Training and Detraining Effects of a Rehabilitation Program with or without Electro-Cryotherapy in Patients with Anterior Knee Pain: A Randomized Trial
Abstract
:1. Introduction
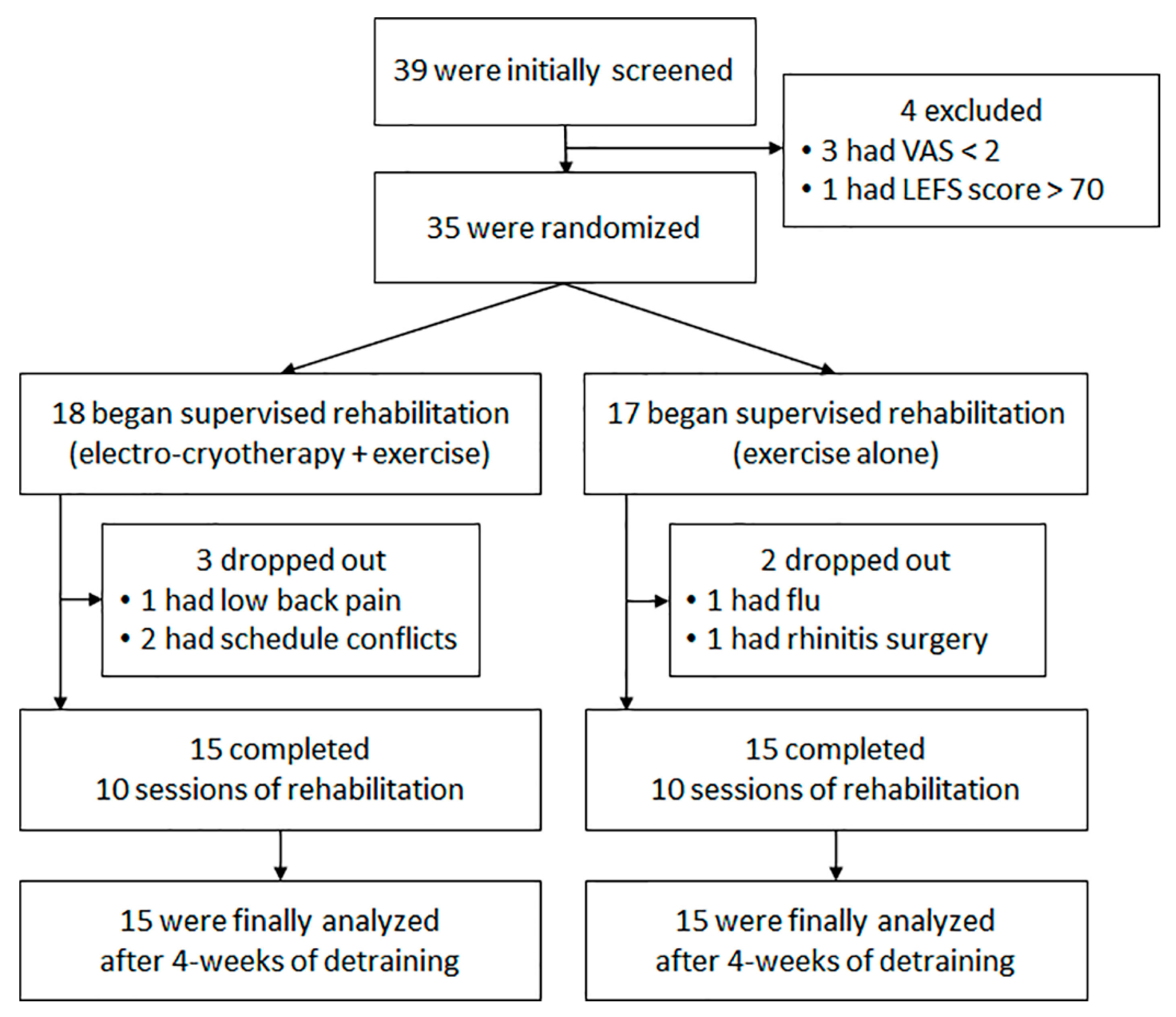
2. Materials and Methods
2.1. Subjects
2.2. Testing Procedures
2.3. Endpoints
2.3.1. Quadriceps Function
2.3.2. Self-Reported Function
2.4. Treatment Conditions
2.4.1. Modality + Exercise
2.4.2. Exercise Alone
2.5. Statistical Analysis
3. Results
3.1. Quadriceps Function
3.2. Self-Reported Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingram, T.G.; Roddick, J.M.; Byrne, J.M. Is gamma loop dysfunction related to bilateral inhibition in anterior knee pain? Muscle Nerve 2016, 53, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Guney, H.; Yuksel, I.; Kaya, D.; Doral, M.N. The relationship between quadriceps strength and joint position sense, functional outcome and painful activities in patellofemoral pain syndrome. Knee. Surg. Sport. Traumatol. Arthrosc. 2016, 24, 2966–2972. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hopkins, J.T. Induced anterior knee pain immediately reduces involuntary and voluntary quadriceps activation. Clin. J. Sport Med. 2013, 23, 19–24. [Google Scholar] [CrossRef]
- Hopkins, J.T.; Ingersoll, C.D.; Edwards, J.; Klootwyk, T.E. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J. Athl. Train. 2002, 37, 25–31. [Google Scholar] [PubMed]
- Garstang, S.V.; Stitik, T.P. Osteoarthritis: Epidemiology, risk factors, and pathophysiology. Am. J. Phys. Med. Rehabil. 2006, 85, S2–S11. [Google Scholar] [CrossRef]
- Hart, J.M.; Pietrosimone, B.; Hertel, J.; Ingersoll, C.D. Quadriceps activation following knee injuries: A systematic review. J. Athl. Train. 2010, 45, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoloni, M.; Mangone, M.; Fratocchi, G.; Murgia, M.; Saraceni, V.M.; Santilli, V. Kinematic and kinetic features of normal level walking in patellofemoral pain syndrome: More than a sagittal plane alteration. J. Biomech. 2010, 43, 1794–1798. [Google Scholar] [CrossRef]
- Weiss, K.; Whatman, C. Biomechanics associated with patellofemoral pain and ACL injuries in sports. Sports Med. 2015, 45, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Kim, H.; Seeley, M.; Feland, J.; Hopkins, J. Effects of transcutaneous electrical nerve stimulation on quadriceps function in individuals with experimental knee pain. Scand. J. Med. Sci. Sports 2016, 26, 1080–1090. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J.; Dalbeth, N. Effects of cryotherapy on arthrogenic muscle inhibition using an experimental model of knee swelling. Arthritis Rheum. 2009, 61, 78–83. [Google Scholar] [CrossRef]
- Hart, J.M.; Kuenze, C.M.; Pietrosimone, B.G.; Ingersoll, C.D. Quadriceps function in anterior cruciate ligament-deficient knees exercising with transcutaneous electrical nerve stimulation and cryotherapy: A randomized controlled study. Clin. Rehabil. 2012, 26, 974–981. [Google Scholar] [CrossRef]
- Hart, J.M.; Kuenze, C.M.; Diduch, D.R.; Ingersoll, C.D. Quadriceps muscle function after rehabilitation with cryotherapy in patients with anterior cruciate ligament reconstruction. J. Athl. Train. 2014, 49, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrosimone, B.G.; Saliba, S.A.; Hart, J.M.; Hertel, J.; Kerrigan, D.C.; Ingersoll, C.D. Effects of disinhibitory transcutaneous electrical nerve stimulation and therapeutic exercise on sagittal plane peak knee kinematics and kinetics in people with knee osteoarthritis during gait: A randomized controlled trial. Clin. Rehabil. 2010, 24, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Saliba, S.A.; Hart, J.M.; Hertel, J.; Kerrigan, D.C.; Ingersoll, C.D. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J. Orthop. Sports Phys. Ther. 2011, 41, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, K.L. Quadriceps strengthening with the DAPRE technique: Case studies with neurological implications. Med. Sci. Sports Exerc. 1985, 17, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Saliba, S.A.; Hart, J.M.; Hertel, J.; Ingersoll, C.D. Contralateral effects of disinhibitory TENS on quadriceps function in people with knee osteoarthritis following unilateral treatment. N. Am. J. Sports Phys. Ther. 2010, 5, 111. [Google Scholar] [PubMed]
- Herrington, L.; Al-Sherhi, A. A controlled trial of weight-bearing versus non—weight-bearing exercises for patellofemoral pain. J. Orthop. Sports Phys. Ther. 2007, 37, 155–160. [Google Scholar] [CrossRef]
- Park, J.; Grindstaff, T.L.; Hart, J.M.; Hertel, J.N.; Ingersoll, C.D. Knee-extension exercise’s lack of immediate effect on maximal voluntary quadriceps torque and activation in individuals with anterior knee pain. J. Sport. Rehabil. 2012, 21, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Tyler, T.F.; Nicholas, S.J.; Mullaney, M.J.; McHugh, M.P. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am. J. Sports Med. 2006, 34, 630–636. [Google Scholar] [CrossRef]
- Alcock, G.; Werstine, M.; Robbins, S.; Stratford, P. Longitudinal changes in the lower extremity functional scale after anterior cruciate ligament reconstructive surgery. Clin. J. Sport Med. 2012, 22, 234–239. [Google Scholar] [CrossRef]
- Grindstaff, T.L.; Hertel, J.; Beazell, J.R.; Magrum, E.M.; Ingersoll, C.D. Effects of lumbopelvic joint manipulation on quadriceps activation and strength in healthy individuals. Man. Ther. 2009, 14, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Le Blanc, R. Quantification of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 1996, 19, 861–869. [Google Scholar] [CrossRef]
- Kline, P.W.; Morgan, K.D.; Johnson, D.L.; Ireland, M.L.; Noehren, B. Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. Am. J. Sports Med. 2015, 43, 2553–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.L.; Aagaard, P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur. J. Appl. Physiol. 2006, 96, 46–52. [Google Scholar] [CrossRef]
- Pincivero, D.M.; Gandaio, C.B.; Ito, Y. Gender-specific knee extensor torque, flexor torque, and muscle fatigue responses during maximal effort contractions. Eur. J. Appl. Physiol. 2003, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Van Den Ham, E.C.H.; Kooman, J.P.; Schols, A.M.; Nieman, F.H.M.; Does, J.D.; Franssen, F.M.E.; Akkermans, M.A.; Janssen, P.P.; van Hooff, J.P. Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. Am. J. Transplant. 2005, 5, 1957–1965. [Google Scholar] [CrossRef]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Hart, J.M.; Saliba, S.A.; Hertel, J.; Ingersoll, C.D. Immediate effects of transcutaneous electrical nerve stimulation and focal knee joint cooling on quadriceps activation. Med. Sci. Sports Exerc. 2009, 41, 1175–1181. [Google Scholar] [CrossRef]
- Pamukoff, D.N.; Pietrosimone, B.G.; Ryan, E.D.; Lee, D.R.; Blackburn, J.T. Quadriceps function and hamstrings co-activation after anterior cruciate ligament reconstruction. J. Athl. Train. 2017, 52, 422–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Quantitative methods in psychology: A power primer. Psychol. Bull. 1992, 112, 1155–1159. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Allen, C.; Avila, P.; Bina, J.; Konrade, J.; Munns, S. Soft tissue thermodynamics before, during, and after cold pack therapy. Med. Sci. Sports Exerc. 2002, 34, 45–50. [Google Scholar] [CrossRef]
- Kwon, S.; Bruening, D.A.; Morrin, S.J.; Kunz, D.M.; Hopkins, J.T.; Seeley, M.K. Simultaneous ice and transcutaneous electrical nerve stimulation decrease anterior knee pain during running but do not affect running kinematics or associated muscle inhibition. Clin. Biomech. 2020, 72, 1–7. [Google Scholar] [CrossRef]
- Cheing, G.L.; Hui-Chan, C.W. Would the addition of TENS to exercise training produce better physical performance outcomes in people with knee osteoarthritis than either intervention alone? Clin. Rehabil. 2004, 18, 487–497. [Google Scholar] [CrossRef]
- Park, J.; Hopkins, J.T. Immediate effects of acupuncture and cryotherapy on quadriceps motoneuron pool excitability: Randomised trial using anterior knee infusion model. Acupunct. Med. 2012, 30, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Cheing, G.L.; Hui-Chan, C.W.; Chan, K. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Clin. Rehabil. 2002, 16, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.J.; Denegar, C.R. Does cryotherapy improve outcomes with soft tissue injury? J. Athl. Train. 2004, 39, 278–279. [Google Scholar] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.S.; Dyhre-Poulsen, P. Increase rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef]
- Winters, J.D.; Rudolph, K.S. Quadriceps rate of force development affects gait and function in people with knee osteoarthritis. Eur. J. Appl. Physiol. 2014, 114, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| n/mean ± 95% CI | Modality + Exercise (n = 15) | Exercise Alone (n = 15) | p-Value |
| Age (year) | 20.6 ± 1.2 | 21.8 ± 1.6 | 0.25 |
| Sex (M/F) | 9/6 | 10/5 | N/A |
| Height (cm) | 170.7 ± 4.6 | 173.0 ± 3.0 | 0.42 |
| Mass (kg) | 74.8 ± 11.9 | 68.7 ± 4.6 | 0.36 |
| BMI (kg/m2) | 25.4 ± 6.3 | 22.9 ± 2.4 | 0.17 |
| LBM (kg) | 56.0 ± 6.7 | 53.7 ± 4.5 | 0.58 |
| Physical activity (hours per week) | 3.3 ± 1.1 | 4.3 ± 1.8 | 0.41 |
| Pain period (months) | 46.1 ± 19.4 | 45.0 ± 16.8 | 0.94 |
| Painful knee (L/R) | 7/8 | 8/7 | N/A |
| Injury type | |||
| Atraumatic | 9 | 8 | N/A |
| Traumatic | 6 | 7 | N/A |
| History of knee surgery | 4 | 3 | N/A |
| Time since surgery (month) | 32.0 ± 12.4 | 56.0 ± 21.5 | N/A |
| Side-side ratio (%) | |||
| MVIC | 92.2 ± 10.9 | 95.1 ± 12.5 | 0.69 |
| CAR | 98.9 ± 8.5 | 96.6 ± 8.4 | 0.62 |
| RTD | 87.0 ± 10.1 | 101.2 ± 12.8 | 0.26 |
| PT | 93.3 ± 6.9 | 97.2 ± 7.6 | 0.37 |
| Exercise | Volume or Duration | Intensity or Progression |
| Jogging | 5-min | Treadmill speed at 6.0 to 8.5 km/h |
| Stretching * | A 30-s hold × 2 sets | Until mild discomfort is felt at the end range of motion |
| SLRs ** | 8 reps × 3 sets | Leg weight |
| Knee extensions **; Lunges **; Squats † | 4 sets | Determined by the DAPRE technique |
| Balance | A 30-second balance × 3 sets | Static (eyes-open or closed; on a balance pad) and dynamic balance |
| Mean ± 95% CI | Modality + Exercise (n = 15) | Exercise Alone (n = 15) | Combined (n = 30) | |
| MVIC (Nm/kg) | Pre-rehabilitation | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.6 ± 0.2 |
| Post-rehabilitation | 2.9 ± 0.4 | 3.0 ± 0.4 | 2.9 ± 0.2 | |
| Detraining | 2.9 ± 0.4 | 3.0 ± 0.4 | 2.9 ± 0.2 * | |
| CAR (%) | Pre-rehabilitation | 81.0 ± 5.1 | 81.2 ± 7.6 | 81.1 ± 3.2 |
| Post-rehabilitation | 85.1 ± 5.2 | 86.5 ± 4.8 | 85.8 ± 2.5 | |
| Detraining | 83.6 ± 4.8 | 87.2 ± 6.1 | 85.4 ± 2.7 | |
| RTD (Nm/kg/s) | Pre-rehabilitation | 10.6 ± 1.7 | 10.8 ± 1.5 | 10.7 ± 0.8 |
| Post-rehabilitation | 13.2 ± 2.1 | 12.4 ± 1.7 | 12.8 ± 0.9 ** | |
| Detraining | 12.7 ± 2.0 | 11.6 ± 2.2 | 12.2 ± 1.0 | |
| PT (Nm/kg) | Pre-rehabilitation | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| Post-rehabilitation | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 ** | |
| Detraining | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.1 ** | |
| Mean ± 95% CI | Modality + Exercise (n = 15) | Exercise Alone (n = 15) | Combined (n = 30) | |
| VAS during ADL (cm) | Pre-rehabilitation | 4.3 ± 0.8 | 4.0 ± 0.9 | 4.2 ± 0.6 |
| Post-rehabilitation | 1.3 ± 0.4 | 1.2 ± 0.3 | 1.3 ± 0.3 * | |
| Detraining | 1.1 ± 0.4 | 1.4 ± 0.7 | 1.2 ± 0.4 * | |
| VAS at least (cm) | Pre-rehabilitation | 1.2 ± 0.6 | 0.9 ± 0.7 | 1.0 ± 0.4 |
| Post-rehabilitation | 0.6 ± 0.4 | 0.5 ± 0.3 | 0.6 ± 0.2 ** | |
| Detraining | 0.5 ± 0.4 | 0.5 ± 0.3 | 0.5 ± 0.2 ** | |
| VAS at worst (cm) | Pre-rehabilitation | 7.3 ± 0.7 | 6.6 ± 0.9 | 7.0 ± 0.6 |
| Post-rehabilitation | 4.1 ± 1.1 | 3.9 ± 0.8 | 4.0 ± 0.7 * | |
| Detraining | 3.2 ± 0.7 | 3.5 ± 1.1 | 3.3 ± 0.6 * | |
| LEFS (scores) | Pre-rehabilitation | 49.3 ± 5.5 | 56.3 ± 5.1 | 52.8 ± 3.9 |
| Post-rehabilitation | 59.1 ± 5.8 | 60.6 ± 6.8 | 59.8 ± 4.4 ** | |
| Detraining | 64.2 ± 5.4 | 64.5 ± 4.5 | 64.4 ± 3.5 *,† | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, Y.; Park, J. Training and Detraining Effects of a Rehabilitation Program with or without Electro-Cryotherapy in Patients with Anterior Knee Pain: A Randomized Trial. Appl. Sci. 2021, 11, 4812. https://doi.org/10.3390/app11114812
Roh Y, Park J. Training and Detraining Effects of a Rehabilitation Program with or without Electro-Cryotherapy in Patients with Anterior Knee Pain: A Randomized Trial. Applied Sciences. 2021; 11(11):4812. https://doi.org/10.3390/app11114812
Chicago/Turabian StyleRoh, Yuyeon, and Jihong Park. 2021. "Training and Detraining Effects of a Rehabilitation Program with or without Electro-Cryotherapy in Patients with Anterior Knee Pain: A Randomized Trial" Applied Sciences 11, no. 11: 4812. https://doi.org/10.3390/app11114812
APA StyleRoh, Y., & Park, J. (2021). Training and Detraining Effects of a Rehabilitation Program with or without Electro-Cryotherapy in Patients with Anterior Knee Pain: A Randomized Trial. Applied Sciences, 11(11), 4812. https://doi.org/10.3390/app11114812






