Effect of Muscle-Specific Fatigue on the Risk of Anterior Cruciate Ligament Injury in Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equipment
2.3. Experimental Procedure
- Baseline measurement: Once the participants were ready, they performed three trials of the pre-DDM task, which involved running at a speed of 4.5 ± 0.2 m/s, 5 m behind the force platform, followed by a DDM on top of the force platform (Figure 2A). The trial was valid only when the subject’s right foot gazed at the front and landed on the force plate and with a left turn at a range of 35°–55° in the direction of travel [6,18,19,40]. The running speed was controlled using 2 timing gates (Witty Microgate, Bolzano, Italy).
- Treatment: Muscle-specific fatigue treatment was performed using an isokinetic strength measurement system (Figure 2B) performing knee flexion/extension at a fixed angular velocity. Initially, the subject’s reference value of the peak knee torque was established, taking the average peak torque value of 3 trials of knee flexion/extension at an angular velocity of 90 °/s. The reference value of the peak knee torque was determined before the pre-DDM task. The fatigue task of the QF muscle involved knee extension and flexion at a fixed angular velocity of 90 °/s and 300 °/s, respectively, whereas the angular velocity for extension (300 °/s) and flexion (90 °/s) was reversed for the fatigue task of the HA muscle [22,41]. The onset of fatigue was set when the peak torque reached 50 ± 2% three times consecutively, with a two-minute break between each trial [41,42]. Participants performed walking for 20 min at their preferred walking speed to avoid any muscle injury during the post DDM trials for the control condition.
- Post measurement: During the muscle-specific fatigue protocol, the post-DDM task was performed immediately once the knee peak torque drop was confirmed. For the control treatment condition, participants performed a post-DDM task immediately after completing the 20-min walk, using the same protocol as the pre-test DDM task. Only the first successful trial was used for further analysis considering the fatigue recovery period.
2.4. Data Analysis
2.4.1. ACL Model
2.4.2. Calculation of Kinematic and Kinetic Variables
- Center of mass (COM): based on the location of COM of each segment expressed in meters (m); COM velocity is the first derivative of COM expressed in meters per second (m/s).
- Knee angle (kneeang (°)): refers to the flexion, valgus, and external rotation angles expressed in degrees (°).
- Knee joint force (NN/kg): anterior tibial shear force (kneeshear), normalized shear force; normalized to body mass.
- Knee torque (kneeptorque) (Nm): mean and peak knee torque for flexion and extension expressed in newtons per meter.
- Knee moment (kneemom) (Nm, Nm/(kg ∗ HT)): extension, adduction, internal rotation; All moments computed in the study are defined as internal moment and normalized with body height and mass.
2.5. Statistical Analysis
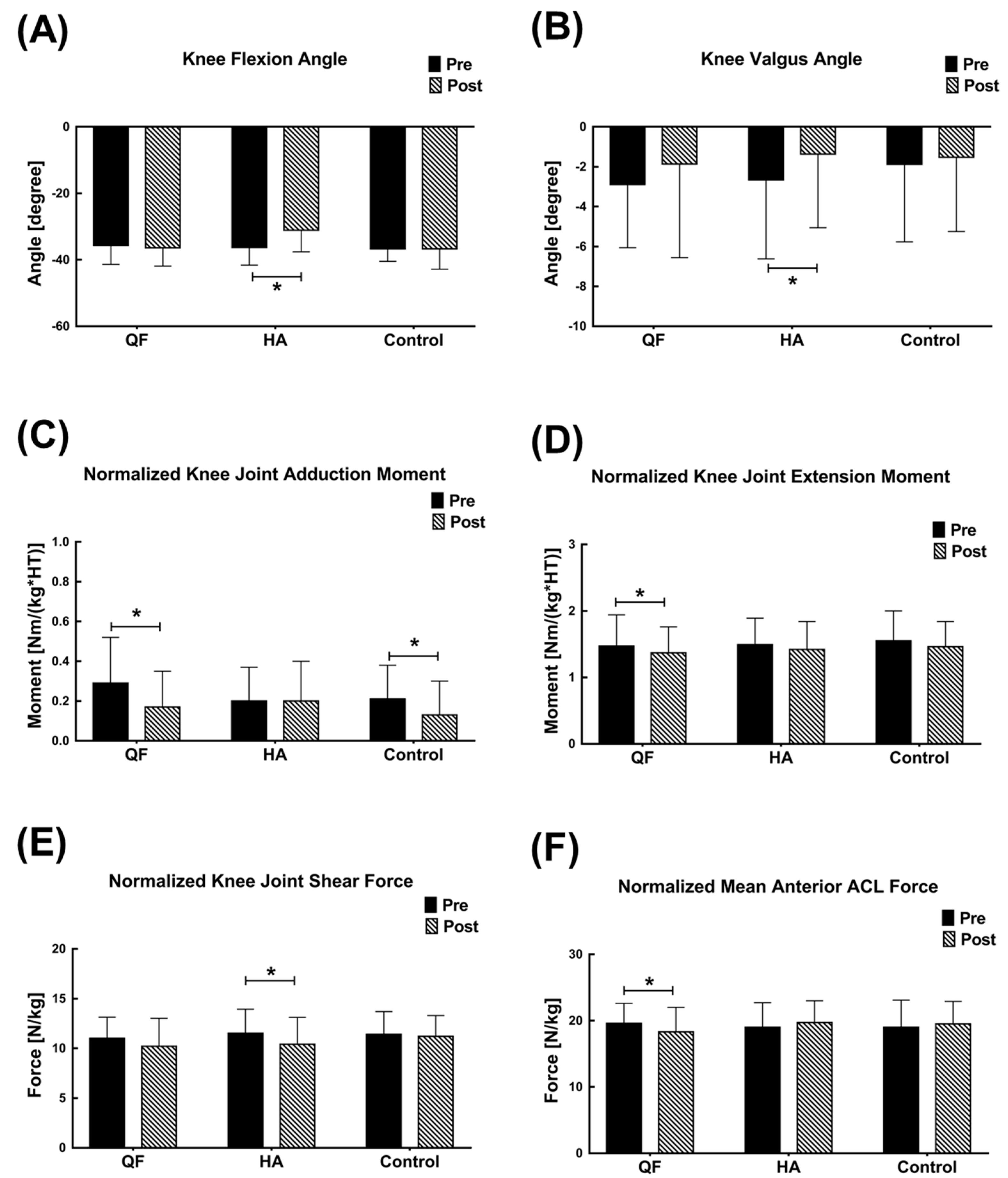
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chappell, J.D.; Herman, D.C.; Knight, B.S.; Kirkendall, D.T.; Garrett, W.E.; Yu, B. Effect of fatigue on knee kinetics and kinematics in stop-jump tasks. Am. J. Sports Med. 2005, 33, 1022–1029. [Google Scholar] [CrossRef]
- Tsai, L.C.; Sigward, S.M.; Pollard, C.D.; Fletcher, M.J.; Powers, C.M. Effects of fatigue and recovery on knee mechanics during side-step cutting. Med. Sci. Sports Exerc. 2009, 41, 1952–1957. [Google Scholar] [CrossRef]
- Enoka, R.M.; Stuart, D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Reed-Jones, R.J.; Vallis, L.A. Proprioceptive deficits of the lower limb following anterior cruciate ligament deficiency affect whole body steering control. Exp. Brain Res. 2007, 182, 249–260. [Google Scholar] [CrossRef]
- Olsen, O.E.; Myklebust, G.; Engebretsen, L.; Bahr, R. Injury mechanisms for anterior cruciate ligament injuries in team handball: A systematic video analysis. Am. J. Sports Med. 2004, 32, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Weinhandl, J.T.; Earl-Boehm, J.E.; Ebersole, K.T.; Huddleston, W.E.; Armstrong, B.S.R.; O’Connor, K.M. Anticipatory effects on anterior cruciate ligament loading during sidestep cutting. Clin. Biomech. 2013, 28, 655–663. [Google Scholar] [CrossRef]
- Joseph, A.M.; Collins, C.L.; Henke, N.M.; Yard, E.E.; Fields, S.K.; Comstock, R.D. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J. Athl. Train. 2013, 48, 810–817. [Google Scholar] [CrossRef] [PubMed]
- McNair, P.; Marshall, R.; Matheson, J. Important features associated with acute anterior cruciate ligament injury. N. Z. Med. J. 1990, 103, 537–539. [Google Scholar] [PubMed]
- Agel, J.; Arendt, E.A.; Bershadsky, B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: A 13-year review. Am. J. Sports Med. 2005, 33, 524–531. [Google Scholar] [CrossRef]
- Bien, D.P. Rationale and implementation of anterior cruciate ligament injury prevention warm-up programs in female athletes. J. Strength Cond. Res. 2011, 25, 271–285. [Google Scholar] [CrossRef]
- Ford, K.R.; Myer, G.D.; Hewett, T.E. Valgus knee motion during landing in high school female and male basketball players. Med. Sci. Sports Exerc. 2003, 35, 1745–1750. [Google Scholar] [CrossRef]
- Imwalle, L.E.; Myer, G.D.; Ford, K.R.; Hewett, T.E. Relationship between hip and knee kinematics in athletic women during cutting maneuvers: A possible link to noncontact anterior cruciate ligament injury and prevention. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2009, 23, 2223. [Google Scholar] [CrossRef]
- Pollard, C.D.; Sigward, S.M.; Ota, S.; Langford, K.; Powers, C.M. The influence of in-season injury prevention training on lower-extremity kinematics during landing in female soccer players. Clin. J. Sport Med. 2006, 16, 223–227. [Google Scholar] [CrossRef]
- Sigward, S.; Powers, C.M. The influence of experience on knee mechanics during side-step cutting in females. Clin. Biomech. 2006, 21, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Newcomer, R.; Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin. Biomech. 2008, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Ford, K.R.; Brent, J.L.; Hewett, T.E. Differential neuromuscular training effects onACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskelet. Disord. 2007, 8, 1–7. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L.J.; Schock, H.J.; Boylan, J.P.; Ashton-Miller, J.A. Gender differences in muscular protection of the knee in torsion in size-matched athletes. JBJS 2003, 85, 782–789. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.G.; Huang, X.; Su, A.; Van Den Bogert, A.J. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin. Biomech. 2004, 19, 828–838. [Google Scholar] [CrossRef]
- McLean, S.G.; Huang, X.; van den Bogert, A.J. Investigating isolated neuromuscular control contributions to non-contact anterior cruciate ligament injury risk via computer simulation methods. Clin. Biomech. 2008, 23, 926–936. [Google Scholar] [CrossRef][Green Version]
- Behrens, M.; Mau-Moeller, A.; Wassermann, F.; Bruhn, S. Effect of fatigue on hamstring reflex responses and posterior-anterior tibial translation in men and women. PLoS ONE 2013, 8, e56988. [Google Scholar] [CrossRef]
- Cortes, N.; Greska, E.; Kollock, R.; Ambegaonkar, J.; Onate, J.A. Changes in lower extremity biomechanics due to a short-term fatigue protocol. J. Athl. Train. 2013, 48, 306–313. [Google Scholar] [CrossRef]
- Geiser, C.F.; O’Connor, K.M.; Earl, J.E. Effects of isolated hip abductor fatigue on frontal plane knee mechanics. Memed. Sci. Sports Exerc. 2010, 42, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Zebis, M.K.; Bencke, J.; Andersen, L.L.; Alkjaer, T.; Suetta, C.; Mortensen, P.; Kjaer, M.; Aagaard, P. Acute fatigue impairs neuromuscular activity of anterior cruciate ligament-agonist muscles in female team handball players. Scand. J. Med. Sci. 2011, 21, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Kernozek, T.W.; Ragan, R.J. Estimation of anterior cruciate ligament tension from inverse dynamics data and electromyography in females during drop landing. Clin. Biomech. 2008, 23, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Malinzak, R.A.; Colby, S.M.; Kirkendall, D.T.; Yu, B.; Garrett, W.E. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin. Biomech. 2001, 16, 438–445. [Google Scholar] [CrossRef]
- Shin, C.S.; Chaudhari, A.M.; Andriacchi, T.P. The influence of deceleration forces on ACL strain during single-leg landing: A simulation study. J. Biomech. 2007, 40, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Navacchia, A.; Ueno, R.; Ford, K.R.; DiCesare, C.A.; Myer, G.D.; Hewett, T.E. EMG-Informed Musculoskeletal Modeling to Estimate Realistic Knee Anterior Shear Force During Drop Vertical Jump in Female Athletes. Ann. Biomed. Eng. 2019, 47, 2416–2430. [Google Scholar] [CrossRef]
- Simonsen, E.B.; Magnusson, S.P.; Bencke, J.; Naesborg, H.; Havkrog, M.; Ebstrup, J.F.; Sørensen, H. Can the hamstring muscles protect the anterior cruciate ligament during a side-cutting maneuver. Scand. J. Med. Sci. 2000, 10, 78–84. [Google Scholar] [CrossRef]
- Lee, J.; Pathak, P.; Panday, S.B.; Moon, J. Effect of Foot-Planting Strategy on Anterior Cruciate Ligament Loading in Women During a Direction Diversion Maneuver: A Musculoskeletal Modeling Approach. Orthop. J. Sports Med. 2020, 8, 2325967120963180. [Google Scholar] [CrossRef]
- Moon, J.; Kim, H.; Lee, J.; Panday, S.B. Effect of wearing a knee brace or sleeve on the knee joint and anterior cruciate ligament force during drop jumps: A clinical intervention study. Knee 2018, 25, 1009–1015. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.J.; Chinnasee, C.; Weir, G.; Sasimontonkul, S.; Alderson, J. Joint dynamics of rear- and fore-foot unplanned sidestepping. J. Sci. Med. Sport 2017, 20, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.M.; Hearn, B.K.; Andriacchi, T.P. Sport-dependent variations in arm position during single-limb landing influence knee loading: Implications for anterior cruciate ligament injury. Am. J. Sports Med. 2005, 33, 824–830. [Google Scholar] [CrossRef]
- Elias, L.J.; Bryden, M.P.; Bulman-Fleming, M.B. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 1998, 36, 37–43. [Google Scholar] [CrossRef]
- Van Melick, N.; Meddeler, B.M.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.; van Cingel, R.E. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS ONE 2017, 12, e0189876. [Google Scholar] [CrossRef] [PubMed]
- Law, R.Y.; Herbert, R.D. Warm-up reduces delayed-onset muscle soreness but cool-down does not: A randomised controlled trial. Aust. J. Physiother. 2007, 53, 91–95. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.D.; Ghoussayni, S.N.; Ewins, D.J.; Kent, J.A. A six degrees-of-freedom marker set for gait analysis: Repeatability and comparison with a modified Helen Hayes set. Gait. Posture 2009, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, M.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Donnelly, C.J.; Lloyd, D.G.; Elliott, B.C.; Reinbolt, J.A. Optimizing whole-body kinematics to minimize valgus knee loading during sidestepping: Implications for ACL injury risk. J. Biomech. 2012, 45, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Hantes, M.E.; Tsarouhas, A.; Giakas, G.; Spiropoulos, G.; Sideris, V.; Christel, P.; Malizos, K.N. Effect of fatigue on tibial rotation after single- and double-bundle anterior cruciate ligament reconstruction: A 3-dimensional kinematic and kinetic matched-group analysis. Am. J. Sports Med. 2012, 40, 2045–2051. [Google Scholar] [CrossRef]
- Bellew, J.W.; Fenter, P.C. Control of balance differs after knee or ankle fatigue in older women. Arch. Phys. Med. Rehabil. 2006, 87, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Dorn, T.W.; Lin, Y.-C.; Pandy, M.G. Estimates of muscle function in human gait depend on how foot-ground contact is modelled. Comput. Methods Biomech. Biomed. Engin. 2012, 15, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Kar, J.; Quesada, P.M. A numerical simulation approach to studying anterior cruciate ligament strains and internal forces among young recreational women performing valgus inducing stop-jump activities. Ann. Biomed. Eng. 2012, 40, 1679–1691. [Google Scholar] [CrossRef]
- Kar, J.; Quesada, P.M. A musculoskeletal modeling approach for estimating anterior cruciate ligament strains and knee anterior-posterior shear forces in stop-jumps performed by young recreational female athletes. Ann. Biomed. Eng. 2013, 41, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.; Marshall, S.; Batterham, A.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA; London, UK, 1988. [Google Scholar]
- DeMorat, G.; Weinhold, P.; Blackburn, T.; Chudik, S.; Garrett, W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am. J. Sports Med. 2004, 32, 477–483. [Google Scholar] [CrossRef]
- Navacchia, A.; Kefala, V.; Shelburne, K.B. Dependence of Muscle Moment Arms on In Vivo Three-Dimensional Kinematics of the Knee. Ann. Biomed. Eng. 2017, 45, 789–798. [Google Scholar] [CrossRef]
- Kellis, E.; Galanis, N.; Kofotolis, N.; Hatzi, A. Effects of hip flexion angle on surface electromyographic activity of the biceps femoris and semitendinosus during isokinetic knee flexion. Muscles Ligaments Tendons J. 2017, 7, 286. [Google Scholar] [CrossRef]
- Navacchia, A.; Bates, N.A.; Schilaty, N.D.; Krych, A.J.; Hewett, T.E. Knee abduction and internal rotation moments increase ACL force during landing through the posterior slope of the tibia. J. Orthop. Res. 2019, 37, 1730–1742. [Google Scholar] [CrossRef]
- Markolf, K.L.; Burchfield, D.M.; Shapiro, M.M.; Shepard, M.F.; Finerman, G.A.; Slauterbeck, J.L. Combined knee loading states that generate high anterior cruciate ligament forces. J. Orthop. Res. 1995, 13, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E. Antagonist moment of force during maximal knee extension in pubertal boys: Effects of quadriceps fatigue. Eur. J. Appl. Physiol. 2003, 89, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bloswick, D.; Merryweather, A. An improved OpenSim gait model with multiple degrees of freedom knee joint and knee ligaments. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Murdock, G.H.; Hubley-Kozey, C.L. Effect of a high intensity quadriceps fatigue protocol on knee joint mechanics and muscle activation during gait in young adults. Eur. J. Appl. Physiol. 2012, 112, 439–449. [Google Scholar] [CrossRef] [PubMed]


| Pre (Nm) | Post (Nm) | Repetition (Frequency) | Ratio (%) | |
|---|---|---|---|---|
| QF fatigue | 146.2 ± 23.9 | 74.9 ± 12.6 | 15.5 ± 5.3 | 51.3 ± 0.5 |
| HA fatigue | 66.3 ± 14.3 | 33.6 ± 5.9 | 18.9 ± 4.0 | 50.7 ± 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Lee, J.; Kim, K.; Koo, D.; Lee, J.; Pathak, P.; Sanchez, G.A.R.; Panday, S.B. Effect of Muscle-Specific Fatigue on the Risk of Anterior Cruciate Ligament Injury in Females. Appl. Sci. 2021, 11, 4969. https://doi.org/10.3390/app11114969
Moon J, Lee J, Kim K, Koo D, Lee J, Pathak P, Sanchez GAR, Panday SB. Effect of Muscle-Specific Fatigue on the Risk of Anterior Cruciate Ligament Injury in Females. Applied Sciences. 2021; 11(11):4969. https://doi.org/10.3390/app11114969
Chicago/Turabian StyleMoon, Jeheon, Jinseok Lee, Keehyun Kim, Dohoon Koo, Jusung Lee, Prabhat Pathak, Gustavo Adrian Ruiz Sanchez, and Siddhartha Bikram Panday. 2021. "Effect of Muscle-Specific Fatigue on the Risk of Anterior Cruciate Ligament Injury in Females" Applied Sciences 11, no. 11: 4969. https://doi.org/10.3390/app11114969
APA StyleMoon, J., Lee, J., Kim, K., Koo, D., Lee, J., Pathak, P., Sanchez, G. A. R., & Panday, S. B. (2021). Effect of Muscle-Specific Fatigue on the Risk of Anterior Cruciate Ligament Injury in Females. Applied Sciences, 11(11), 4969. https://doi.org/10.3390/app11114969








