1. Introduction
Cranioplasty is a surgical procedure that is performed to repair a defect in the human skull. In the presence of a large lacuna in the cranial vault, surgery usually aims to (i) restore the anatomy by recomposing the skull bone or (ii) by make use of a synthetic substitute (e.g., plate, mesh).
Whatever the cause of the defect (trauma, congenital, complication of an earlier surgery), the goals of cranioplasty are to mitigate defect-related symptoms (headaches or, in some cases, neurological impairment), normalize the intracranial pressure, ensure adequate protection for the brain and restore the normal oval shape of the head. This last aspect cannot be overlooked. An esthetic outcome that is negatively judged by the patient can potentially be the source of psychological and social issues that greatly affect quality of life.
Cranioplasty is not exempt from critical aspects. Related literature reports that complications affect a large percentage of patients (up to over 20%) [
1,
2,
3]. Although an early surgery can avoid complications caused by changes in cerebral blood flow and cerebrospinal fluid dynamics [
4], it cannot be performed before the full recovery of the wound of the primary procedure (e.g., in decompressive craniectomy). Moreover, by performing an early cranioplasty, the infection rate related to an interruption of the wound healing increases [
1,
5,
6,
7]. Consequently, patients may undergo cranioplasty long after the defect appears (in some cases, years pass before undergoing a cranioplasty), increasing the risks associated with the presence of the lacuna. For this reason, normal physiological conditions are not assured dealing with the anatomical tissues in the area of the defect.
Accordingly, the most common complications involve the skin flap, wound infection/dehiscence, ulcer and necrosis [
5]. The scalp conditions must not be underestimated in planning cranioplasty. Loss of elasticity, presence of much scar tissue, multiple interventions in the same region are the main factors that can affect surgery and subsequent rehabilitation. In extreme cases, damaged skin tissue can lead to ruptures, failure of suture stitches and, ultimately, exposure of the implant. Moreover, the incidence of complications is accentuated when dealing with large defects due to the dimensions of the surgical incisions and to the higher difference in skin tension caused by introducing an implant.
These problems must be considered when designing cranioplasty implants. To avoid any complications, scalp tissue expansion is usually considered as a solution in the case of a previously infected cranioplasty or delayed reconstruction of large defects [
8]. Scalp skin expansion is performed by inserting one or more smooth devices, named expanders, under the skin and next to the defect to stretch the surrounding tissue. Expanders remain in place long enough for skin expansion to be considered sufficient. This approach usually allows to overcome the skin tissue complications. Still, it is not exempt from complications that include infection, exposure, rupture of the expander and hematoma [
8]. Furthermore, it requires a two-stage cranioplasty, hence increasing the risks for the patient and the cost of the procedure.
Since skin expansion requires such a cumbersome and invasive operation, there is the need to explore alternatives strategies to relieve scalp skin tension. Unfortunately, there is little literature about other feasible possible options. To the authors’ knowledge, the only notable example is proposed in [
9]. The authors have experimented with the idea of an implant with a controlled curvature tailored to mitigate skin tension; however, this strategy heavily affects the esthetic result and should be avoided.
To show a different approach that can achieve an adequate esthetic result through a one-step cranioplasty and avoiding expanders, the following authors propose a strategy for an accurate assessment of scalp tissue by exploiting techniques based on reverse engineering (RE) and additive manufacturing (AM) technologies. The introduction of RE and AM techniques in the medical field has greatly improved the quality of the treatment, unlocking the possibility of producing custom-made prostheses that perfectly fit the patient’s anatomy in a preliminary stage. The design of a custom implant starting from diagnostic imaging allows the surgeon to accurately plan the intervention, rather than model a mesh implant directly in the operating theatre. Along with the possibility of tailoring the devices to the specific needs of the single patient, these technologies can be exploited to improve the evaluations performed in the presurgical stage by enabling quantitative assessment of both hard and soft tissue as well as by enabling in silico and physical simulations. Consequently, surgeons can test different devices and approaches for the same operation in a presurgical stage and in a safe way for the patient. This approach has been shown to reduce surgical time and connected risks while also reducing the occurrences of unforeseen events. Moreover, even the esthetic outcome results improved, as designers can exploit imaging data to compute the shape of the plate that best fits the patient’s skull.
The proposed strategy is focused on evaluating the scalp contracture. It provides quantitative indications about the difference between the skin flap needed to cover the prosthesis and the actual skin flap. This is a significant achievement, as the literature analysis highlights the lack of objective metrics used in assessing preoperative conditions. If the surgeon is prone to using expanders, these can be precisely designed in type, size and position. Alternatively, the present procedure allows experimenting with alternative approaches for solving possible criticalities regarding the scalp in a one-step cranioplasty, thus avoiding further intervention. This last point is the most interesting. Tt enables the possibility of customizing the surgical approach and the devices to the specific needs of the patient.
In the following, starting from an in-depth description of the problems that must be faced when approaching the design of a cranioplasty implant, the procedure is presented in all its steps. Then, a case study is reported to show how such procedure has allowed a one-step minimally invasive cranioplasty for a patient with a large cranial defect with an overlying fibrotic inextensible tissue that had caused three failures in previous cranioplasties because of wound dehiscence. In the case study, the methodology delivered a precise evaluation of the critical scalp region. This information enabled the planning of a minimally invasive intervention and the design of related medical devices that were tailored both on the anatomy of the patient and on the surgical strategy.
2. Materials and Methods
As discussed in the introduction, cosmetics are a critical indication for cranioplasty to restore the integrity and appearance of the skull and, thus, to avoid psychological and social impairments for the patient [
10,
11]. However, restoring the normal shape of the cranial vault is not a trivial task since the original cranial vault shape is commonly unknown, especially when the defect is caused by a trauma or the craniotomy had occurred a long time before. In these cases, it is necessary to retrieve the missing region coherently with the surrounding healthy bone, by minimizing the cranial asymmetries and by ensuring surface continuity with the corrective device [
12,
13]. When the cranioplasty is performed making use of a pre-designed artificial implant (typically produced using additive manufacturing techniques), the device can be designed and manufactured in a preoperative stage and tailored to the very patient by exploiting its diagnostic images (usually, computed tomography (CT) images). This approach enables better esthetic results than an intraoperative methyl-methacrylate reconstruction (whose result depends entirely on the surgeon’s ability). In addition, entering into the theatre with a ready-to-use device results in a shorter surgery.
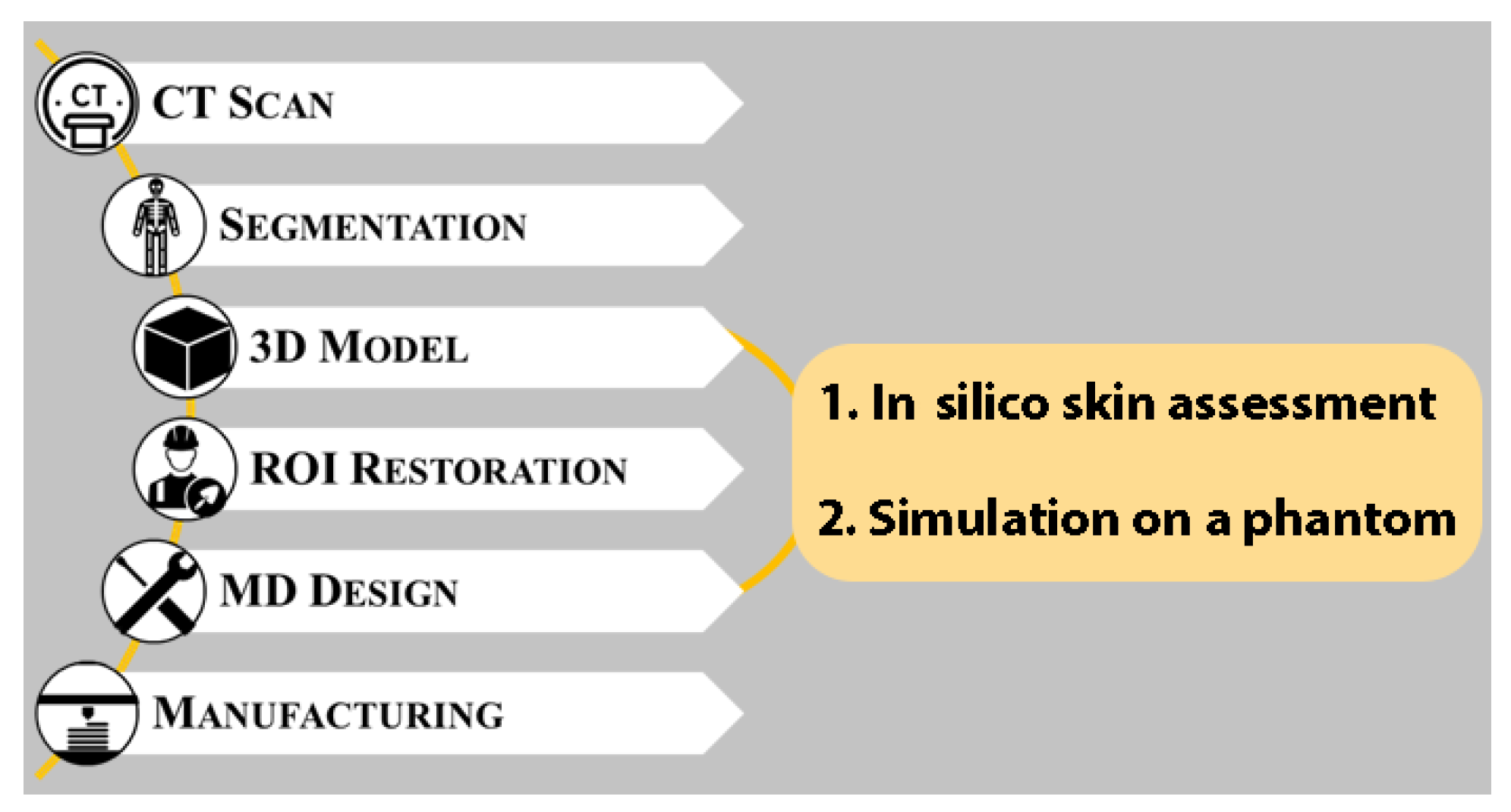
The presurgical design process of an artificial cranial prosthesis usually follows the typical workflow shown in
Figure 1. The first step involves the segmentation of the patient’s diagnostic images to obtain a 3D model of the defective cranial vault. The model is then virtually restored in three dimensions ensuring, as said, both cranial symmetry and bone continuity as far as possible. On a practical level, this task is carried out by digitally reconstructing the missing external surface of the skull; this will define the final shape of the implant and, ultimately, of the patient’s head at the end of the operation. This step is by far the most critical task in the design process because of the complexity and variability of human anatomy as well as the importance of the esthetic outcome of the intervention. To obtain the actual device, the retrieved surface is trimmed, thickened and modeled using typical computer-aided design (CAD) tools to meet all of the surgeon’s specifications, such as external profile and fixation strategy.
Considering its importance, in the last years, many procedures able to perform a coherent cranial vault virtual reconstruction of the three-dimensional (3D) defective model have been proposed in the literature with the specific intent to serve as a reference for the prosthesis design [
14]. Such procedures can be categorized by the type of reference data used to model the surface of the plate in the defect area. When contralateral skull surface information is available, mirroring approaches can be employed; otherwise, surface interpolation methods or template-based methods remain the only viable options. As recent literature on the design and insertion of synthetic cranial implant shows, in silico 3D skull reconstruction can commonly ensure enough information to design a reliable device to guarantee a good outcome.
Indeed, using refined CAD reconstruction techniques that consider both esthetic and functional aspects is the main element of the whole procedure. Still, in some cases, additional constraints must be considered. As said in the previous section, one of the most critical variables to be considered is whether the skin flap will be able to effectively cover the designed prosthesis. This is particularly critical, especially for those patients who present previous complications (especially wounds infections) or in delayed cranioplasty, when scalp tissue shrinkage and scalp atrophy or tense suture from previous surgeries may lead to a skin flap no more able to cover the designed prosthesis [
8,
15].
Accordingly, a careful planning of the intervention and a quantitative assessment of the scalp conditions is critical to avoid complications related to the skin flaps. On the other hand, such an assessment is not trivial since it is very difficult to predict and simulate the actual quality and condition of the tissue above the defect and, consequently, its ability to cover the prosthesis.
Proposing an alternative approach to using skin expanders that can ensure both esthetical and functional effective outcomes as well as minimize the likelihood of complications related to excessive skin tension is very difficult because such an approach is strongly dependent on the specific condition of each patient. As a consequence, it can be achieved only by considering a quantitative evaluation of the actual condition of the specific patient. This evaluation must provide surgeons and engineers with all the information to define all the existing constraints to minimize complications occurrences for the specific case considered. Such constraints will evidently affect both the characteristic of the device as well as the surgical strategy.
The approach proposed in this paper aims to integrate the traditional workflow depicted in
Figure 1 by introducing two additional steps between the generation of the 3D models of the structures of interest and the design of the personalized medical devices (see
Figure 2). The first step to be integrated is a fully in silico evaluation that allows quantitative considerations about the shrinkage’s presence, positioning, and extensions. This information can give additional constraints regarding the corrective devices but also regarding the possible surgical strategy.
The information provided by the first in silico analysis can then be confirmed and integrated with a second step that involves simulations on physical phantoms. A series of personalized devices recreating the a priori condition of the skull and the expected result are used to perform an in-depth evaluation on multiple aspects related to the condition of the scalp and on the whole surgical procedure. This step aims also to demonstrate, preoperatively and in a safe way for the patient, the effectiveness of the developed devices and surgical strategy, comparing different alternatives or combinations of both of them. Moreover, introducing a physical simulation step in the planning of a cranioplasty leads to other advantages. Secondary aspects, such as access to the fixation regions, easiness of the positioning of the implant, can be evaluated. Finally, the surgeon benefits from the experience acquired during the simulation by registering different haptic and visual stimuli, which can be helpful in proportion to the realism of the simulacrum used in the simulation.
In the following, the two steps of the proposed procedure are described in detail with special attention to material and methods. In the following section, we discuss a case study to show how such procedure allowed a one-step minimally invasive cranioplasty for a patient with a large cranial defect with an overlying fibrotic inextensible tissue that had caused three failures in previous cranioplasties because of wound dehiscence.
2.1. In-Silico Skin Assessment
The first step is a fully in silico procedure to provide a quantitative comparison between the actual scalp and the scalp needed to cover the restored cranial shape. It aims to investigate the presence of critical regions where the skin flap could be shrunken. The whole analysis can be carried out in CAD software by working on the 3D models obtained applying the traditional design framework for AM-fabricated cranial implants. The analysis is carried out by comparing the 3D model of the actual internal surface of the scalp and the reconstructed cranial vault.
Both the 3D models can be retrieved from the patient’s diagnostic image; the defect can be restored by following one of the procedures presented in the literature and discussed in the introduction. The models involved in the analysis must be registered, i.e., they must have the correct spatial relationship with each other. Usually, all the models are retrieved from the same diagnostic study, and, consequently, they are already registered.
The analysis is based on the assumption that the internal surface of the scalp must have the same dimension or being larger than the outer surface of the skull with which it is in contact. Such comparison is carried out by measuring the length of a set of corresponding paths obtained on both surfaces. Operatively, the paths are found by defining two perpendicular sets of parallel planes (
Figure 3). The intersection of each plane with the skin surface or with the reconstructed surface defines a path. As a result, two patterns of corresponding paths are obtained, as shown in
Figure 4. The more the planes, the higher the resolution of the analysis. The planes are defined parallel to the three anatomical planes (sagittal, coronal, and transverse). Depending on the location of the defect, only two planes groups must be considered. For a lateral defect, planes parallel to coronal and transversal; for a frontal or posterior defect, planes parallel to sagittal and transversal; for a defect in the upper part of the cranial vault, planes parallel to sagittal and coronal planes must be considered.
To make the corresponding paths directly comparable, identical extremities should be identified. Accordingly, the user needs to define the endpoints of the paths outside the defective region where the surfaces are perfectly superimposed. This way, the endpoints belong to both surfaces and, consequently, to both the corresponding paths. Once that the extremities are identified, the paths can be trimmed to obtain their final definition.
Subsequently, typical CAD functions can be used to query all the lengths of the so-generated trimmed paths and compute the differences between the lengths of each couple of corresponding paths. These data are then presented to the user in a series of tables and used as quantitative indications about the possible shrinkage of the scalp. If the analysis shows, for each path, a value of skin tissue higher than the one required by the preliminary skull reconstruction (i.e., each path on the inner surface of the scalp is longer than the corresponding path on the reconstructed skull), the device can be designed as typical on the restored skull, and the cranioplasty can be done without risk of subsequent complications of the skin flap. Otherwise, in the case of comparable values or smaller scalp length, the assessment provides the surgeon quantitative indications on the location and severity of any potential skin shrinkage. This information can help find alternative surgical pathways or constructive solutions for corrective devices that avoid using expanders.
It is worth noting that, because of the difficulty to evaluate and simulate in silico the actual quality of the scalp and take its mechanical properties into consideration, assumptions on the skin flap elasticity are entirely avoided by this procedure. The authors have considered it convenient to hypothesize and model the skin as a rigid body, postponing and isolating all the evaluations on the elasticity of the tissue to the subsequent physical simulation.
2.2. Physical Simulation
To verify and integrate the results provided by the in silico simulation, the procedure considers an additional step with introducing a physical simulation on a patient-specific phantom reproducing the defective bone, the cranial implant, and the scalp. The authors have experimented with many materials and technologies to find the correct balance between costs, fidelity and times required for producing the different components. One of the cheapest and effective is by manufacturing the bone out of plastic via AM and the skin by casting a silicone with skin-like properties. The molds for the skin can also be produced via AM, which results in the best choice due to the limited costs associated with production in limited numbers and for the complex shapes that must be fabricated. The most suitable AM technologies for fabricating the skull and molds are fused-filament fabrication (FFF) or selective laser sintering (SLS). No specific properties are required from the plastic material. In both cases, state-of-the-art machines are perfectly capable of assuring the required accuracy, which could be estimated in a maximum deviation of 0.5 mm point-to-point from the digital surface.
Regarding the scalp, it is important to mimic its real geometry (easily retrieved from the CT scan). Indeed, the quality and resolution of the anatomy acquisition affect the quality of the resulting virtual and physical model, and, consequently, the effectiveness of the subsequent evaluations. For this reason, CT is the data source of choice as it provides the data with the highest resolution. Nevertheless, an extreme resolution is not necessary. According to the authors’ experience, a slice thickness up to 2 mm (which is an average resolution for a CT acquisition) is sufficient to obtain a satisfactory quality of the resulting 3D anatomic model. Accordingly, whenever CT images of the patient are available, there is usually no need for a new, higher resolution acquisition.
The material used to reproduce the skin should be easily cut and sutured to allow the surgeon to perform all the required operations. The choice of a silicone able to effectively mimic the properties of the skin (especially by not overestimating its elasticity) is obviously the critical task. Much literature deals with this topic and can be taken as a reference to help in this choice [
16].
To assemble the phantom, the skull (defective or restored) and the scalp are mounted together to replicate the patient’s head (see
Figure 5 for a schematic representation of the phantom). For the skull, a section performed on the axial plane is typically the best choice to isolate the area of the cranium that is of interest. This allows for an easier model to fabricate; whenever possible, it is convenient to eliminate the anterior skull bones to obtain simple anatomy. The models are fabricated, taking into consideration that the silicone skin and the skull should be placed in the correct position both in the presence of the defect and of the implant. Accordingly, the skin should possibly embrace the plastic skull around the area of the axial cut (red detail in
Figure 5) to assure a correct position.
Thanks to these models, surgeons can confirm the effectiveness of the assumptions suggested by the in silico analysis. In addition, physical reproduction is a powerful tool that integrates the data provided by the in silico analysis by introducing the mimicry of anatomical tissues and their mechanical properties. Moreover, surgeons can develop and simulate alternatives surgical strategies as well as devices to find the right combination that maximizes the chances of a positive outcome. The manufactured phantom allows the surgeon to:
Assess preoperative conditions—by using the models of the defective skull and of the skin tissue (
Figure 4, left);
Evaluate the planned cranioplasty result—by using the model of the defective skull with the implant in the correct position and the skin tissue repositioned on the skull (
Figure 4, center);
Simulate the intervention by working with actual surgical instruments on the preoperative model and performing the planned surgery on the simulator (
Figure 4, right).
Surgeons can then rely on an unprecedented array of information able to help them in developing every aspect surrounding cranioplasty (surgery pathways, device shape, device thickness, fixation, and so on), and test whether the impaired skin can cover the oval shape of the reconstructed skull with proper skin tension and thus avoiding skin expanders.
3. Results
The proposed procedure was performed on a 27-year-old woman with a wide cranial vault lacuna in the upper part of the skull and slightly crossing the sagittal plane, as shown in
Figure 6.
The patient underwent surgery in 2000 when she was nine-year-old for a low-grade brain neoplasm and, again, four years later for a tumor recurrence. In both cases, the cranial void was closed by performing an autograft cranioplasty. A major wound infection a few months after the second surgery necessitated the removal of the autograft.
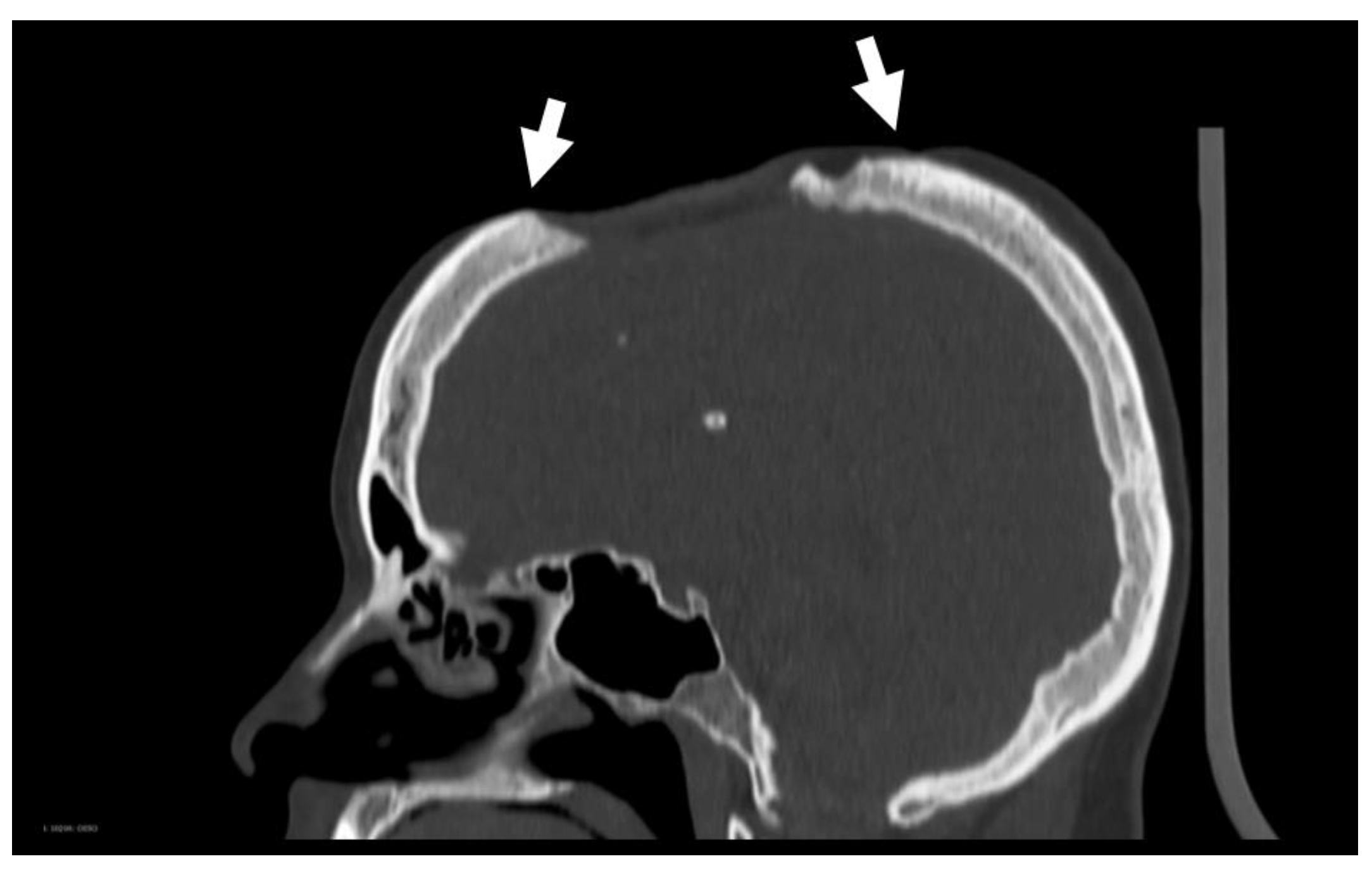
In 2012 and 2014, two further cranioplasties were attempted, in both cases with methyl methacrylate allografts. Both attempts failed due to wound-closure breakdown with subsequent prosthesis exposure. When the patient was seen in April 2018, the defect was covered by a sunken skin flap with dystrophic and very thin skin. The scalp conditions were so critical that a new cranioplasty was not possible without risking skin necrosis. In particular, there was significant atrophy of the scalp, with a thin and fragile skin flap, especially on the boundary of the defect (see
Figure 7).
As a consequence, careful skin assessment and surgical planning were required to avoid additional and more serious complications. The procedure started with the segmentation of the patient’s diagnostic images CT images are particularly suitable because cranial bones and scalp appear with two well-identifiable grayscale intensity windows, as shown in
Figure 7. The CT imaging acquisition parameters were slice thickness equal to 2 mm with a spacing of 1 mm. The pixel spacing was equal to 0.48 mm × 0.48 mm.
To perform the required analyses, it is unnecessary to reproduce scalp anatomy in details describing all its layers. Still, it is instead important to duplicate its overall geometry and consistency. Consequently, all the scalp is segmented (and then modeled) as a single body. The segmentation was carried out using the threshold method in Materialise® Mimics software. The same software also allows automatically obtaining and exporting the STLs from the segmentation masks.
The defective skull was then restored by applying the procedure presented by the authors in [
12]. It is a semi-automatic template-based approach that can restore unilateral or quasi-unilateral (i.e., single defect slightly passing the sagittal plane, as in this case) cranial defects, ensuring to maximize symmetry and continuity with the healthy surrounding bones. Such procedure exploits the contralateral anatomy to provide a reliable template to guide the reconstruction of the missing bone. Unlike existing mirroring-based approaches, the contralateral part is only used as a reference to guide a subsequent reconstruction based on surface approximation/interpolation. The procedure automatically mirrors the contralateral points within the defect boundary around the skull’s symmetry plane. The symmetry plane is automatically computed through the method proposed by Di Angelo et al. in [
13]. The method only requires as input the 3D model of the skull in STL format and can find a good approximation of its symmetry plane in a fully automatic way also in the case of a large defective area.
The points retrieved from the mirrored counterpart are defined as approximation centers for the reconstructive surface to ensure a symmetrical reconstruction as far as possible without over-constraining the surface, which is essential to obtain a smooth patch. The points on the defect boundary are defined as interpolation centers to ensure the continuity on the boundary between the patch and the surrounding bone. The points on the boundary are automatically retrieved by the algorithm starting from a seed point manually selected by the user. This operation is the only interaction required to the user during the reconstruction, in addition to the segmentation and the preprocessing of the model.
Figure 8 shows the defective skull and the restored one.
Case Study: The Skin Assessment
Once the cranial vault had been restored, assessing the skin flap was performed, in the first stage, with the in silico analysis. The analysis was carried out in the Geomagic® Design X environment. First, the three anatomical planes were defined on the skull to serve as a reference for the definition of the paths. The sagittal plane was defined as the previously computed symmetry plane. The transverse plane was defined as the Frankfurt plane, i.e., the plane drawn through the top point of both the ear canals (the anatomical landmarks named porions) and the bottom border of the left orbital (the anatomical landmark named left orbital). The last plane, the coronal one, was defined perpendicular to the others and passing through the top point of the left ear canal.
Since the defect was located in the upper part of the skull, the planes used for the analysis were sagittal and coronal. With the aim to cover a larger region than the defective one, 21 planes parallel to the sagittal and 30 planes parallel to the coronal was defined in both directions concerning the corresponding reference planes. All planes were equally spaced 5 mm apart. The resulting path pattern is shown in
Figure 9.
Once the path pattern had been defined, the difference between lengths of the corresponding paths was calculated.
Table 1 and
Table 2 report such differences for the sagittal-oriented and the coronal-oriented paths.
Figure 9 visually resumes the results of the assessment. Red-colored paths represent a skin shrinkage greater than 0.5 mm, and the orange-colored paths a skin shrinkage between 0 and 0.5 mm.
Figure 9 shows that the skin shrinkage was mainly located on the lateral and frontal boundaries of the defect, with a particularly critical region along the 20th coronal path, where more than 4 mm of skin was missing compared to the reconstructed skull shape. The analysis also showed the presence of a region in the center of the defect (frontal paths #12 to #19 and sagittal paths #8 to #12). The skin path was up to 10 mm longer than the corresponding bone-related path. This was probably due to the long-lasting suction action to which the skin flap had been subjected.
This stretched zone could have provided the extra skin enabling a safe cranioplasty. Still, the presence of such a particularly impaired scalp did not allow to ensure such an assumption.
To further explore and integrate these results, both the patient’s defective and restored cranial vaults and her scalp were reproduced. An FFF printer (MakerBot Replicator+, Banbury, UK) was used to manufacture out of plastic the skull and the devices and the molds to cast silicone and reproduce the skin. FFF parameters used in the process were layer height of 0.2 mm, extruder of 0.4 mm, and printing temperature of 215 °C. Non-soluble PLA supports were used in all prints and later removed by hand. Fabricated models were, in all cases, manually cleaned and refined. The silicone used in this application was the Smooth-On Ecoflex 00–50 (Smooth-On-Macungie, PA, USA). We chose this material after having qualitatively testing various specimens following the indications described in [
16]. The skull and the skin molds were printed in polylactide (PLA). The skin was designed to fit over the plastic model of the skull, as shown in
Figure 10a. As with the skull, prototypes of the implant can also be made from plastic using a 3D printer.
First, the physical simulation confirmed that the conditions were borderline. The same suction action that stretched the skin in the most sunken region had, however, worsened the condition of the skin, particularly thin and fragile along the edges of the defect (white regions visible in
Figure 10a). The Frontal region, in particular, did not allow further incisions without encountering certain complications after surgery short of introducing expanders.
Consequently, the assessment suggested devising a minimal incision that would avoid the frontal, posterior and lateral regions as far as possible and designing a device that would not increase skin tension in the same areas.
Figure 10b shows in green the eligible region for the skin incision. Red indicates the areas that must be avoided according to the skin assessment. The only solution to avoid skin expanders was then to plan a minimally invasive surgery and, consequently, to design a cranial prosthesis able to be correctly positioned despite the small incision. In addition, the device should have avoided increasing skin tension in the lateral, posterior and frontal sides.
Many attempts with various surgical strategies and different implants and supplementary devices for positioning the implant were simulated on the produced models to find the best combination of surgery pathways and device features able to fulfill the constraints defined through the skin assessment without using skin expanders. Different iterations of the devices have been tested with physical simulations (e.g., see
Figure 11), with the surgeon performing surgery on the phantom.
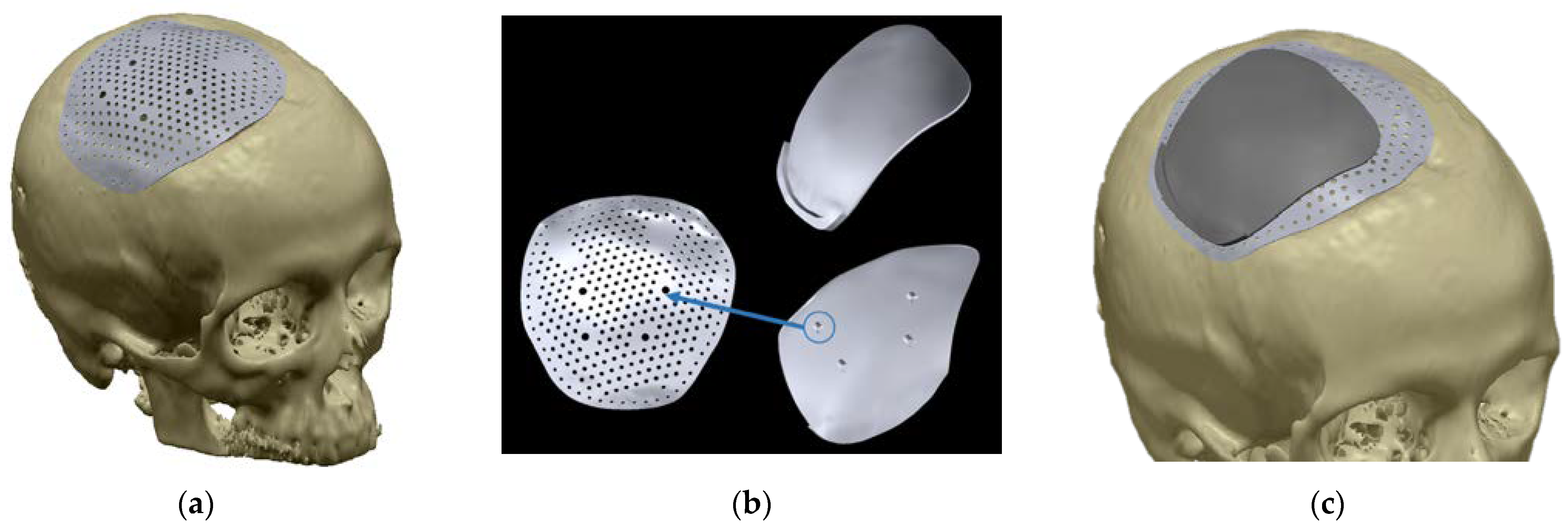
All these attempts resulted in an innovative minimally invasive procedure with a surgical cut that was 1/3 smaller than a traditional cranioplasty. The pathways were maintained within the established limits, planned to be the smallest possible to allow the prosthesis to pass. The patient-specific device had to slide inside the dissection to its resting position. Physical references properly designed on the implant ensured its right positioning. However, the small size of the incision had essentially made the surgery a blind operation. The prosthesis was 0.4 mm thin; the implant did not exceed the frontal boundary of the defect, while it leaned 15 mm on the other 3 sides, allowing the fixation through screws on the bone. Because of the minimal incision located in the upper part of the skull, screws were inserted transcutaneously in the lateral side. To make this possible, an additional device was designed to provide physical references that allowed effortless identification of the holes through the skin. The additional device could be mounted on the plate through four pins (
Figure 12b) and then easily removed once the fixing is realized.
Simulations demonstrated that the developed procedure and devices would have reduced surgical incisions and the skull surface exposed during surgery. Furthermore, the stresses imposed on the skin flap were significantly lowered. Hence the risks of damages caused to the tissues were minimized. The chances of a full recovery in a narrower time window improved.
The patient underwent surgery in October 2018, after 6 months of evaluations and simulations. EBM technology (Arcam Q10+ EBM machine–made by Arcam–Gothenburg, Sweden) was used to manufacture the implanted device, which is made of surgical-grade titanium alloy (Ti-6Al-4V, which is among the implantable materials provided by Arcam). The selection of fabrication parameters was performed by the manufacturer. Due to their complex shapes, biocompatibility requirements, and considering that only one specimen needs to be fabricated for each device, powder-based metal additive manufacturing techniques are obviously the elective technologies to be involved in the process. On this aspect, although EBM and Ti-6Al-4V were chosen for the fabrication of the specific devices, any biocompatible metallic material produced by powder-based systems would have been satisfactory. Indeed, state-of-the-art metallic AM systems of major companies (Arcam-Gothenburg, Sweden, EOS-Krailling, DE, Concept Laser GmbH–Lichtenfels, Germany) are currently characterized by technical specifications (quality and chemical characteristics of powders, mechanical properties of resulting parts, accuracy of fabrication) that easily satisfy the requirements imposed by this kind of application.
The postoperative CT was analyzed and compared to the design predictions, revealing that the implant was properly positioned (see
Figure 13). At the date of writing, there were no adverse events or complications to report more than two years after surgery.
4. Discussion and Conclusions
Cranioplasty is an established surgical technique to restore the integrity and appearance of the skull by the insertion of a prosthesis. While applying RE and AM techniques have revolutionized how cranioplasties are performed with great benefits for the patients, full exploitation of the beneficial effects achievable through applying such technologies cannot disregard proper planning of the intervention that considers all the major aspects of the surgery. Access to the ROI, the shape of the implant, fixation of the device, conditions of the tissues. Among the documented critical aspects that characterize the treatment, skin flap complications can be identified as a major cause of problems for a fast and full recovery.
Literature studies have presented various solutions to mitigate skin tension problems. Preliminary interventions studied to expand skin tissue in the defect area are sometimes a viable option to ease applying an implant. However, this solution requires a two-stage cranioplasty, hence increasing the risks for the patient and the cost of the procedure. A proposed alternative is to control the implant curvature, tailored to mitigate skin tension; by following this strategy, however, the implant’s geometry partially loses its accuracy with respect to the anatomy to be reconstructed.
This paper presents a procedure for designing patient-specific implants for cranioplasty introducing a quantitative assessment of scalp conditions. Such a procedure allows investigating any criticalities that could lead to complications of the skin flap following surgery. The procedure exploits the potentialities given by AM and RE technologies introducing both in silico and on physical phantom simulations. Consequently, surgeons can test different devices and different approaches for the same operation in a presurgical stage with no risks for the patient. This possibility allows the surgeon to customize the device and the surgery approach to the specific needs of the patient. The proposed procedure represents an effective tool for assessing the scalp condition before a cranioplasty, providing a quantitative evaluation of the real need for expanders. To date, using expanders is always necessary when there might be complications involving the skin flap.
The case study demonstrates how RE and AM can be operatively used to plan and simulate surgery, allowing doctors to evaluate all possible strategies and related risks in advance and safely for the patient. The experimentation on the case study has led to developing a novel type of implant composed of two different devices (the implant itself and a guiding device used to help the surgeon identify the position for the fixation holes). As a result, it is now also possible to tailor the entire surgery and devices to minimize scalp wound area, the volume of blood loss, complexity and duration of the intervention, and predict and eliminate possible causes of complications.
Depending on the specific conditions faced on the intervention, the adoption of the proposed procedure can be considered as an alternative to using expanders whenever the condition of the skin tissue in the area of the defect poses a serious problem to the full integration of the implant.
As discussed in the paper, proper planning of the cranioplasty should consider the esthetical outcome as the principal aspect by tailoring the device geometry on the very patient. Nevertheless, actual conditions of the tissues in the ROI and possible complications must be considered to minimize foreseeable related criticalities, which could compromise the correct evolution of the treatment. The described approach highlights RE and AM potentialities in allowing the surgeons, through synergistic work with engineers, to tailor the device and the surgery to the patient’s specific needs.
Future developments of the proposed approach could concern the automation of the in silico measurements and the standardization of the materials and methods of fabrication of the physical models to carry out the simulation.