density Planting Enhance the Cost-Efficiency of Halimione Portulacoides Extraction Units for Integrated Aquaculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Setup
2.3. Growth Performance
2.4. Nutrient Extraction Efficiency
2.5. Photosynthetic Pigments
2.6. Statistical Analysis
3. Results
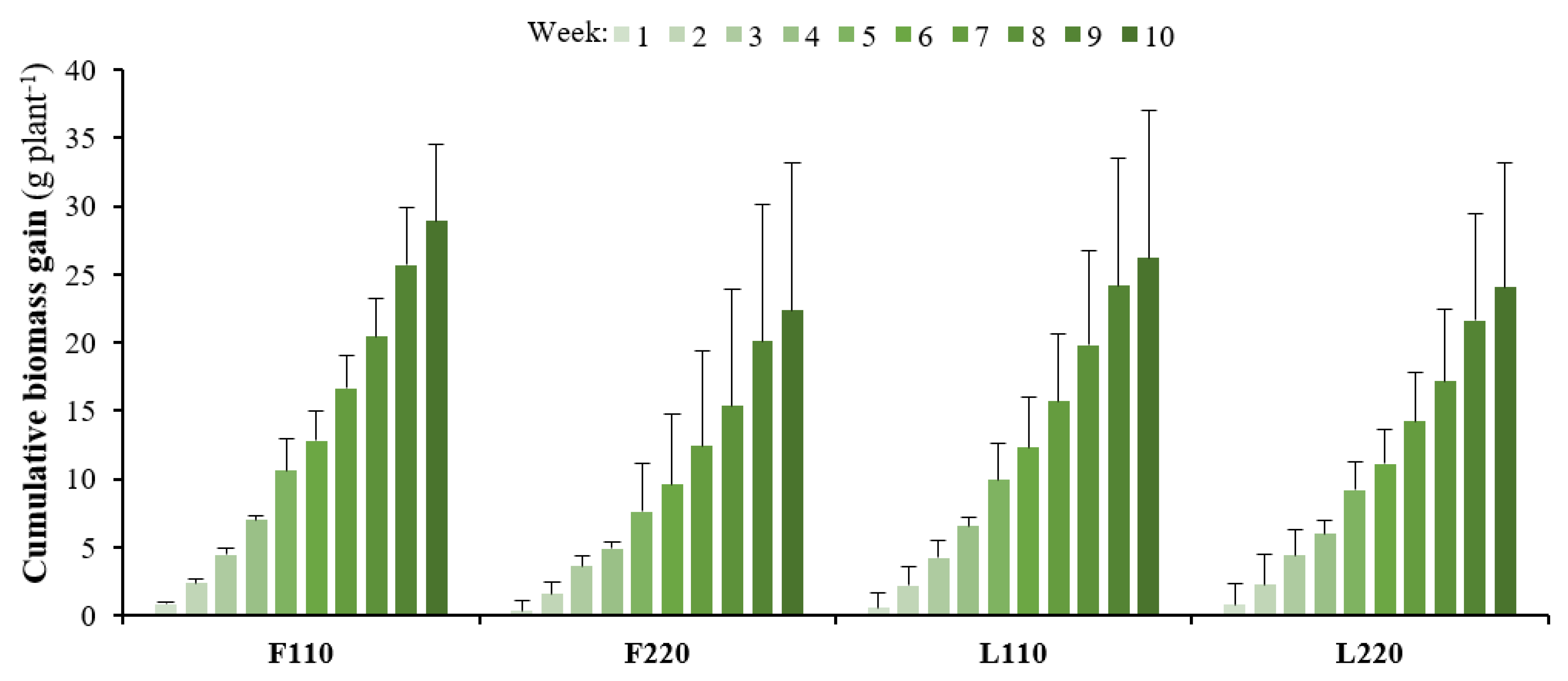
3.1. Growth Parameters and Productivity
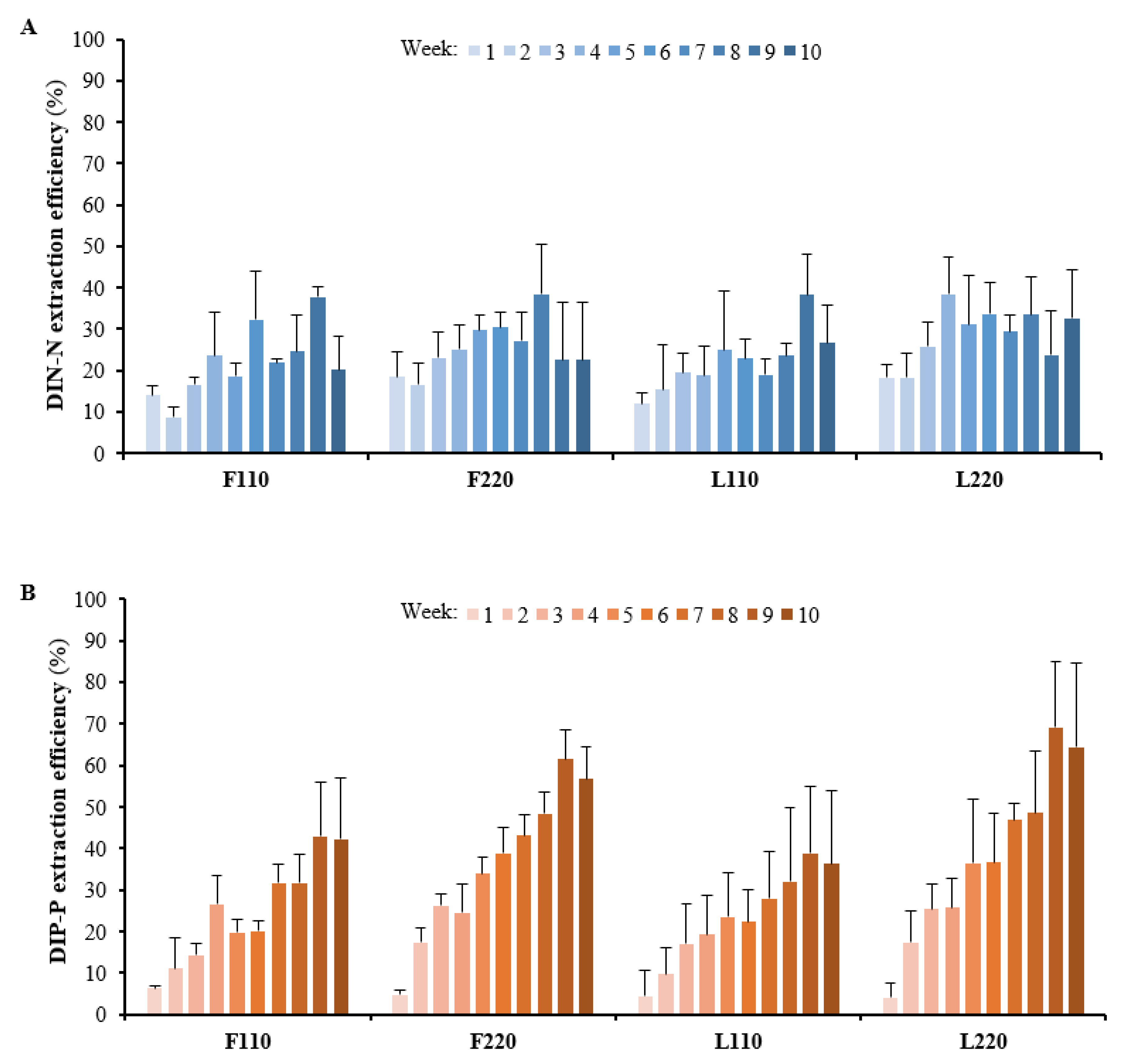
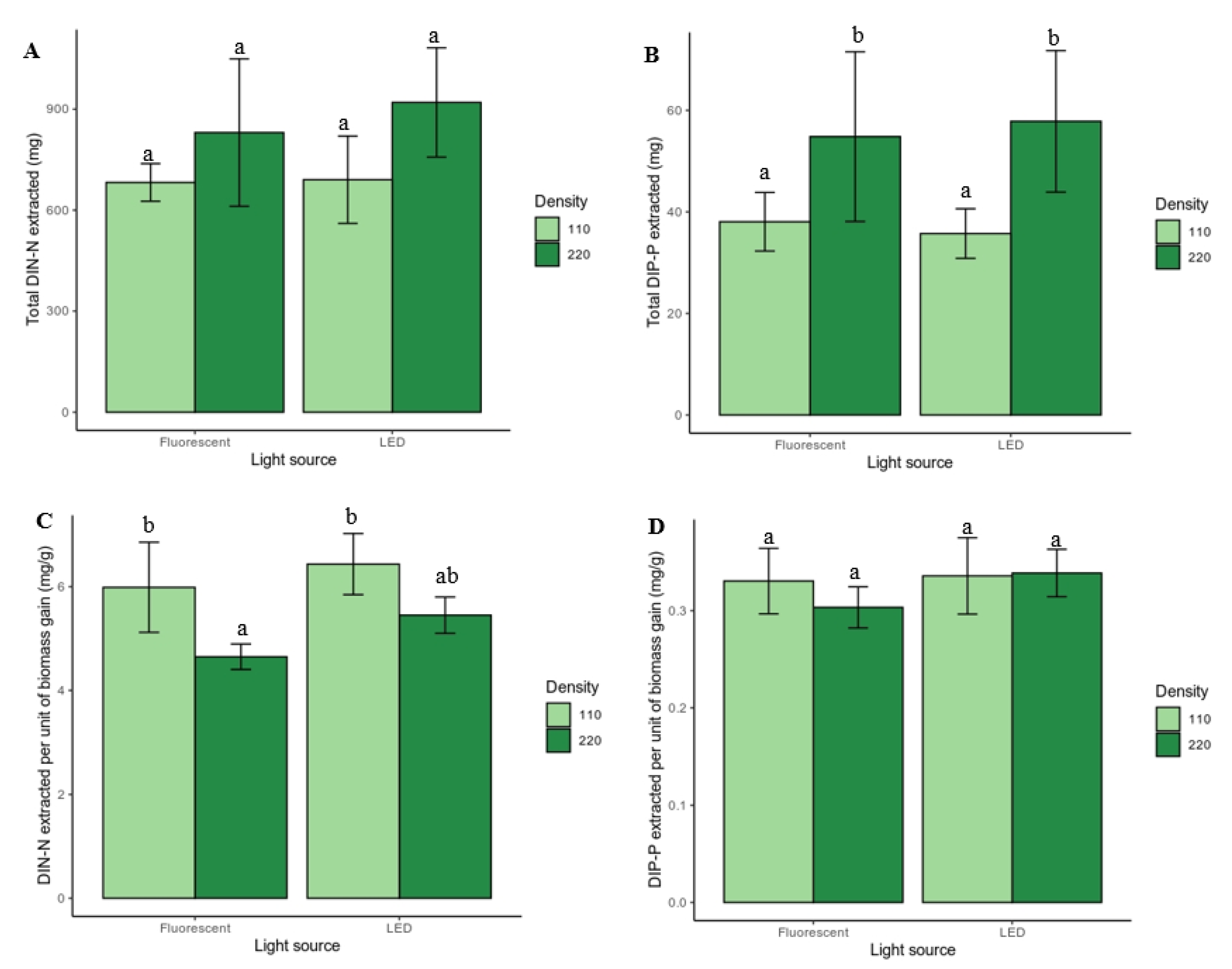
3.2. Extraction of Dissolved Inorganic N and P
3.3. Photosynthetic Pigments
4. Discussion
4.1. Artificial Lighting
4.2. Planting Density
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Chopin, T.; Robinson, S.M.C.; Troell, M.; Neori, A.; Buschmann, A.H.; Fang, J. Multitrophic Integration for Sustainable Marine Aquaculture. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2463–2475. ISBN 978-0-08-045405-4. [Google Scholar]
- Custódio, M.; Villasante, S.; Cremades, J.; Calado, R.; Lillebø, A.I. Unravelling the Potential of Halophytes for Marine Integrated Multi-Trophic Aquaculture (IMTA)—A Perspective on Performance, Opportunities and Challenges. Aquac. Environ. Interact. 2017, 9, 445–460. [Google Scholar] [CrossRef]
- Gunning, D.; Maguire, J.; Burnell, G. The Development of Sustainable Saltwater-Based Food Production Systems: A Review of Established and Novel Concepts. Water 2016, 8, 598. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-15942-9. [Google Scholar]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New Developments in Recirculating Aquaculture Systems in Europe: A Perspective on Environmental Sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Ruiz-Salmón, I.; Margallo, M.; Laso, J.; Villanueva-Rey, P.; Mariño, D.; Quinteiro, P.; Dias, A.C.; Nunes, M.L.; Marques, A.; Feijoo, G.; et al. Addressing Challenges and Opportunities of the European Seafood Sector under a Circular Economy Framework. Curr. Opin. Environ. Sci. Health 2020, 13, 101–106. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Maciel, E.; Costa Leal, M.; Lillebø, A.I.; Domingues, P.; Domingues, M.R.; Calado, R. Bioprospecting of Marine Macrophytes Using MS-Based Lipidomics as a New Approach. Mar. Drugs 2016, 14, 49. [Google Scholar] [CrossRef]
- Sharma, R.; Wungrampha, S.; Singh, V.; Pareek, A.; Sharma, M.K. Halophytes As Bioenergy Crops. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- WHO. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150483-6. [Google Scholar]
- WHO; Regional Office for Europe. European Food and Nutrition Action Plan 2015–2020; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2015; ISBN 978-92-890-5123-1. [Google Scholar]
- Zanella, L.; Vianello, F. Functional Food from Endangered Ecosystems: Atriplex portulacoides as a Case Study. Foods 2020, 9, 1533. [Google Scholar] [CrossRef]
- Custódio, M.; Villasante, S.; Calado, R.; Lillebø, A.I. Testing the Hydroponic Performance of the Edible Halophyte Halimione portulacoides, a Potential Extractive Species for Coastal Integrated Multi-Trophic Aquaculture. Sci. Total Environ. 2020, 144378. [Google Scholar] [CrossRef]
- Custódio, M.; Maciel, E.; Domingues, M.R.; Lillebø, A.I.; Calado, R. Nutrient Availability Affects the Polar Lipidome of Halimione portulacoides Leaves Cultured in Hydroponics. Sci. Rep. 2020, 10, 6583. [Google Scholar] [CrossRef]
- Maciel, E.; Domingues, P.; Domingues, M.R.M.; Calado, R.; Lillebø, A. Halophyte Plants from Sustainable Marine Aquaponics Are a Valuable Source of Omega-3 Polar Lipids. Food Chem. 2020, 320, 126560. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.; Lillebø, A.; Domingues, P.; da Costa, E.; Calado, R.; Domingues, M.R.M. Polar Lipidome Profiling of Salicornia ramosissima and Halimione portulacoides and the Relevance of Lipidomics for the Valorization of Halophytes. Phytochemistry 2018, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.; Calado, R.; Lillebø, A.I. New Species for the Biomitigation of a Super-Intensive Marine Fish Farm Effluent: Combined Use of Polychaete-Assisted Sand Filters and Halophyte Aquaponics. Sci. Total Environ. 2017, 599–600, 1922–1928. [Google Scholar] [CrossRef]
- Martins-Noguerol, R.; Cambrollé, J.; Mancilla-Leytón, J.M.; Puerto-Marchena, A.; Muñoz-Vallés, S.; Millán-Linares, M.C.; Millán, F.; Martínez-Force, E.; Figueroa, M.E.; Pedroche, J.; et al. Influence of Soil Salinity on the Protein and Fatty Acid Composition of the Edible Halophyte Halimione portulacoides. Food Chem. 2021, 352, 129370. [Google Scholar] [CrossRef]
- Cha, M.-K.; Jeon, Y.A.; Son, J.E.; Cho, Y.-Y. Development of Planting-Density Growth Harvest (PGH) Charts for Quinoa (Chenopodium quinoa Willd.) and Sowthistle (Ixeris dentata Nakai) Grown Hydroponically in Closed-Type Plant Production Systems. Hortic. Environ. Biotechnol. 2016, 57, 213–218. [Google Scholar] [CrossRef]
- van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-Analyses of Interspecific Variation and Environmental Control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Truax, B.; Fortier, J.; Gagnon, D.; Lambert, F. Planting Density and Site Effects on Stem Dimensions, Stand Productivity, Biomass Partitioning, Carbon Stocks and Soil Nutrient Supply in Hybrid Poplar Plantations. Forests 2018, 9, 293. [Google Scholar] [CrossRef]
- Jones, M.A. Using Light to Improve Commercial Value. Hortic. Res. 2018, 5, 47. [Google Scholar] [CrossRef]
- Viršilė, A.; Olle, M.; Duchovskis, P. LED Lighting in Horticulture. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 113–147. ISBN 978-981-10-5807-3. [Google Scholar]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Cammarisano, L.; Donnison, I.S.; Robson, P.R.H. The Effect of Red & Blue Rich LEDs vs. Fluorescent Light on Lollo Rosso Lettuce Morphology and Physiology. Front. Plant Sci. 2021, 12, 603411. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Kook, H.-S.; Park, S.-H.; Jang, Y.-J.; Lee, G.-W.; Kim, J.S.; Kim, H.M.; Oh, B.-T.; Chae, J.-C.; Lee, K.-J. Blue LED (Light-Emitting Diodes)-Mediated Growth Promotion and Control of Botrytis Disease in Lettuce. Acta Agric. Scand. B Soil Plant Sci. 2013, 63, 271–277. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-Scale Vegetable Production and the Rise of Microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High Performance of Vegetables, Flowers, and Medicinal Plants in a Red-Blue LED Incubator for Indoor Plant Production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- He, J. Integrated Vertical Aeroponic Farming Systems for Vegetable Production in Space Limited Environments. Acta Hortic. 2017, 25–36. [Google Scholar] [CrossRef]
- Sanoubar, R.; Calone, R.; Noli, E.; Barbanti, L. Data on Seed Germination Using LED versus Fluorescent Light under Growth Chamber Conditions. Data Brief 2018, 19, 594–600. [Google Scholar] [CrossRef]
- Weeplian, T.; Yen, T.-B.; Ho, Y.-S. Growth, Development, and Chemical Constituents of Edible Ice Plant (Mesembryanthemum crystallinum L.) Produced under Combinations of Light-Emitting Diode Lights. HortScience 2018, 53, 865–874. [Google Scholar] [CrossRef]
- Wang, L.-W.; Showalter, A.M.; Ungar, I.A. Effects of Intraspecific Competition on Growth and Photosynthesis of Atriplex prostrata. Aquat. Bot. 2005, 83, 187–192. [Google Scholar] [CrossRef]
- Boxman, S.E.; Nystrom, M.; Capodice, J.C.; Ergas, S.J.; Main, K.L.; Trotz, M.A. Effect of Support Medium, Hydraulic Loading Rate and Plant Density on Water Quality and Growth of Halophytes in Marine Aquaponic Systems. Aquac. Res. 2017, 48, 2463–2477. [Google Scholar] [CrossRef]
- Khan, M.A.; Aziz, S. Some Aspects of Salinity, Plant Density, and Nutrient Effects on Cressa cretica L. J. Plant Nutr. 1998, 21, 769–784. [Google Scholar] [CrossRef][Green Version]
- Webb, J.M.; Quintã, R.; Papadimitriou, S.; Norman, L.; Rigby, M.; Thomas, D.N.; Le Vay, L. The Effect of Halophyte Planting Density on the Efficiency of Constructed Wetlands for the Treatment of Wastewater from Marine Aquaculture. Ecol. Eng. 2013, 61, 145–153. [Google Scholar] [CrossRef]
- Buhmann, A.K.; Waller, U.; Wecker, B.; Papenbrock, J. Optimization of Culturing Conditions and Selection of Species for the Use of Halophytes as Biofilter for Nutrient-Rich Saline Water. Agric. Water Manag. 2015, 149, 102–114. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of Seawater Concentration on the Productivity and Nutritional Value of Annual Salicornia and Perennial Sarcocornia Halophytes as Leafy Vegetable Crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Cruz, S.; Calado, R.; Serôdio, J.; Jesus, B.; Cartaxana, P. Pigment Profile in the Photosynthetic Sea Slug Elysia viridis (Montagu, 1804). J. Mollus. Stud. 2014, 80, 475–481. [Google Scholar] [CrossRef][Green Version]
- Izzo, L.G.; Arena, C.; De Micco, V.; Capozzi, F.; Aronne, G. Light Quality Shapes Morpho-Functional Traits and Pigment Content of Green and Red Leaf Cultivars of Atriplex hortensis. Sci. Hortic. 2019, 246, 942–950. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-Emitting Diodes in Horticulture. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–88. ISBN 978-1-119-10778-1. [Google Scholar]
- Oliver, L.P.; Coyle, S.D.; Bright, L.A.; Shultz, R.C.; Hager, J.V.; Tidwell, J.H. Comparison of Four Artificial Light Technologies for Indoor Aquaponic Production of Swiss Chard, Beta vulgaris, and Kale, Brassica oleracea. J. World Aquacult. Soc. 2018, 49, 837–844. [Google Scholar] [CrossRef]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of Light-Emitting Diode Irradiation on Photosynthesis, Phytochemical Composition and Mineral Element Content of Lettuce Cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Chen, X.; Guo, W.; Xue, X.; Wang, L.; Qiao, X. Growth and Quality Responses of ‘Green Oak Leaf’ Lettuce as Affected by Monochromic or Mixed Radiation Provided by Fluorescent Lamp (FL) and Light-Emitting Diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Namgyel, T.; Khunarak, C.; Siyang, S.; Pobkrut, T.; Norbu, J.; Kerdcharoen, T. Effects of Supplementary LED Light on the Growth of Lettuce in a Smart Hydroponic System. In Proceedings of the 10th International Conference on Knowledge and Smart Technology (KST 2018), Chiang Mai, Thailand, 31 January–3 February 2018; pp. 216–220. [Google Scholar]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of Light Quality on the Accumulation of Phytochemicals in Vegetables Produced in Controlled Environments: A Review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Bugbee, B. Toward an Optimal Spectral Quality for Plant Growth and Development: The Importance of Radiation Capture. Acta Hortic. 2016, 1–12. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. The Role of Xanthophyll Cycle Carotenoids in the Protection of Photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Metallo, R.M.; Kopsell, D.A.; Sams, C.E.; Bumgarner, N.R. Influence of Blue/Red vs. White LED Light Treatments on Biomass, Shoot Morphology, and Quality Parameters of Hydroponically Grown Kale. Sci. Hortic. 2018, 235, 189–197. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue Light-Emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Kitayama, M.; Nguyen, D.T.P.; Lu, N.; Takagaki, M. Effect of Light Quality on Physiological Disorder, Growth, and Secondary Metabolite Content of Water Spinach (Ipomoea aquatica Forsk) Cultivated in a Closed-Type Plant Production System. Korean J. Hortic. Sci. Technol. 2019, 37, 206–218. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting Broccoli Accumulate Higher Concentrations of Nutritionally Important Metabolites under Narrow-Band Light-Emitting Diode Lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting Green or Far-Red Radiation for Blue Radiation Induces Shade Avoidance and Promotes Growth in Lettuce and Kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef]
- Lin, K.; Huang, Z.; Xu, Y. Influence of Light Quality and Intensity on Biomass and Biochemical Contents of Hydroponically Grown Lettuce. HortScience 2018, 53, 1157–1163. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The Effects of Red, Blue, and White Light-Emitting Diodes on the Growth, Development, and Edible Quality of Hydroponically Grown Lettuce (Lactuca sativa L. Var. Capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for Energy Efficient Greenhouse Lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of Environment Lighting on the Growth, Photosynthesis, and Quality of Hydroponic Lettuce in a Plant Factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Duarte, B.; Silva, H.; Dias, J.M.; Sleimi, N.; Marques, J.C.; Caçador, I. Functional and Ecophysiological Traits of Halimione portulacoides and Sarcocornia perennis Ecotypes in Mediterranean Salt Marshes under Different Tidal Exposures. Ecol. Res. 2018, 33, 1145–1156. [Google Scholar] [CrossRef]
- Davy, A.J.; Bishop, G.F.; Costa, C.S.B. Salicornia L. (Salicornia pusilla J. Woods, S. ramosissima J. Woods, S. europaea L., S. obscura P.W. Ball & Tutin, S. nitens P.W. Ball & Tutin, S. fragilis P.W. Ball & Tutin and S. dolichostachya Moss). J. Ecol. 2001, 89, 681–707. [Google Scholar] [CrossRef]
- Shpigel, M.; Ben-Ezra, D.; Shauli, L.; Sagi, M.; Ventura, Y.; Samocha, T.; Lee, J.J. Constructed Wetland with Salicornia as a Biofilter for Mariculture Effluents. Aquaculture 2013, 412–413, 52–63. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Potential of Producing Salicornia bigelovii Hydroponically as a Vegetable at Moderate NaCl Salinity. HortScience 2014, 49, 1154–1157. [Google Scholar] [CrossRef]
- Waller, U.; Buhmann, A.K.; Ernst, A.; Hanke, V.; Kulakowski, A.; Wecker, B.; Orellana, J.; Papenbrock, J. Integrated Multi-Trophic Aquaculture in a Zero-Exchange Recirculation Aquaculture System for Marine Fish and Hydroponic Halophyte Production. Aquacult. Int. 2015, 23, 1473–1489. [Google Scholar] [CrossRef]
- Smaje, C. The Strong Perennial Vision: A Critical Review. Agroecol. Sustain. Food Syst. 2015, 39, 471–499. [Google Scholar] [CrossRef]
- Pinheiro, I.; Arantes, R.; do Espírito Santo, C.M.; do Nascimento Vieira, F.; Lapa, K.R.; Gonzaga, L.V.; Fett, R.; Barcelos-Oliveira, J.L.; Seiffert, W.Q. Production of the Halophyte Sarcocornia ambigua and Pacific White Shrimp in an Aquaponic System with Biofloc Technology. Ecol. Eng. 2017, 100, 261–267. [Google Scholar] [CrossRef]
- Pinheiro, I.; Carneiro, R.F.S.; Vieira, F. do N.; Gonzaga, L.V.; Fett, R.; Costa, A.C. de O.; Magallón-Barajas, F.J.; Seiffert, W.Q. Aquaponic Production of Sarcocornia ambigua and Pacific White Shrimp in Biofloc System at Different Salinities. Aquaculture 2020, 519, 734918. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of Plant Competition for Nutrients, Water and Light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Lüderitz, V.; Gerlach, F. Phosphorus Removal in Different Constructed Wetlands. Acta Biotechnol. 2002, 22, 91–99. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen Transformations in Aquaponic Systems: A Review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.B.; Hassan, A. A Study on the Optimal Hydraulic Loading Rate and Plant Ratios in Recirculation Aquaponic System. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef]

| Fluorescent Lamp | LED Tile | |
|---|---|---|
| Reference name | Philips 54W/830 Min Bipin T5 HO ALTO UNP | AquaBeam 1500 Ultima NP Ocean Blue Light |
| Power (W) | 54 | 30 |
| Luminous flux (lm) | 5000 | 1965 |
| Luminous efficacy (lm/W) | 93 | 66 |
| Correlated color temperature (K) | 3000 | 20,000 |
| Chromaticity coordinates | X: 0.436; Y: 0.404 | X: 0.250; Y: 0.253 |
| Photosynthetic photon flux density (µmol m−2 s−1) | 371.0 ± 12.0 | |
| Unit | F110 | F220 | L110 | L220 | |
|---|---|---|---|---|---|
| Growth per plant | |||||
| Initial biomass | g FW | 6.8 ± 0.3 | 6.8 ± 0.2 | 6.8 ± 0.1 | 6.8 ± 0.1 |
| Final biomass | g FW | 30.2 ± 5.0 | 24.7 ± 4.5 | 28.4 ± 4.9 | 23.8 ± 3.6 |
| Final aboveground biomass | g FW | 25.7 ± 4.4 a | 21.1 ± 4.0 ab | 24.4 ± 4.5 ab | 20.3 ± 3.2 b |
| Final belowground biomass | g FW | 4.5 ± 0.7 | 3.6 ± 0.5 | 4.1 ± 0.4 | 3.5 ± 0.5 |
| Leaves | n | 243 ± 36 | 205 ± 34 | 261 ± 33 | 218 ± 28 |
| Stems | mm | 55.2 ± 7.0 a | 41.2 ± 5.5 b | 50.3 ± 8.3 ab | 40.9 ± 3.8 b |
| Growth per hydroponic unit | |||||
| Final biomass | g FW | 150.8 ± 25.2 ac | 246.9 ± 44.9 b | 142.2 ± 24.3 c | 237.6 ± 36.4 ab |
| Final aboveground biomass | g FW | 128.5 ± 21.8 a | 210.7 ± 40.4 b | 121.9 ± 22.5 a | 202.9 ± 32.1 b |
| Final belowground biomass | g FW | 22.3 ± 3.4 a | 36.2 ± 5.3 b | 20.3 ± 2.0 a | 34.7 ± 4.6 b |
| Leaves | n | 1215 ± 178 a | 2050 ± 344 b | 1305 ± 167 a | 2176 ± 284 b |
| Stems | mm | 276.0 ± 35.2 a | 412.0 ± 54.9 b | 251.5 ± 41.4 a | 409.2 ± 38.4 b |
| Root: shoot ratio | - | 0.17 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.02 | 0.17 ± 0.01 |
| Relative growth rate | mg g−1 day−1 FW | 21.1 ± 2.1 | 18.1 ± 2.5 | 20.3 ± 2.3 | 17.8 ± 2.1 |
| Productivity | g m−2 day−1 FW | 37.1 ± 7.8 ac | 56.6 ± 14.0 b | 34.4 ± 7.6 c | 54.0 ± 11.5 ab |
| Unit | F110 | F220 | L110 | L220 | |
|---|---|---|---|---|---|
| 9′c-Neoxanthin | mg g−1 DW | 0.292 ± 0.028 | 0.294 ± 0.030 | 0.287 ± 0.016 | 0.295 ± 0.031 |
| Violaxanthin | 0.415 ± 0.042 | 0.415 ± 0.048 | 0.427 ± 0.032 | 0.468 ± 0.026 | |
| Antheraxanthin | 0.038 ± 0.003 | 0.037 ± 0.004 | 0.040 ± 0.007 | 0.032 ± 0.005 | |
| Lutein | 0.875 ± 0.085 | 0.855 ± 0.105 | 0.801 ± 0.061 | 0.861 ± 0.071 | |
| Zeaxanthin | 0.042 ± 0.006 | 0.039 ± 0.002 | 0.051 ± 0.013 | 0.036 ± 0.007 | |
| Chlorophyll b | 2.243 ± 0.217 | 2.141 ± 0.190 | 2.126 ± 0.155 | 2.252 ± 0.236 | |
| Chlorophyll a | 6.282 ± 0.533 | 5.950 ± 0.508 | 6.032 ± 0.451 | 6.269 ± 0.594 | |
| β,β-Carotene | 0.335 ± 0.012 | 0.323 ± 0.038 | 0.320 ± 0.035 | 0.354 ± 0.043 | |
| ratios | |||||
| Chl b:Chl a | 0.357 ± 0.005 | 0.360 ± 0.002 | 0.350.003 | 0.359 ± 0.008 | |
| β,β-Car: Chla | 0.053 ± 0.004 | 0.054 ± 0.002 | 0.053 ± 0.003 | 0.056 ± 0.003 | |
| Xant:Chl a | 0.265 ± 0.003 | 0.275 ± 0.009 | 0.266 ± 0.005 | 0.270 ± 0.008 |
| Species | Hydroponic Technique | Growth Period (Days) | PHOTOPERIOD L/D (h) | PAR (mol m−2 s−1) | Shoot Biomass per Plant (g) | Photosynthetic Pigments * | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FL | LED Blue | LED Red | LED R+B | LED White | |||||||
| Beta vulgaris | Aquaponics | 3 weeks | - | 200 | 33.3 | - | - | - | 117.7 | No differences | [47] |
| Brocolli oleacea var. italica | DWC | 20 | 16/8 | 250 | 51.0 | - | - | 71.8 | - | Higher: LED R+B Lower: FL | [57] |
| Ipomoea aquatica | DWC | 14 | 14/10 | 200 | - | 6.1 | 8.5 | 8.7 | - | Higher: LED R+B, R Lower: LED B | [56] |
| Lactuca sativa var. capitata | DWC | 35 | 16/8 | 210 | 149.0 | - | - | 136.3 | 164.1 (+RB) | No differences | [61] |
| L. sativa var. capitata | NFT | 35 | 16/8 | - | - | 69.7 | 51.0 | 64.5 | - | - | [50] |
| L. sativa var. crispa | DWC | 50 | 14/10 | 133 | 32.1 | 23.5 | 46.9 | 24.4 | - | Higher: LED R+B Lower: LED R | [49] |
| L. sativa var. Korea | NFT | 3 weeks | 16/8 | 150 | 29.5 | - | - | 21.2–42.6 | - | No differences | [60] |
| L. sativa var. Ziwei | DWC | 18 | 16/8 | 300 | 49.3 | - | - | 40.0 | - | - | [63] |
| Species | Life Cycle | Production System | Salinity (ppt) | Growth Period (Weeks) | Retention Time | Initial N (mg L−1) | Initial P (mg L−1) | Plant Density (Plants m−2) | Yields (g m−2 day−1) | N Extracted (%) | P Extracted (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Halimione portulacoides | P | Hydro | 20 | 10 | 1 week | 63.3 | 3.1 | 110 | 36 | 22 | 24 | Present study |
| 220 | 55 | 28 | 36 | |||||||||
| Hydro | 20 | 10 | 1 week | 55.6 | 11.9 | 220 | 73 | 35 | 5 | [16] | ||
| 20.8 | 2.8 | 220 | 63 | 79 | 52 | |||||||
| Aqua | 20 | 22 | 12 h | 8.6 | 0.4 | - | 112 | 65 | 0 | [20] | ||
| Hydro | 15 | 5 | 5 weeks | 50 | 9.8 | 38 | 33 | 50 | 45 | [42] | ||
| Batis maritima | P | Aqua | 15 | 4 | <2 h | variable | - | 92 | 11 * | 89 | - | [39] |
| 184 | 11 * | 15 | - | |||||||||
| Salicornia bigelovii | A | Hydro | 12 | 4 | 1 week | 278.3 | 36.7 | 260 | 73 | - | - | [67] |
| Salicornia dolichostachya | A | Hydro | 15 | 5 | 5 weeks | 50 | 9.8 | 38 | 60 | 48 | 46 | [42] |
| Aqua | 15 | 5 | 1 day | 19.4 | 2.8 | 38 | 60 | 17 | 0 | [68] | ||
| Salicronia europaea | A | CW | ~28 | 3 | 2 days | ~26 | ~10 | 20 | 105 | 48 | 70 | [41] |
| 10,000 | 124 | 45 | 64 | |||||||||
| Salicornia persica | A | CW | 35 | 13 | 1.5 days | 12.2 | 1.6 | 100 | 55 | 53 | 13 | [66] |
| Hydro | 26 | 26 | 1 week | 200 | 200 | 1000 | 87 | - | - | [43] | ||
| Sarcocornia ambigua | P | Aqua | 36 | 10 | - | 22.3 | 5.3 | 100 | 110 | - | - | [70] |
| Sesuvium portulacastrum | P | Aqua | 15 | 4 | <2 h | variable | - | 92 | 18 * | 18 | - | [39] |
| 184 | 18 * | 70 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custódio, M.; Cartaxana, P.; Villasante, S.; Calado, R.; Lillebø, A.I. LED Lighting and High-Density Planting Enhance the Cost-Efficiency of Halimione Portulacoides Extraction Units for Integrated Aquaculture. Appl. Sci. 2021, 11, 4995. https://doi.org/10.3390/app11114995
Custódio M, Cartaxana P, Villasante S, Calado R, Lillebø AI. LED Lighting and High-Density Planting Enhance the Cost-Efficiency of Halimione Portulacoides Extraction Units for Integrated Aquaculture. Applied Sciences. 2021; 11(11):4995. https://doi.org/10.3390/app11114995
Chicago/Turabian StyleCustódio, Marco, Paulo Cartaxana, Sebastián Villasante, Ricardo Calado, and Ana Isabel Lillebø. 2021. "LED Lighting and High-Density Planting Enhance the Cost-Efficiency of Halimione Portulacoides Extraction Units for Integrated Aquaculture" Applied Sciences 11, no. 11: 4995. https://doi.org/10.3390/app11114995
APA StyleCustódio, M., Cartaxana, P., Villasante, S., Calado, R., & Lillebø, A. I. (2021). LED Lighting and High-Density Planting Enhance the Cost-Efficiency of Halimione Portulacoides Extraction Units for Integrated Aquaculture. Applied Sciences, 11(11), 4995. https://doi.org/10.3390/app11114995









