Thermodynamics of Soil Microbial Metabolism: Applications and Functions
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermodynamic Characterization of SOM
2.2. Thermodynamics of the Soil Microbial Metabolism (SMM)
3. Results
3.1. Thermodynamic Characterization of SOM
3.2. Thermodynamics of SOM Microbial Decomposition
3.2.1. Thermodynamic State Variables and Soil Microbial Diversity
3.2.2. Microbial Diversity and Metabolic Carbon Use Efficiency
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Addiscott, T. Entropy, non-linearity and hierarchy in ecosystems. Geoderma 2010, 160, 57–63. [Google Scholar] [CrossRef]
- Hansen, L.D.; Popović, M.; Tolley, H.D.; Woodfield, B.F. Laws of evolution parallel the laws of thermodynamics. J. Chem. Thermodyn. 2018, 124, 141–148. [Google Scholar] [CrossRef]
- Schrödinger, E. What Is Life? The Physical Aspects of a Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Odum, E.P. The Strategy of Ecosystem Development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Fallou, F.A. First Principles of Soil Science; G. Schönfield Buchandlung: Dresden, Germany, 1857. [Google Scholar]
- Conant, R.T. Challenges and Opportunities for Carbon Sequestration in Grasslands Systems: A Technical Report on Grassland Management and Climate Change Mitigation; FAO: Rome, Italy, 2010; Volume 9. [Google Scholar]
- Hedges, J.; Eglinton, G.; Hatcher, P.; Kirchman, D.; Arnosti, C.; Derenne, S.; Evershed, R.; Kögel-Knabner, I.; de Leeuw, J.; Littke, R.; et al. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 2000, 31, 945–958. [Google Scholar] [CrossRef]
- Tfaily, M.M.; Chu, R.K.; Tolić, N.; Roscioli, K.M.; Anderton, C.; Paša-Tolić, L.; Robinson, E.W.; Hess, N.J. Advanced Solvent Based Methods for Molecular Characterization of Soil Organic Matter by High-Resolution Mass Spectrometry. Anal. Chem. 2015, 87, 5206–5215. [Google Scholar] [CrossRef]
- Šimon, T. Quantitative and qualitative characterization of soil organic matter in the long-term fallow experiment with different fertilization and tillage. Arch. Agron. Soil Sci. 2007, 53, 241–251. [Google Scholar] [CrossRef]
- Ljungholm, K.; Norén, B.; Sköld, R.; Wadsö, I. Use of Microcalorimetry for the Characterization of Microbial Activity in Soil. Oikos 1979, 33, 15. [Google Scholar] [CrossRef]
- Barros, N.; Feijoo, S.; Salgado, J. Calorimetry and soil. Thermochim. Acta 2007, 458, 11–17. [Google Scholar] [CrossRef]
- Suurkuusk, J.; Suurkuusk, M.; Vikegard, P. A multichannel microcalorimetric system: The third generation Thermal Activity Monitor (TAM III). J. Thermal. Anal. Calorim. 2017, 15, 1–18. [Google Scholar] [CrossRef]
- Battley, E.H. Energetics of Microbial Growth; John Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
- Heijnen, J.J.; Kleerebezem, R. Bioenergetics of Microbial Growth. In Encyclopedia of Industrial Biotechnology; Wiley: Hoboken, NJ, USA, 2010; pp. 1–24. [Google Scholar]
- Von Stockar, U.; Maskow, T.; Liu, J.; Marison, I.W.; Patiño, R. Thermodynamics of microbial growth and metabolism: An analysis of the current situation. J. Biotechnol. 2006, 121, 517–533. [Google Scholar] [CrossRef] [PubMed]
- LaRowe, D.E.; Van Cappellen, P. Degradation of natural organic matter: A thermodynamic analysis. Geochim. Cosmochim. Acta 2011, 75, 2030–2042. [Google Scholar] [CrossRef]
- Leifeld, J.; Klein, K.; Wüst-Galley, C. Soil organic matter stoichiometry as indicator for peatland degradation. Sci. Rep. 2020, 10, 7634–7642. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.; et al. Persistence of soil organic matter as an ecosystem property. Nat. Cell Biol. 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sciuba, E. What did Lotka really say? A critical reassessment of the maximum power principle. Ecol. Modell. 2011, 222, 1347–1353. [Google Scholar] [CrossRef]
- Song, H.-S.; Stegen, J.C.; Graham, E.B.; Lee, J.-Y.; Garayburu-Caruso, V.A.; Nelson, W.C.; Chen, X.; Moulton, J.D.; Scheibe, T.D. Representing Organic Matter Thermodynamics in Biogeochemical Reactions via Substrate-Explicit Modeling. Front. Microbiol. 2020, 11, 531756. [Google Scholar] [CrossRef] [PubMed]
- Gary, C.; Frossard, J.; Chenevard, D. Heat of combustion, degree of reduction and carbon content: 3 interrelated methods of estimating the construction cost of plant tissues. Agronomie 1995, 15, 59–69. [Google Scholar] [CrossRef]
- Jung, H.-J.G.; Varel, V.H.; Weimer, P.J.; Ralph, J. Accuracy of Klason lignin and acid detergent lignin methods as assessed by bomb calorimetry. J. Agric. Food Chem. 1999, 47, 2005–2008. [Google Scholar] [CrossRef] [PubMed]
- Rovira, P.; Henriques, R. Energy content of soil organic matter as studied by bomb calorimetry. Soil Biol. Biochem. 2008, 40, 172–185. [Google Scholar] [CrossRef]
- Baraldi, P.; Beltrami, C.; Cassai, C.; Molinari, L.; Zunarelli, R. Measurements of combustion enthalpy of solids by DSC. Mater. Chem. Phys. 1998, 53, 252–255. [Google Scholar] [CrossRef]
- Malucelli, L.C.; Silvestre, G.F.; Carneiro, J.; Vasconcelos, E.C.; Guiotoku, M.; Maia, C.M.B.F.; Filho, M.A.S.C. Biochar higher heating value estimative using thermogravimetric analysis. J. Therm. Anal. Calorim. 2019, 139, 2215–2220. [Google Scholar] [CrossRef]
- Barros, N.; Fernandez, I.; Byrne, K.A.; Jovani-Sancho, A.J.; Ros-Mangriñan, E.; Hansen, L.D. Thermodynamics of soil organic matter decomposition in semi-natural oak (Quercus) woodland in southwest Ireland. Oikos 2020, 129, 1632–1644. [Google Scholar] [CrossRef]
- Roels, J.A. Energetics and Kinetics in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Sandler, S.I.; Orbey, H. On the thermodynamics of microbial growth processes. Biotechnol. Bioeng. 1991, 38, 697–718. [Google Scholar] [CrossRef]
- Ludovisi, A.; Pandolfi, P.; Taticchi, M.I. The strategy of ecosystem development: Specific dissipation as an indicator of ecosystem maturity. J. Theor. Biol. 2005, 235, 33–43. [Google Scholar] [CrossRef]
- Battley, E.H. An empirical method for estimating the entropy of formation and the absolute entropy of dried microbial biomass for use in studies on the thermodynamics of microbial growth. Thermochim. Acta 1999, 326, 7–15. [Google Scholar] [CrossRef]
- Chakrawal, A.; Herrmann, A.M.; Šantrůčková, H.; Manzoni, S. Quantifying microbial metabolism in soils using calorespirometry—A bioenergetics perspective. Soil Biol. Biochem. 2020, 148, 107945. [Google Scholar] [CrossRef]
- Maskow, T.; Kemp, R.B.; Buchholz, F.; Schubert, T.; Kiesel, B.; Harms, H. What heat is telling us about microbial conversions in nature and technology: From chip- to megacalorimetry. Microb. Biotechnol. 2009, 3, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Wadsö, L.; Hansen, L.D. Calorespirometry of terrestrial organisms and ecosystems. Methods 2015, 76, 11–19. [Google Scholar] [CrossRef]
- Barros, N.; Feijóo, S.; Hansen, L.D. Calorimetric determination of metabolic heat, CO2 rates and the calorespirometric ratio of soil basal metabolism. Geoderma 2011, 160, 542–547. [Google Scholar] [CrossRef]
- Herrmann, A.M.; Bölscher, T. Simultaneous screening of microbial energetics and CO2 respiration in soil samples from different ecosystems. Soil Biol. Biochem. 2015, 83, 88–92. [Google Scholar] [CrossRef]
- Barros, N.; Hansen, L.; Piñeiro, V.; Pérez-Cruzado, C.; Villanueva, M.; Proupín, J.; Añón, J.A.R. Factors influencing the calorespirometric ratios of soil microbial metabolism. Soil Biol. Biochem. 2016, 92, 221–229. [Google Scholar] [CrossRef]
- Hansen, L.D.; Macfarlane, C.; McKinnon, N.; Smith, B.N.; Criddle, R.S. Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochim. Acta 2004, 422, 55–61. [Google Scholar] [CrossRef]
- Barros, N. Calorimetry and soil biodegradation: Experimental procedures and thermodynamic models. In Toxicity and Biodegradation Testing; Humana Press: Totowa, NJ, USA, 2018; pp. 123–145. [Google Scholar]
- Harris, J.A.; Ritz, K.; Coucheney, E.; Grice, S.; Lerch, T.Z.; Pawlett, M.; Herrmann, A.M. The thermodynamic efficiency of soil microbial communities subject to long-term stress is lower than those under conventional input regimes. Soil Biol. Biochem. 2012, 47, 149–157. [Google Scholar] [CrossRef]
- Geyer, K.M.; Dijkstra, P.; Sinsabaugh, R.; Frey, S.D. Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol. Biochem. 2019, 128, 79–88. [Google Scholar] [CrossRef]
- Chakrawal, A.; Herrmann, A.M.; Manzoni, S. Leveraging energy flows to quantify microbial traits in soils. Soil Biol. Biochem. 2021, 155, 108169. [Google Scholar] [CrossRef]
- Xu, J.; Feng, Y.; Barros, N.; Zhong, L.; Chen, R.; Lin, X. Exploring the potential of microcalorimetry to study soil microbial metabolic diversity. J. Therm. Anal. Calorim. 2016, 127, 1457–1465. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Wu, M.; Chen, R.; Li, Z.; Lin, X.; Zhu, Y.; Delgado-Baquerizo, M. Evaluation of Microbe-Drive Soil Organic Matter Quantity and Quality by Thermodynamic Theory. mBIO 2021, 12, e03252-20. [Google Scholar] [CrossRef]
- Grosskopf, T.; Soyer, O.S. Microbial diversity arising from thermodynamic constrains. ISME J. 2016, 10, 2725–2733. [Google Scholar] [CrossRef]
- Hansen, L.D.; Tolley, H.D.; Woodfield, B.F. Tranformation of matter in living organisms during growth and evolution. Biophys. Chem. 2021, 106550. [Google Scholar] [CrossRef] [PubMed]
- Bölscher, T.; Paterson, E.; Freitag, T.; Thornton, B.; Herrmann, A.M. Temperature sensitivity of substrate-use efficiency can result from altered microbial physiology without change to community composition. Soil Biol. Biochem. 2017, 109, 59–69. [Google Scholar] [CrossRef]
- Hansen, L.D.; Barros, N.; Transtrum, M.K.; Rodríguez-Añón, J.A.; Proupín, J.; Piñeiro, V.; Arias-González, A.; Gartzia-Bengoetxea, N. Effect of extreme temperatures on soil: A calorimetric approach. Thermochim. Acta 2018, 670, 128–135. [Google Scholar] [CrossRef]
- Hardin, G. The Competitive Exclusion Principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Barros, N.; Feijóo, S. A combined mass and energy balance to provide bioindicators of soil microbiological quality. Biophys. Chem. 2003, 104, 561–572. [Google Scholar] [CrossRef]
- Barros, N.; Salgado, J.; Rodríguez-Añón, J.A.; Proupín, J.; Villanueva, M.; Hansen, L.D. Calorimetric approach to metabolic carbón conversión efficiency in soils: Comparison of experimental and theoretical models. J. Thermal. Anal. Calorim. 2010, 99, 771–777. [Google Scholar] [CrossRef]
- Barros, N.; Feijoo, S.; Simoni, J.A.; Airoldi, C.; Ramajo, B.; Espina, A.; García, J.R. A mass and energy balance to provide microbial growth yield efficiency in soil: Sensitivity to metal layering phospates. J. Therm. Anal. Calorim. 2008, 93, 657–665. [Google Scholar] [CrossRef]
- Soares, M.; Rousk, J. Microbial growth and carbon use efficiency in soil: Links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 2019, 131, 195–205. [Google Scholar] [CrossRef]
- Masiello, C.A.; Gallagher, M.E.; Randerson, J.T.; Deco, R.M.; Chadwick, O.A. Evaluating two experimental approaches for measuring ecosystem carbon oxidation state and oxidative ratio. J. Geophys. Res. Space Phys. 2008, 113, 03010. [Google Scholar] [CrossRef]
- Chavez-Vergara, B.; Merino, A.; Vázquez-Marrufo, G.; García-Oliva, F. Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico. Geoderma 2014, 235–236, 133–145. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nat. Cell Biol. 2015, 528, 60–68. [Google Scholar] [CrossRef]
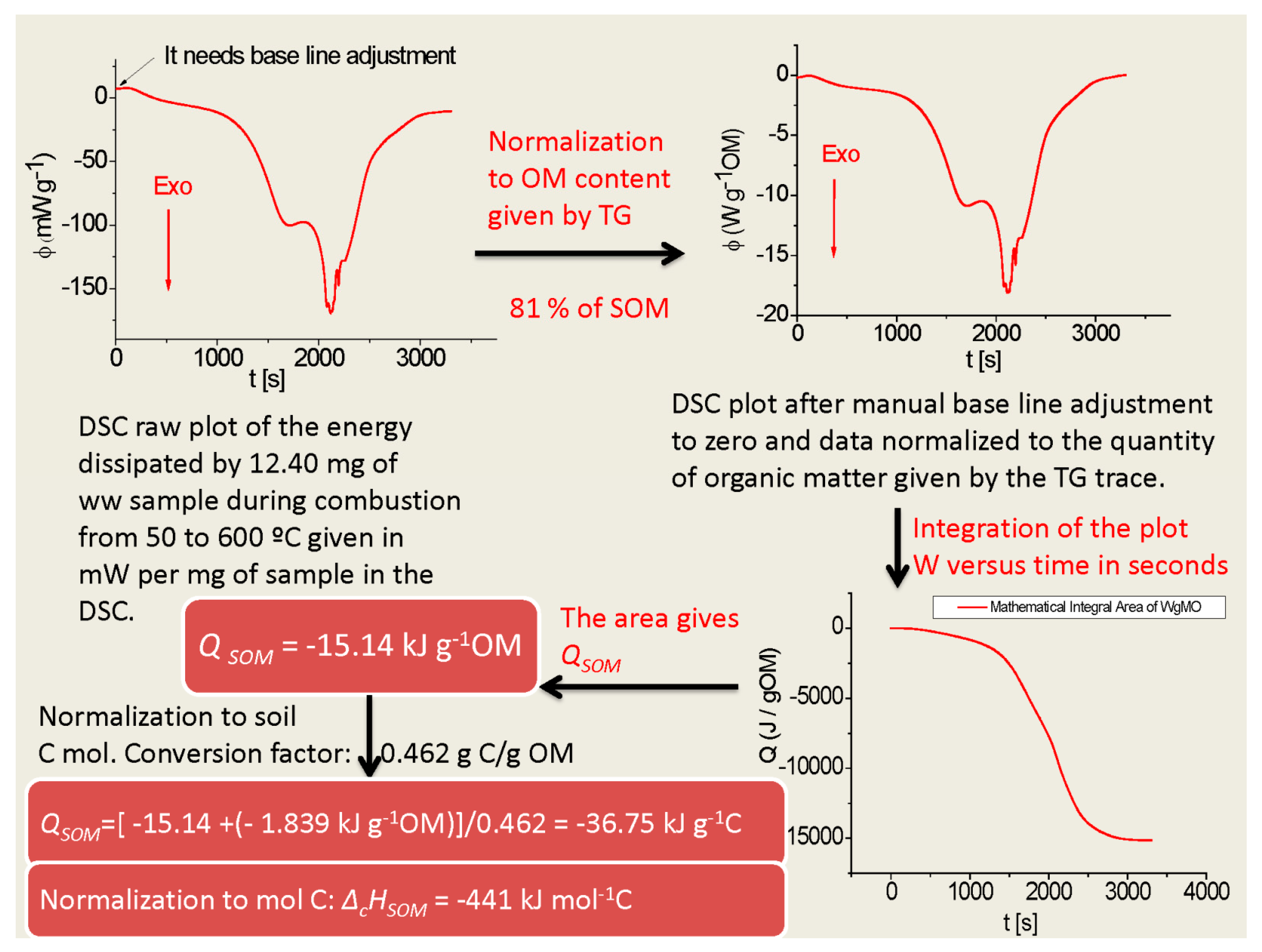
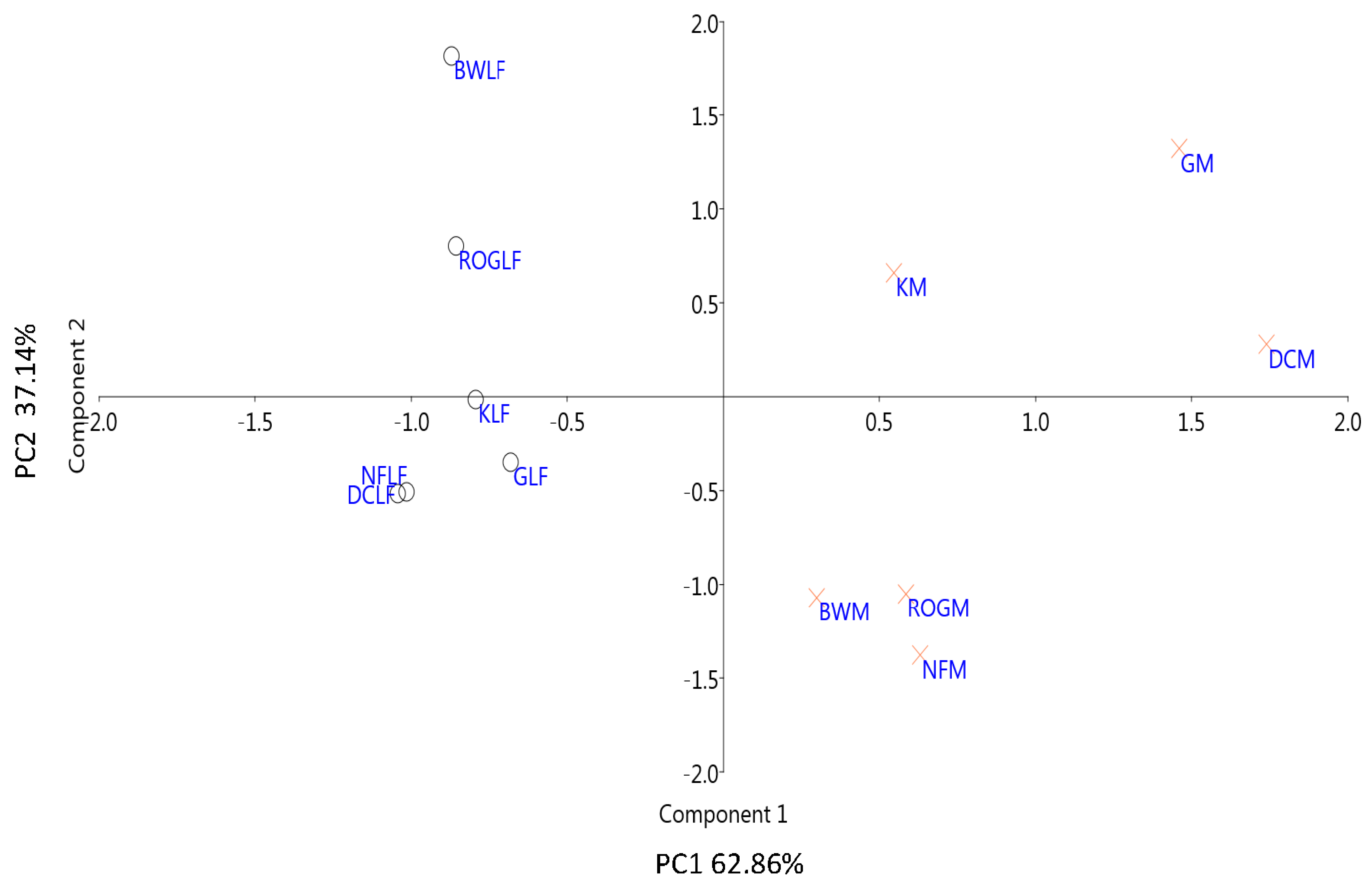
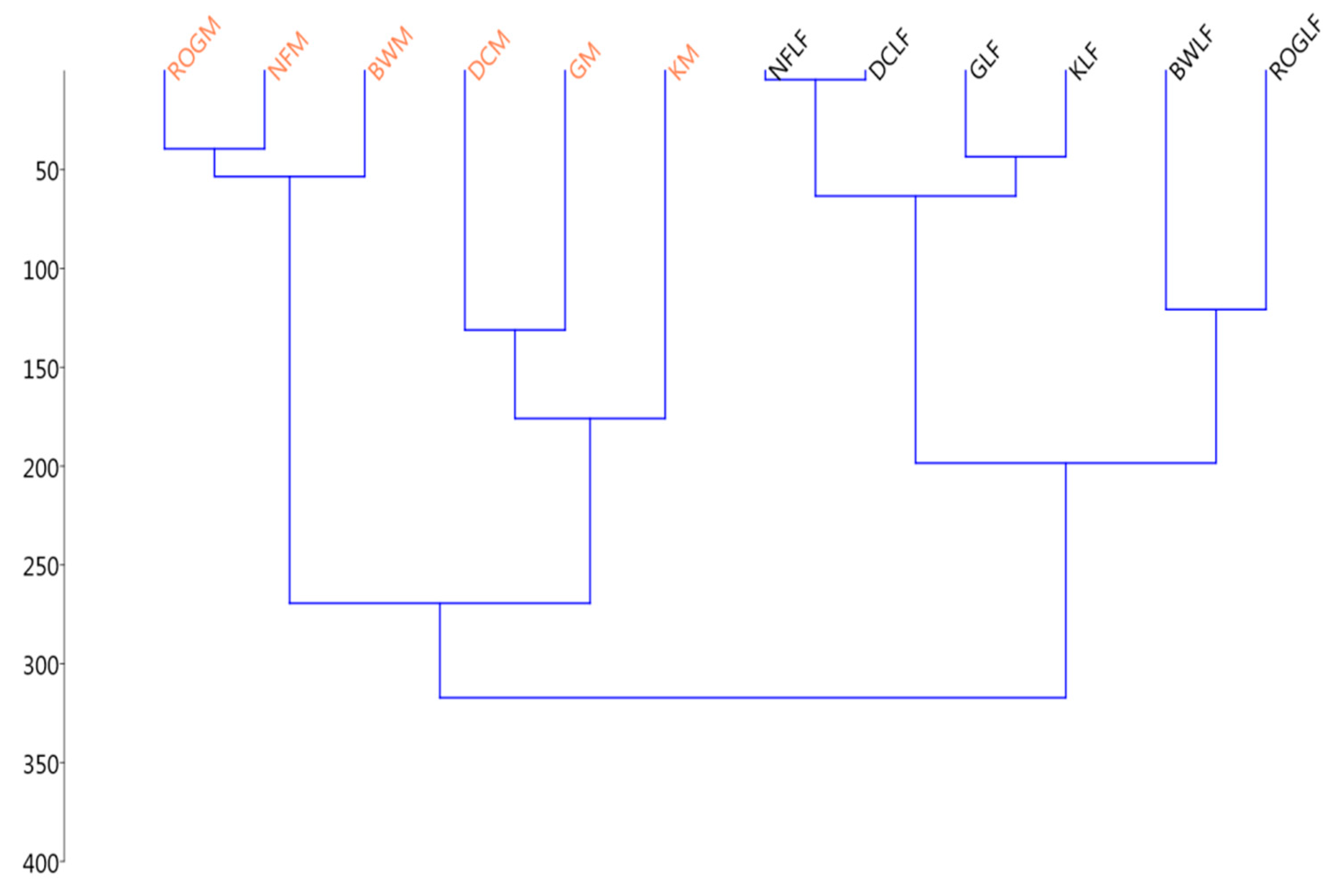
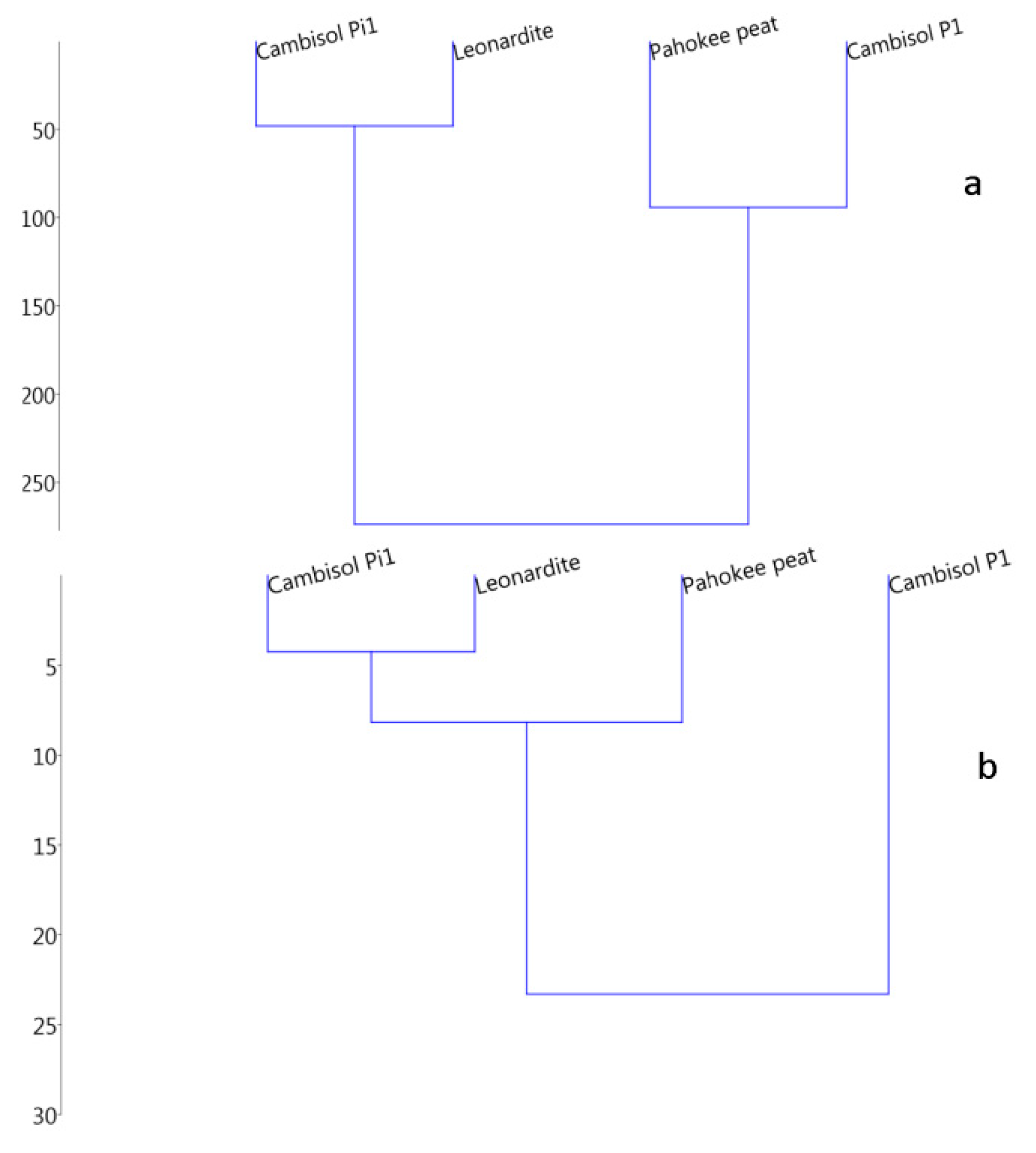
| Samples | Ctot (%) | Corg (%) | H (%) | N (%) | SOM (%) |
|---|---|---|---|---|---|
| ROG LF | 39 | 34 | 5.0 | 1.6 | 73 |
| BW LF | 46 | 41 | 6.5 | 2.5 | 81 |
| NF LF | 44 | 42 | 6.5 | 2.0 | 80 |
| DC LF | 50 | 42 | 5.5 | 1.4 | 95 |
| G LF | 51 | 46 | 5.3 | 1.7 | 97 |
| K LF | 51 | 40 | 6.4 | 1.7 | 96 |
| ROG M | 5 | 5 | 0.3 | 0.3 | 10 |
| BWM | 11 | 9 | 1.0 | 0.6 | 23 |
| NFM | 12 | 8 | 1.4 | 0.5 | 16 |
| DCM | 6 | 4 | 0.7 | 0.3 | 7 |
| G M | 5 | 4 | 0.5 | 0.2 | 9 |
| KM | 10 | 10 | 1.4 | 0.5 | 21 |
| Samples | −QSOM kJ g−1SOM LF | −QSOM kJ g−1SOM M | −∆cHSOM kJ mol−1C LF | −∆cHSOM kJ mol−1C M |
|---|---|---|---|---|
| ROG | 15.4 | 21.9 | 449 | 617 |
| BW | 15.1 | 20.7 | 441 | 586 |
| NF | 15.1 | 22.2 | 440 | 624 |
| DC | 15.0 | 26.4 | 437 | 734 |
| G | 16.4 | 25.0 | 475 | 697 |
| K | 15.9 | 21.3 | 461 | 602 |
| Samples | γSOMR LF | γSOMS&O LF | γSOMR M | γSOMS&O M |
| ROG | 3.90 | 4.11 | 5.36 | 5.66 |
| BW | 3.83 | 4.05 | 5.10 | 5.39 |
| NF | 3.83 | 4.04 | 5.43 | 5.72 |
| DC | 3.80 | 4.01 | 6.38 | 6.73 |
| G | 4.13 | 4.36 | 6.06 | 6.39 |
| K | 4.01 | 4.23 | 5.23 | 5.22 |
| Samples | −∆cGSOMR kJ mol−1C LF | −∆cGSOMS&O kJ mol−1C LF | −∆cGSOMR kJ mol−1C M | −∆cGSOMS&O kJ mol−1C M |
|---|---|---|---|---|
| ROG | 455 | 454 | 594 | 624 |
| BW | 448 | 446 | 568 | 593 |
| NF | 448 | 445 | 599 | 631 |
| DC | 445 | 442 | 689 | 742 |
| G | 477 | 481 | 659 | 705 |
| K | 465 | 466 | 581 | 609 |
| Samples | ∆cSSOMR J K−1mol−1C LF | ∆cSSOMS&O J K−1mol−1C LF | ∆cSSOMR J K−1mol−1C M | ∆cSSOMS&O J K−1mol−1C M |
| ROG | 20.1 | 16.8 | −79.3 | 23.5 |
| BW | 23.5 | 16.8 | −60.4 | 23.5 |
| NF | 26.8 | 16.8 | −83.9 | 23.5 |
| DC | 26.9 | 16.8 | −151.0 | 26.9 |
| G | 6.7 | 20.1 | −127.5 | 26.9 |
| K | 13.4 | 16.8 | −70.5 | 23.5 |
| Samples | −CR kJ mol−1CO2-C LF | −CR kJ mol−1CO2-C M | γ LF | γ M | −∆rGSOM kJmol−1C LF | −∆rGSOM kJmol−1C M | ∆rSSOM JK−1mol−1C LF | ∆rSSOM JK−1mol−1C M |
|---|---|---|---|---|---|---|---|---|
| ROG | 423 ± 30 | 280 ± 7 | 3.88 | 2.57 | 427 | 283 | 16.0 | 10.6 |
| BW | 507 ± 19 | 276 ± 9 | 4.65 | 2.53 | 513 | 279 | 19.2 | 10.5 |
| NF | 312 ± 10 | 253 ± 12 | 2.86 | 2.32 | 316 | 256 | 11.8 | 9.6 |
| DC | 310 ± 44 | 400 ± 68 | 2.85 | 3.67 | 315 | 405 | 11.8 | 15.2 |
| G | 327 ± 41 | 485 ± 9 | 3.00 | 4.45 | 332 | 490 | 12.4 | 18.4 |
| K | 355 ± 51 | 421 ± 17 | 3.26 | 3.87 | 359 | 427 | 13.4 | 16.0 |
| Samples | Taxa_S Bacteria | Taxa_S Fungi | Shannon Bacteria | Shannon Fungi | −∆cHSOM kJmol−1C | −∆cGSOM kJmol−1C | −CR kJmol−1CO2-C | −∆rGSOM kJmol−1C |
|---|---|---|---|---|---|---|---|---|
| Leonardite | 7 | 21 | 1.92 | 2.07 | 538 | 554 | 273 ± 14 | 276 |
| Pahokee peat | 8 | 15 | 1.93 | 2.19 | 545 | 551 | 463 ± 20 | 468 |
| Cambisol pasture | 28 | 11 | 3.07 | 2.20 | 489 | 495 | 495 ± 21 | 500 |
| Cambisol pine | 6 | 21 | 1.67 | 2.90 | 539 | 544 | 239 ± 18 | 242 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, N. Thermodynamics of Soil Microbial Metabolism: Applications and Functions. Appl. Sci. 2021, 11, 4962. https://doi.org/10.3390/app11114962
Barros N. Thermodynamics of Soil Microbial Metabolism: Applications and Functions. Applied Sciences. 2021; 11(11):4962. https://doi.org/10.3390/app11114962
Chicago/Turabian StyleBarros, Nieves. 2021. "Thermodynamics of Soil Microbial Metabolism: Applications and Functions" Applied Sciences 11, no. 11: 4962. https://doi.org/10.3390/app11114962
APA StyleBarros, N. (2021). Thermodynamics of Soil Microbial Metabolism: Applications and Functions. Applied Sciences, 11(11), 4962. https://doi.org/10.3390/app11114962






