Geometric and Volumetric Measurements of Orbital Structures in CT Scans in Thyroid Eye Disease Classification
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Subjects
2.2.1. Inclusion Criteria
2.2.2. Classification of Thyroid Eye Disease (TED) Patients
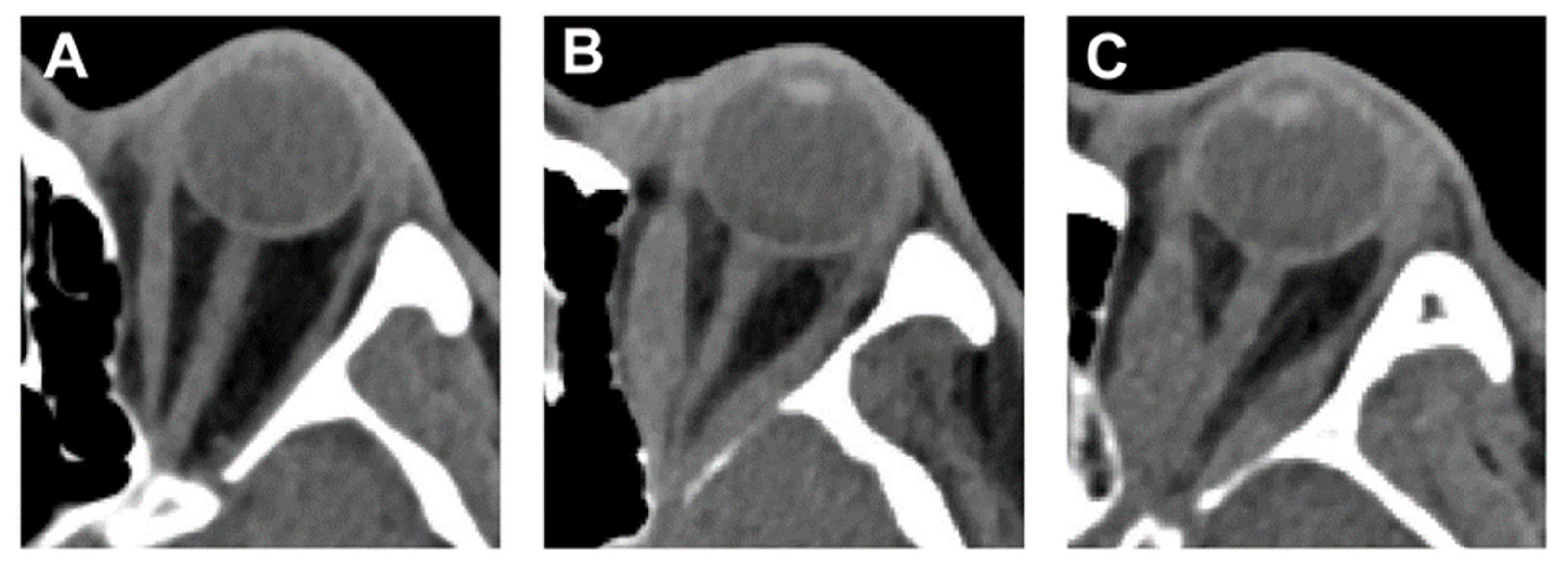
2.3. Orbital Computed Tomography (CT) Scan
2.4. Volume and Angle Measurements of Orbital Tissue
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Comparison of TED-IM Group and TED-NM Group
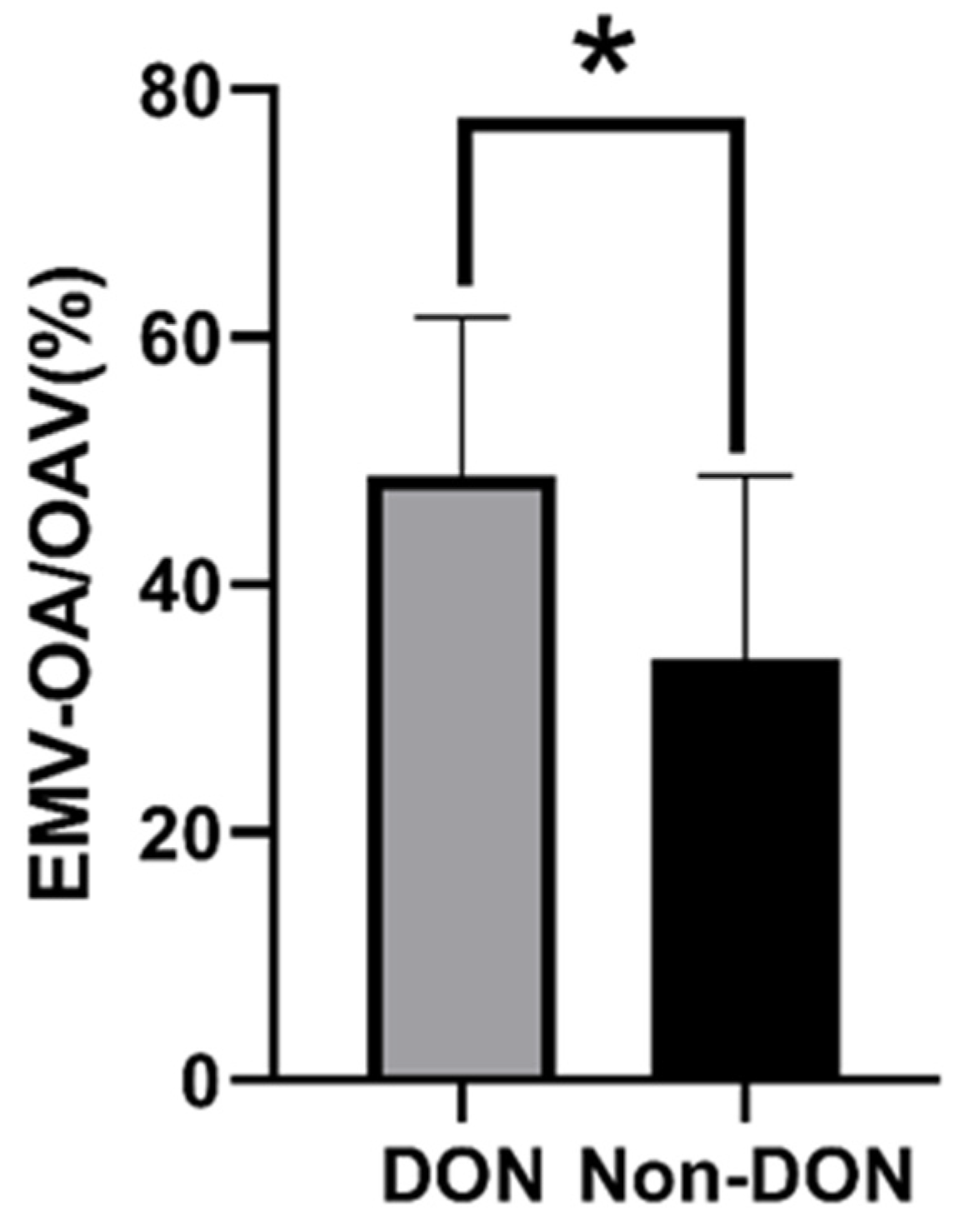
3.3. Comparison of DON Group and Non-DON Group in TED-IM Patients
3.4. Distribution by Population about the AMR-ON and ALR-ON
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lazarus, J.H. Epidemiology of Graves’ orbitopathy (GO) and relationship with thyroid disease. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 273–279. [Google Scholar] [CrossRef]
- Gharib, S.; Moazezi, Z.; Bayani, M.A. Prevalence and severity of ocular involvement in Graves’ disease according to sex and age: A clinical study from Babol, Iran. Casp. J. Intern. Med. 2018, 9, 178–183. [Google Scholar] [CrossRef]
- Bahn, R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef]
- Saeed, P.; Tavakoli Rad, S.; Bisschop, P. Dysthyroid Optic Neuropathy. Ophthalmic Plast. Reconstr. Surg. 2018, 34, S60–S67. [Google Scholar] [CrossRef]
- Bingham, C.M.; Harris, M.A.; Realini, T.; Nguyen, J.; Hogg, J.P.; Sivak-Callcott, J.A. Calculated computed tomography volumes of lacrimal glands and comparison to clinical findings in patients with thyroid eye disease. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, S.; Mundy, K.; DeLisi, M.P.; Nelson, K.M.; Harrigan, R.L.; Galloway, R.L.; Landman, B.A.; Mawn, L.A. Assessment of Orbital Computed Tomography (CT) Imaging Biomarkers in Patients with Thyroid Eye Disease. J. Digit. Imaging 2019, 32, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.B.; Gorman, C.A. Diagnostic criteria for Graves’ ophthalmopathy. Am. J. Ophthalmol. 1995, 119, 792–795. [Google Scholar] [CrossRef]
- Regensburg, N.I.; Kok, P.H.; Zonneveld, F.W.; Baldeschi, L.; Saeed, P.; Wiersinga, W.M.; Mourits, M.P. A new and validated CT-based method for the calculation of orbital soft tissue volumes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.T.; Jameyfield, E.; Aakalu, V.K. Optic neuropathy and diplopia from thyroid eye disease: Update on pathophysiology and treatment. Curr. Opin. Neurol. 2021, 34, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Silva, L.N.; Gebrim, E.M.; Monteiro, M.L. Quantification of orbital apex crowding for screening of dysthyroid optic neuropathy using multidetector CT. AJNR Am. J. Neuroradiol. 2012, 33, 1602–1607. [Google Scholar] [CrossRef]
- Monteiro, M.L.; Gonçalves, A.C.; Silva, C.T.; Moura, J.P.; Ribeiro, C.S.; Gebrim, E.M. Diagnostic ability of Barrett’s index to detect dysthyroid optic neuropathy using multidetector computed tomography. Clinics 2008, 63, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Weis, E.; Heran, M.K.; Jhamb, A.; Chan, A.K.; Chiu, J.P.; Hurley, M.C.; Rootman, J. Clinical and soft-tissue computed tomographic predictors of dysthyroid optic neuropathy: Refinement of the constellation of findings at presentation. Arch. Ophthalmol. 2011, 129, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Linquist, R.A.; Symons, R.C.; O’Bryhim, B.; Whittaker, T.J.; Sokol, J.A. Cytokine profiles in clinical subtypes of ophthalmic Graves’ disease. Orbit 2014, 33, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Khong, J.J.; McNab, A.A.; Ebeling, P.R.; Craig, J.E.; Selva, D. Pathogenesis of thyroid eye disease: Review and update on molecular mechanisms. Br. J. Ophthalmol. 2016, 100, 142–150. [Google Scholar] [CrossRef]
- Taylor, P.N.; Zhang, L.; Lee, R.W.J.; Muller, I.; Ezra, D.G.; Dayan, C.M.; Kahaly, G.J.; Ludgate, M. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat. Rev. Endocrinol. 2020, 16, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Łacheta, D.; Poślednik, K.B.; Czerwaty, K.; Ludwig, N.; Molińska-Glura, M.; Kantor, I.; Jabłońska-Pawlak, A.; Miśkiewicz, P.; Głuszko, A.; Stopa, Z.; et al. RAGE and HMGB1 Expression in Orbital Tissue Microenvironment in Graves’ Ophthalmopathy. Mediat. Inflamm. 2021, 2021, 8891324. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wang, T.; Guo, H.; Liu, Y.; Dang, N.; Hu, S.; Wu, L.; Zhang, C.; Ye, K.; et al. A novel CD4+ CTL subtype characterized by chemotaxis and inflammation is involved in the pathogenesis of Graves’ orbitopathy. Cell. Mol. Immunol. 2021, 18, 735–745. [Google Scholar] [CrossRef]
- Byun, J.S.; Moon, N.J.; Lee, J.K. Quantitative analysis of orbital soft tissues on computed tomography to assess the activity of thyroid-associated orbitopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 413–420. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef]
- Dolman, P.J. Dysthyroid optic neuropathy: Evaluation and management. J. Endocrinol. Investig. 2021, 44, 421–429. [Google Scholar] [CrossRef]
- Yu, B.; Gong, C.; Ji, Y.F.; Xia, Y.; Tu, Y.H.; Wu, W.C. Predictive parameters on CT scan for dysthyroid optic neuropathy. Int. J. Ophthalmol. 2020, 13, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Regensburg, N.I.; Wiersinga, W.M.; Berendschot, T.T.; Potgieser, P.; Mourits, M.P. Do subtypes of graves’ orbitopathy exist? Ophthalmology 2011, 118, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Tan, H.E.; Fook-Chong, S.; Teo, T.H.; Lim, L.H.; Seah, L.L. Graves ophthalmopathy: The bony orbit in optic neuropathy, its apical angular capacity, and impact on prediction of risk. Ajnr. Am. J. Neuroradiol. 2009, 30, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Boboridis, K.G.; Uddin, J.; Mikropoulos, D.G.; Bunce, C.; Mangouritsas, G.; Voudouragkaki, I.C.; Konstas, A.G. Critical Appraisal on Orbital Decompression for Thyroid Eye Disease: A Systematic Review and Literature Search. Adv. Ther. 2015, 32, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.Q.; Leong, Y.Y.; Lang, S.S.; Htoon, Z.M.; Young, S.M.; Sundar, G. Radiologic Parameters of Orbital Bone Remodeling in Thyroid Eye Disease. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2527–2533. [Google Scholar] [CrossRef]
- Weis, E.; Heran, M.K.; Jhamb, A.; Chan, A.K.; Chiu, J.P.; Hurley, M.C.; Rootman, J. Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: A volumetric analysis. Ophthalmology 2012, 119, 2174–2178. [Google Scholar] [CrossRef]
- Engin, Ö.; Adriaensen, G.; Hoefnagels, F.W.A.; Saeed, P. A systematic review of the surgical anatomy of the orbital apex. Surg. Radiol. Anat. 2021, 43, 169–178. [Google Scholar] [CrossRef]
- Choe, C.H.; Cho, R.I.; Elner, V.M. Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 4–11. [Google Scholar] [CrossRef]
- McCann, J.D.; Goldberg, R.A.; Anderson, R.L.; Burroughs, J.R.; Ben Simon, G.J. Medial wall decompression for optic neuropathy but lateral wall decompression with fat removal for non vision-threatening indications. Am. J. Ophthalmol. 2006, 141, 916–917. [Google Scholar] [CrossRef]





| Characteristics | TED-IM | TED-NM | p Value |
|---|---|---|---|
| Number of orbits | 62 | 30 | |
| Age (years) | 47.83 ± 11.49 | 39.11 ± 12.86 | <0.05(0.012) |
| Sex (male: female) Parameters of orbits | 31:31 | 8:22 | 0.309 |
| OV (cm3) | 22.31 ± 0.38 | 23.06 ± 0.65 | 0.357 |
| EMV (cm3) | 4.59 ± 0.25 | 3.23 ± 0.43 | <0.05(0.014) |
| RFV (cm3) | 7.60 ± 0.46 | 9.86 ± 0.64 | <0.05(0.012) |
| EMV/OV (%) | 20.44 ± 1.06 | 13.68 ± 1.82 | <0.05(0.004) |
| RFV/OV (%) | 33.89 ± 20.36 | 42.95 ± 2.84 | <0.05(0.023) |
| OAV (cm3) | 1.79 ± 0.91 | 1.70 ± 0.15 | 0.813 |
| EMV-OA (cm3) | 0.80 ± 0.58 | 0.34 ± 0.08 | 0.064 |
| EMV-OA/OAV (%) | 42.43 ± 15.37 | 20.46 ± 5.41 | <0.05(0.001) |
| Characteristics | DON | Non-DON | p Value |
|---|---|---|---|
| Number of orbits | 30 | 32 | |
| Age (years) | 48.65 ± 11.79 | 47.09 ± 11.43 | 0.666 |
| Sex (male: female) Parameters of orbits | 16:14 | 15:17 | 0.775 |
| OV (cm3) | 23.02 ± 2.51 | 21.97 ± 2.04 | 0.172 |
| EMV (cm3) | 4.93 ± 1.15 | 4.44 ± 2.05 | 0.386 |
| RFV (cm3) | 8.38 ± 2.07 | 7.57 ± 3.00 | 0.466 |
| EMV/OV (%) | 21.45 ± 4.65 | 19.93 ± 8.26 | 0.504 |
| RFV/OV (%) | 36.22 ± 9.80 | 34.47 ± 14.10 | 0.711 |
| OAV (cm3) | 1.68 ± 0.79 | 1.94 ± 1.06 | 0.391 |
| EMV-OA (cm3) | 0.83 ± 0.49 | 0.77 ± 0.70 | 0.759 |
| EMV-OA/OAV (%) | 48.81 ± 12.79 | 34.07 ± 14.30 | <0.05(0.003) |
| AMR-ON | 0° | <5° | 5–10° | 10–15° | >15° | Amount | |
|---|---|---|---|---|---|---|---|
| TED-NM (orbits, %) | 0(0) | 0(0) | 10(33) | 15(50) | 5(17) | 30(100) | |
| TED-IM (orbit number, %) | non-DON | 3(9) | 12(38) | 15(47) | 2(6) | 0(0) | 32(100) |
| DON | 24(80) | 4(13) | 2(7) | 0(0) | 0(0) | 30(100) | |
| ALR-ON | 0° | <5° | 5–10° | 10–15° | >15° | Amount | |
|---|---|---|---|---|---|---|---|
| TED-NM (orbit number, %) | 0(0) | 0(0) | 1(3) | 7(23) | 22(73) | 30(100) | |
| TED-IM (orbit number, %) | non-DON | 1(3) | 2(6) | 7(22) | 18(56) | 4(13) | 32(100) |
| DON | 9(30) | 4(13) | 7(23) | 8(27) | 2(7) | 30(100) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Y.; Zhang, Z.; Li, C.; Ma, H.; Yin, P.; Wang, Y.; Luo, G.; Lu, R. Geometric and Volumetric Measurements of Orbital Structures in CT Scans in Thyroid Eye Disease Classification. Appl. Sci. 2021, 11, 4873. https://doi.org/10.3390/app11114873
Bao Y, Zhang Z, Li C, Ma H, Yin P, Wang Y, Luo G, Lu R. Geometric and Volumetric Measurements of Orbital Structures in CT Scans in Thyroid Eye Disease Classification. Applied Sciences. 2021; 11(11):4873. https://doi.org/10.3390/app11114873
Chicago/Turabian StyleBao, Yuekun, Zhihui Zhang, Cheng Li, Huan Ma, Pan Yin, Yinghao Wang, Guangwei Luo, and Rong Lu. 2021. "Geometric and Volumetric Measurements of Orbital Structures in CT Scans in Thyroid Eye Disease Classification" Applied Sciences 11, no. 11: 4873. https://doi.org/10.3390/app11114873
APA StyleBao, Y., Zhang, Z., Li, C., Ma, H., Yin, P., Wang, Y., Luo, G., & Lu, R. (2021). Geometric and Volumetric Measurements of Orbital Structures in CT Scans in Thyroid Eye Disease Classification. Applied Sciences, 11(11), 4873. https://doi.org/10.3390/app11114873






