Facile Synthesis and Characterization of Palladium@Carbon Catalyst for the Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Catalyst Preparation
2.2.2. Suzuki–Miyaura Reaction Catalyzed by a Pd/C Nanocatalyst
2.3. Mizoroki–Heck Cross-Coupling Reaction
2.4. Characterization
3. Results and Discussion
3.1. XRD Analysis
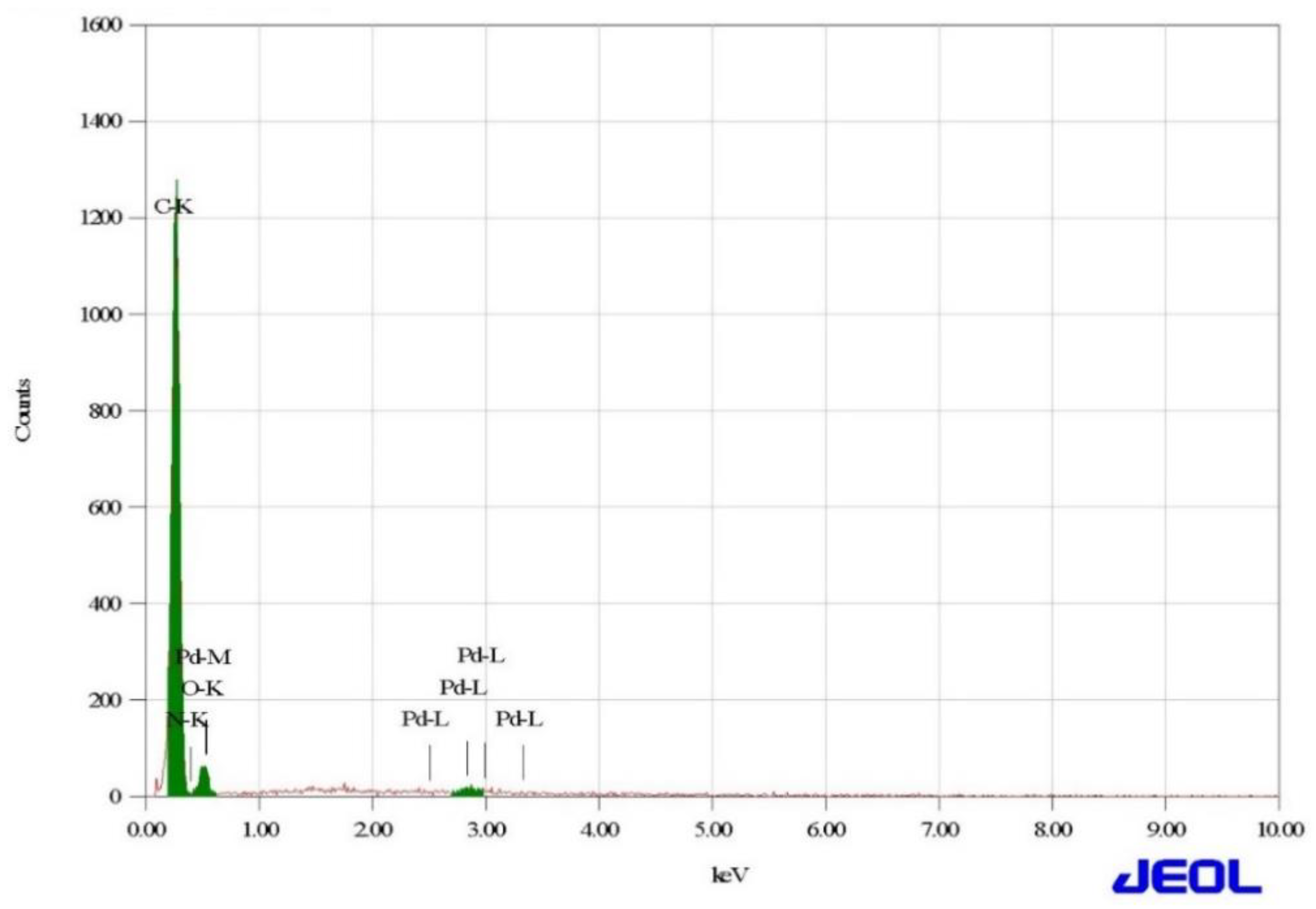
3.2. SEM and EDX Analysis
3.3. BET Analysis
3.4. Suzuki Reaction Catalyzed by the Pd/C Catalyst
3.5. Mizoroki–Heck Cross-Coupling Reaction by the Pd/C Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, A. Organoboron compounds in new synthetic reactions. Pure Appl. Chem. 1985, 57, 1749–1758. [Google Scholar] [CrossRef]
- Heck, R.F. Palladium-catalyzed vinylation of organic halides. Org. React. 2004, 27, 345–390. [Google Scholar]
- Milstein, D.; Stille, J. Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism. J. Am. Chem. Soc. 1979, 101, 4992–4998. [Google Scholar] [CrossRef]
- Negishi, E. Palladium-or nickel-catalyzed cross coupling. A new selective method for carbon-carbon bond formation. Acc. Chem. Res. 1982, 15, 340–348. [Google Scholar] [CrossRef]
- Nakao, Y.; Hiyama, T. Silicon-based cross-coupling reaction: An environmentally benign version. Chem. Soc. Rev. 2011, 40, 4893–4901. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Handa, S.; Wang, Y.; Gallou, F.; Lipshutz, B.H. Sustainable Fe–ppm Pd nanoparticle catalysis of Suzuki-Miyaura cross-couplings in water. Science 2015, 349, 1087–1091. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, A.; Suzuki, H.; Kubota, Y.; Ohuchi, K.; Higashimura, H.; Yokozawa, T. Chain-growth polymerization for the synthesis of polyfluorene via suzuki-miyaura coupling reaction from an externally added initiator unit. J. Am. Chem. Soc. 2007, 129, 7236–7237. [Google Scholar] [CrossRef]
- Heravi, M.M.; Hashemi, E. Recent applications of the Suzuki reaction in total synthesis. Tetrahedron 2012, 68, 9145–9178. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.; Adil, S.F.; Shaik, M.R.; Khan, M.; Assal, M.E.; Siddiqui, M.R.H.; Al-Warthan, A. One-Pot synthesized Pd@ N-doped graphene: An efficient catalyst for suzuki–miyaura couplings. Catalysts 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki–Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Ocansey, E.; Darkwa, J.; Makhubela, B.C. Synthesis, characterization and evaluation of bulky bis (pyrazolyl) palladium complexes in Suzuki–Miyaura cross-coupling reactions. RSC Adv. 2018, 8, 13826–13834. [Google Scholar] [CrossRef] [Green Version]
- Wolf, C.; Xu, H. Efficient synthesis of sterically crowded biaryls by palladium-phosphinous acid-catalyzed cross-coupling of aryl halides and aryl grignards. J. Org. Chem. 2008, 73, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tobisu, M.; Xu, T.; Shimasaki, T.; Chatani, N. Nickel-catalyzed Suzuki–Miyaura reaction of aryl fluorides. J. Am. Chem. Soc. 2011, 133, 19505–19511. [Google Scholar] [CrossRef] [PubMed]
- Meise, M.; Haag, R. A highly active water-soluble cross-coupling catalyst based on dendritic polyglycerol n-heterocyclic carbene palladium complexes. ChemSusChem Chem. Sustain. Energy Mater. 2008, 1, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Doucet, H.; Hierso, J.-C. Palladium coupling catalysts for pharmaceutical applications. Curr. Opin. Drug Dis. Dev. 2007, 10, 672–690. [Google Scholar]
- King, A.O.; Yasuda, N. Palladium-catalyzed cross-coupling reactions in the synthesis of pharmaceuticals. In Organometallics in Process Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 205–245. [Google Scholar]
- Torborg, C.; Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Mülhaupt, R. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki−Miyaura coupling reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.; Friedrich, H.B. The current status of heterogeneous palladium catalysed Heck and Suzuki cross-coupling reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Lorenzo, M. Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Das, P.; Linert, W. Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord. Chem. Rev. 2016, 311, 1–23. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, M.; Lee, W.Y.; Hyeon, T. Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for Suzuki coupling reactions. J. Am. Chem. Soc. 2002, 124, 7642–7643. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Green Suzuki–Miyaura coupling reaction catalyzed by palladium nanoparticles supported on graphitic carbon nitride. Appl. Catal. B Environ. 2015, 165, 661–667. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lu, K.-L.; Liu, S.-B.; Rajagopal, S. Functionalized silica matrices and palladium: A versatile heterogeneous catalyst for Suzuki, Heck, and Sonogashira reactions. ACS Sustain. Chem. Eng. 2017, 5, 6357–6376. [Google Scholar] [CrossRef]
- Astakhov, A.V.; Khazipov, O.V.; Chernenko, A.Y.; Pasyukov, D.V.; Kashin, A.S.; Gordeev, E.G.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. A new mode of operation of Pd-NHC systems studied in a catalytic Mizoroki–Heck reaction. Organometallics 2017, 36, 1981–1992. [Google Scholar] [CrossRef]
- Bej, A.; Ghosh, K.; Sarkar, A.; Knight, D.W. Palladium nanoparticles in the catalysis of coupling reactions. RSC Adv. 2016, 6, 11446–11453. [Google Scholar] [CrossRef]
- Khan, M.; Kuniyil, M.; Shaik, M.; Khan, M.; Adil, S.; Al-Warthan, A.; Alkhathlan, H.; Tremel, W.; Tahir, M.; Siddiqui, M. Plant extract mediated eco-friendly synthesis of Pd@ graphene nanocatalyst: An efficient and reusable catalyst for the Suzuki-Miyaura coupling. Catalysts 2017, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Oger, N.; Felpin, F.X. Heterogeneous palladium catalysts for Suzuki–Miyaura coupling reactions involving aryl diazonium salts. ChemCatChem 2016, 8, 1998–2009. [Google Scholar] [CrossRef]
- Movahed, S.K.; Dabiri, M.; Bazgir, A. Palladium nanoparticle decorated high nitrogen-doped graphene with high catalytic activity for Suzuki–Miyaura and Ullmann-type coupling reactions in aqueous media. Appl. Catal., A 2014, 488, 265–274. [Google Scholar] [CrossRef]
- Asadi, S.; Sedghi, R.; Heravi, M.M. Pd nanoparticles immobilized on supported magnetic GO@ PAMPS as an auspicious catalyst for Suzuki–Miyaura coupling reaction. Catal. Lett. 2017, 147, 2045–2056. [Google Scholar] [CrossRef]
- Yoshii, T.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of Pd–graphene–Au nanorod nanocomposite catalyst for boosting suzuki–miyaura coupling reaction by assistance of surface plasmon resonance. J. Phys. Chem. C 2019, 123, 24575–24583. [Google Scholar] [CrossRef]
- Khalili, D.; Banazadeh, A.R.; Etemadi-Davan, E. Palladium stabilized by amino-vinyl silica functionalized magnetic carbon nanotube: Application in suzuki–miyaura and heck–mizoroki coupling reactions. Catal. Lett. 2017, 147, 2674–2687. [Google Scholar] [CrossRef]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in carbon nanotubes as efficacious supports for palladium-catalysed carbon–carbon cross-coupling reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Lakshminarayana, B.; Mahendar, L.; Ghosal, P.; Satyanarayana, G.; Subrahmanyam, C. Nano-sized Recyclable PdO supported carbon nanostructures for heck reaction: Influence of carbon materials. ChemSelect 2017, 2, 2700–2707. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Wang, H.; Cao, Y.; He, J. Facile deposition of Pd nanoparticles on carbon nanotube microparticles and their catalytic activity for Suzuki coupling reactions. J. Phys. Chem. C 2008, 112, 8172–8176. [Google Scholar] [CrossRef]
- Felpin, F.-X. Ten years of adventures with Pd/C catalysts: From reductive processes to coupling reactions. Synlett 2014, 25, 1055–1067. [Google Scholar] [CrossRef]
- Cini, E.; Petricci, E.; Taddei, M. Pd/C catalysis under microwave dielectric heating. Catalysts 2017, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.; Riemer, M. Suzuki–Miyaura coupling of halophenols and phenol boronic acids: Systematic investigation of positional isomer effects and conclusions for the synthesis of phytoalexins from pyrinae. J. Org. Chem. 2014, 79, 4104–4118. [Google Scholar] [CrossRef]
- Yuan, Y.-Q.; Guo, S.-R. Remarkably facile Heck reactions in aqueous two-phase system catalyzed by reusable Pd/C under ligand-free conditions. Synth. Commun. 2012, 42, 1059–1069. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Chen, X.; Wang, L.-G. Recycled Pd/C-catalyzed heck reaction of 2-Iodoanilines under ligand-free conditions. Synthesis 2017, 49, 5364–5370. [Google Scholar] [CrossRef] [Green Version]
- Seki, M. Practical synthesis of multifunctional compounds through Pd/C-catalyzed coupling reactions. J. Synth. Org. Chem Jpn. 2006, 64, 853–866. [Google Scholar] [CrossRef] [Green Version]
- Dighe, M.G.; Lonkar, S.L.; Degani, M.S. Mechanistic insights into palladium leaching in novel Pd/C-catalyzed boron-Heck reaction of arylboronic acid. Synlett 2013, 24, 347–350. [Google Scholar]
- Horikoshi, S.; Serpone, N. Role of microwaves in heterogeneous catalytic systems. Catal. Sci. Technol. 2014, 4, 1197–1210. [Google Scholar] [CrossRef]
- Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Palladium on carbon-catalyzed Suzuki-Miyaura coupling reaction using an efficient and continuous flow system. Catalysts 2015, 5, 18–25. [Google Scholar] [CrossRef] [Green Version]
- García-Suárez, E.J.; Lara, P.; García, A.B.; Ojeda, M.; Luque, R.; Philippot, K. Efficient and recyclable carbon-supported Pd nanocatalysts for the Suzuki–Miyaura reaction in aqueous-based media: Microwave vs. conventional heating. Appl. Catal. A 2013, 468, 59–67. [Google Scholar] [CrossRef]





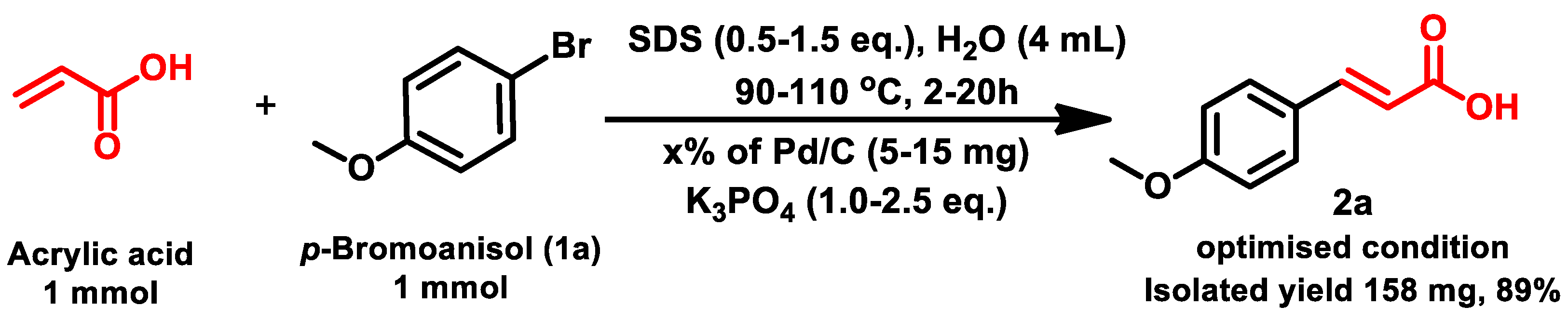



| Sl. No. | Catalyst | Wt. of Catalyst | K3PO4 | SDS | Temp. (°C) | Time (h) | Isolated Yield |
|---|---|---|---|---|---|---|---|
| 1 | 3.0% Pd/C | 10 mg | 1.0 eq. | - | 100 | 10 h | No reaction |
| 2 | 3.0% Pd/C | 10 mg | 1.0 eq. | 0.5 eq. | 100 | 10 h | 27% |
| 3 | 3.0% Pd/C | 10 mg | 1.0 eq. | 1.0 eq. | 100 | 10 h | 46% |
| 4 | 3.0% Pd/C | 10 mg | 1.0 eq. | 1.5 eq. | 100 | 10 h | 47% |
| 5 | 3.0% Pd/C | 10 mg | 1.5 eq. | 1.0 eq. | 100 | 10 h | 63% |
| 6 | 3.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 10 h | 89% |
| 7 | 3.0% Pd/C | 15 mg | 2.5 eq. | 1.0 eq. | 100 | 10 h | 87% |
| 8 | 0.5% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 10 h | 45% |
| 9 | 1.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 10 h | 57% |
| 10 | 2.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 10 h | 76% |
| 11 | 3.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 2 h | 17% |
| 12 | 3.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 5 h | 34% |
| 13 | 3.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 100 | 16 h | 86% |
| 14 | 3.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 90 | 20 h | 11% |
| 15 | 3.0% Pd/C | 10 mg | 2.0 eq. | 1.0 eq. | 110 | 20 h | 83% |
| Sl. No. | Aryl Halides | 1a–f | 2a–e | Isolated Yield |
|---|---|---|---|---|
| 1 | 4-bromoanisol | 1a | 2a | 89% |
| 2 | 3-bromoanisol | 1b | 2b | 85% |
| 3 | 2-bromoanisol | 1c | 2c | 72% |
| 4 | 4-Iodotoluene | 1d | 2d | 87% |
| 5 | 4-Iodobenzene | 1e | 2e | 91% |
| 6 | 4-bromobenzene | 1f | 2e | 88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, H.M.; Aldosari, O.F.; Alotaibi, M.H.; Alotaibi, R.L.; Alhumaimess, M.S.; Morad, M.H.; Adil, S.F.; Shaik, M.R.; Islam, M.S.; Khan, M.; et al. Facile Synthesis and Characterization of Palladium@Carbon Catalyst for the Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions. Appl. Sci. 2021, 11, 4822. https://doi.org/10.3390/app11114822
Alshammari HM, Aldosari OF, Alotaibi MH, Alotaibi RL, Alhumaimess MS, Morad MH, Adil SF, Shaik MR, Islam MS, Khan M, et al. Facile Synthesis and Characterization of Palladium@Carbon Catalyst for the Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions. Applied Sciences. 2021; 11(11):4822. https://doi.org/10.3390/app11114822
Chicago/Turabian StyleAlshammari, Hamed M., Obaid F. Aldosari, Mohammad Hayal Alotaibi, Raja L. Alotaibi, Mosaed S. Alhumaimess, Moataz H. Morad, Syed Farooq Adil, Mohammed Rafi Shaik, Mohammad Shahidul Islam, Mujeeb Khan, and et al. 2021. "Facile Synthesis and Characterization of Palladium@Carbon Catalyst for the Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions" Applied Sciences 11, no. 11: 4822. https://doi.org/10.3390/app11114822
APA StyleAlshammari, H. M., Aldosari, O. F., Alotaibi, M. H., Alotaibi, R. L., Alhumaimess, M. S., Morad, M. H., Adil, S. F., Shaik, M. R., Islam, M. S., Khan, M., & Alwarthan, A. (2021). Facile Synthesis and Characterization of Palladium@Carbon Catalyst for the Suzuki-Miyaura and Mizoroki-Heck Coupling Reactions. Applied Sciences, 11(11), 4822. https://doi.org/10.3390/app11114822









