1. Introduction
Well-being refers to the experience of health, happiness, and prosperity. There are many examples of well-being, such as having good mental health, high satisfaction with life, and a sense of meaning or purpose. Well-being is also a long-term asset for cosmetics. Several studies have demonstrated the positive emotional influence of cosmetics on well-being and self-esteem [
1]. For example, an increase in positive and a decrease in negative emotions have been observed in women after professional hairdressing [
2]. The main reason consumers use cosmetics is to feel good, and wearing make-up or applying a cosmetic product are sources of positive emotion.
Although the skin and emotions are closely linked, the emotions engendered by the specific step of application of a cosmetic product have been under-researched. The spreading of a cosmetic product to the skin generates emotions, whatever the role or effectiveness of the product. Various approaches have been developed to measure the emotions resulting from the application of a cosmetic product. Traditionally, these have relied on subjective response methodologies, such as questionnaires, surveys, or focus groups [
3,
4]. However, these measures are only reflections, not direct real-time proof, of the emotions felt by the individual. Verbal or written opinions can be flawed by the respondent’s cognitive processes.
This is why more objective methods for measuring emotions and based on psychophysiological or neural measures could be used. The techniques used in neuroscience research can overcome some of the limitations associated with the methodologies that are often applied to investigate well-being and emotions. By measuring processes and behaviors that are outside of the individual’s awareness, neuroscience techniques can overcome many of the limitations of subjective and behavioral responses. Objective parameters such as skin conductance and heart rate have been used to measure the emotional effect on subjects of the color and odor of cosmetic products [
5,
6]. Skin response (GSR) measurements have been used to evaluate the capacity of different lip balms to induce different emotions when applied on the lips [
7]. The measure of cerebral activity is another method, tracking emotions directly within the brain. Functional neuroimaging allows the brain’s information processing to be visualized directly, because activity in the involved area induces local electrical, magnetic, or metabolic changes that can be detected with the appropriate method. Most common methods for measuring brain activity are functional Magnetic Resonance Imaging (fMRI), Positron Emission Tomography (PET), Near Infrared Spectroscopy (NIRS), and electroencephalography (EEG). Each of these methods has its pros and cons. Some of these techniques have a high spatial resolution (a few millimeters), but a poor temporal resolution, whereas others have a high temporal resolution (i.e., milliseconds) albeit with a poorer spatial resolution [
8,
9]. Functional neuroimaging can be particularly powerful in the cosmetic domain; it has shown, for example, that the application of a cosmetic product on the skin activates different sensory areas in the brain compared to when the skin alone is touched [
10]. EEG is one of the cerebral recording methods that can advantageously reveal the emotions felt by users when applying a cosmetic product. This is an electrophysiological monitoring method that records electrical activity in the brain. It is typically non-invasive, with the electrodes placed along the scalp. Most of the electrical activity collected in the EEG is generated by groups of pyramidal neurons. These neurons have cell bodies primarily in layers three and five of the cerebral cortex. When they are activated, local current flows are produced. The electrical activity recorded on the scalp is generated by the summation of the excitatory and inhibitory postsynaptic potentials. This activity can be represented by dipoles parallel to the orientation of the pyramidal cells. Only large populations of active neurons can generate recordable electrical activity at the surface of the head. Electrical signal must propagate from the brain, through the skull, skin, and hair before reaching the electrode. The weak electrical signals detected by the scalp electrodes are massively amplified and then recorded in the computer. Because of its ability to assess normal and abnormal electrical activity in the brain in real-time, EEG has proven to be a very powerful tool in the field of neuroscience and clinical neurophysiology [
11]. Its temporal resolution is below the millisecond, which makes it a good candidate for the real-time study of emotions.
The use of EEG in the measurement of emotions has a long history, although not in the area of cosmetics. Dimensional models have been employed extensively to measure emotions with EEG. They have suggested that emotion is best understood as occurring within a dimensional space, most commonly a two-dimensional space, spanning valence and arousal [
12]. Emotional valence describes the extent to which an emotion is positive or negative, whereas arousal refers to its intensity, i.e., the strength of the associated emotional state [
13,
14,
15]. The activity of the prefrontal cortex, which acts as a modulator of primary emotional responses through its connections with deep brain structures [
16], is fundamental in the evaluation of emotional valence. Dominant activity in right versus left prefrontal areas is associated with withdrawal behavior and negative emotions, while the opposite representation (i.e., left versus right) accompanies approach behaviors and positive emotions [
17,
18,
19]. Reduced alpha activity in the right prefrontal cortex is associated with negative emotions, for example after viewing unpleasant films [
19,
20]. On the other hand, reduced alpha activity in the left hemisphere is related to positive emotions, for example after viewing happy movies or listening to pleasant music [
20,
21]. Frontal asymmetries associated with emotions and motivation have also been observed at the theta band level [
22,
23] and the upper beta band level [
24,
25]. In addition to the measurement of emotional valence, Ramirez and Vamvakousis added an additional parameter in determining arousal by calculating the ratio of beta and alpha oscillations, to conform to the dimensional models of emotion studies [
26,
27].
The purpose of this pilot study was to use EEG to compare the emotions induced by the application of two cosmetic products. We hypothesized that the preferred application would generate emotions of more positive valence, as detected with EEG. Our second objective was to offer to the experimenter a real-time representation of participants’ emotional states while they were applying the products. To that end, we developed a visual representation of emotions based on a collaboration between scientists and digital artists [
28]. With this system, we expected to visualize the particles moving to the right in real-time when participants were applying the products.
2. Materials and Methods
2.1. Population
Fifteen female subjects, all aged between 18 and 65 years, participated in the study. All were healthy, and none had a lesion, wound, or dermatosis at the site of application of the products (dominant hand). All declared that they were neither pregnant nor breastfeeding at the time of the study. They received information regarding the aim and procedures of the experiment, and they gave their written informed consent to their participation. In accordance with French law, the study was classified as psychology observational research outside of the Jardé law and so did not require submission to an ethics committee. Since the measure of emotions induced by the application cosmetic product on the skin has never been performed with EEG, a power analysis could not be directly calculated. The goal of this pilot study was to obtain the required preliminary data for the calculation of a sample size for the primary outcome in a larger study. The number of participants was chosen in accordance with studies advocating that 12 participants are enough to properly conduct a pilot study [
29]. The justifications for this sample size are based on rationale about precision about the mean and variance; but also the feasibility and regulatory considerations. Moreover this number was roughly similar to other studies evaluating brain responses following tactile stimulations.
2.2. Product Description
Two cosmetic products were evaluated: two compositions with aqueous continuous phase containing a low amount of oil and characterized by a similar consistency. Benchmark 1 (CP1): viscosity measured by a Brookfield viscometer (spindle 4, speed 6) at 28,000 mPa-s. Benchmark 2 (CP2): viscosity measured by a Brookfield viscometer (spindle 4, speed 6) at 21,000 mPa-s. Neither the participants nor the researchers were aware of the composition of each product.
2.3. Course of the Experiment
Participants were comfortably seated in a chair, informed of the procedure, and instructed to remain as calm as possible and not to move for the duration of the experiment. An EEG headset was then installed with an impedance check lasting approximately 5 min. A 2-min rest recording was then made to normalize the data obtained from each subjects, by delimiting the inferior and superior limits of valence and arousal values. A cross-over design was used, with each subject having to apply the two creams successively in randomized order. The two main advantages of a crossover trial is that it effectively doubles the number of people in a trial and eliminates individual subject differences from the overall treatment effect. This makes it easier for researchers to obtain statistically significant results. The creams were applied from the dominant hand to the non-dominant hand. Each product was applied to the hand for 1 min and repeated 3 times (
Figure 1). Participants were instructed to close their eyes, to remain still, and to focus on their emotions felt while they were gently applying the product. Between each application of the same product, they had to remove the residue on their hand with a towel. They were given a break and instructed to wash their hands. They then had to color a neutral picture for approximately 10 min with colored pencils. During each product application (3 times × 2 products), the neural activity of each participant was recorded. In the second repetition of each product, we video-captured in real-time the representation of their emotions.
At the end of the experiment, participants were asked which one of the two cosmetic products was preferred. After the last repetition of each, their appreciation was also evaluated by completing a questionnaire on a 5-point scale (from not agree at all to totally agree). Participants had to judge six proposals: ‘I liked the whole product’, ‘I liked the texture of the product’, ‘I like the look of the product’, ‘The application of the product was easy’, ‘My skin is soft after its application’ and ‘The product leaves a pleasant film on the skin’.
2.4. Brain Data Acquisition
The EEG data were acquired from an EEG Emotiv EPOC+ system. This consists of 16 saline-based electrodes and a wireless amplifier. The electrodes were located at positions AF3, F7, F3, FC5, T7, P7, O1, O2, P8, T8, FC6, F4, F8, AF4, according to the international 10–20 system. The 10–20 system is an internationally recognized method that allows EEG electrode placement to be standardized. It ensures that inter-electrode spacing is equal and that electrode placements are proportional to skull size and shape. Two electrodes located just above the ears (P3, P4) were used as a reference. The data were collected at a sampling rate of 128 Hz and transferred to the computer via Bluetooth. The choice of this device was based on several advantages compared to more conventional EEG systems. The main advantages of such technologies include quick and easy positioning due to saline electrodes and wireless Bluetooth data transmission, opening new perspectives for the study of cerebral activity in natural or ecological settings.
The installation time of each Emotiv Epoc+ system is considerably shorter, about 5 min less than gel-based systems, where the gel application for each electrode can take up to one hour, which extends considerably the duration of experiments.
2.5. Processing of EEG Data
The EEG processing of valence and arousal was based on methods from previous studies [
26,
27] and involved a two-dimensional arousal-valence design [
30]. Data were averaged over 2 s windows. To determine the valence level, the activation levels of the cortical hemispheres were compared. The F3 and F4 electrodes were used to compare alpha activity on the right and left hemispheres located above the prefrontal lobe. Valence was thus calculated by comparing the alpha power at the electrodes F3 and F4, i.e., by applying the following formula: AlphaF4—AlphaF3. The arousal level was determined by calculating the ratio of beta (12−28 Hz) and alpha (8−12 Hz) band power, which can be a reasonable indicator of an individual’s arousal level [
26,
31,
32]. We chose to use this indicator instead of the more conventional theta/beta ratio because recent studies suggest that theta/beta ratio may rather be a marker of cognitive processing capacity [
33]. The EEG signal was measured at electrodes AF3, AF4, F3, F4, which were located above the prefrontal cortex. Arousal was calculated as follows: (BetaF3 + BetaF4 + BetaAF3 + BetaAF4)/(AlphaF3 + AlphaF4 + AlphaAF3 + AlphaAF4).
Figure 2 shows the location of electrodes used for processing the EEG data.
In this pilot study, no method of correcting or removing artifacts was applied to the EEG signal. To minimize eye movement, participants were asked to close their eyes and the experimenter was monitoring that participants kept their eyes closed during the experiment. To minimize muscle artifacts, participants were asked to move only (and gently) the hand that was applying the cream. No interferences through eye or muscle movement were observed on the interface during the experimental sessions.
2.6. Data Visualization
A visual representation of emotions based on a collaboration between scientists and digital artists was developed to evaluate in real-time the emotions felt by the participants (see [
25] for more details). Emotions were represented as moving particles in a white sphere and tinged slightly according to their location. Groups of particles moved to the right or left according to the valence level and up or down according to the arousal level. For example, for a negative emotion with low arousal, the particles moved to the lower left level of the sphere. Only the researcher (but not the participants) could observe the emotions felt by the participants as they were applying the product.
2.7. Data Analysis
EEG data collected in real-time were automatically processed to indicate the number of times a participant had achieved a positive or negative valence and positive or negative arousal. For each cosmetic product, data of all repetitions were grouped. Four modalities were then obtained: top left, bottom left, top right, bottom right. We used a two-way ANOVA, with factors Product (CP1 or CP2) and Sequence (CP1 followed by CP2, and CP2 followed by CP2) to compare the representation of emotions in positive valence, negative valence, top arousal, low arousal, positive high emotion, positive low emotion, negative high emotion, and negative low emotion. Because this was an exploratory study, we did not use a correction factor to take into account the multiple comparisons. For each proposal on the questionnaire, participants’ responses were rearranged in two modalities (agree vs. do not agree). McNemar’s test was used to compare responses for the two cosmetic products.
3. Results
Three participants spontaneously reported being bothered by the smell of the products when they were applied. Because of this, they reported having difficulties in filling out the questionnaires and judging which cosmetic product was preferred. This was confirmed by the recording of their cerebral activity, with large negative emotions recorded for both CP in two subjects and one CP in one subject. Because the smell may have prevented the participants from feeling the emotions related to the application of the products, analyses were consequently performed on the 12 remaining subjects (who were not bothered by the smell).
3.1. Behavioral Evaluation of the Two Cosmetic Products
Of the participants, 66% preferred CP2, and 33% preferred CP1. The assessment criteria for each product were analyzed based on the questionnaires.
Table 1 shows the percentage of responses from participants for each product. For all questionnaire parameters analyzed, no significant difference in assessment was observed between each product (
p > 0.1 for proposals).
3.2. Neural Evaluation of the Two Cosmetic Products
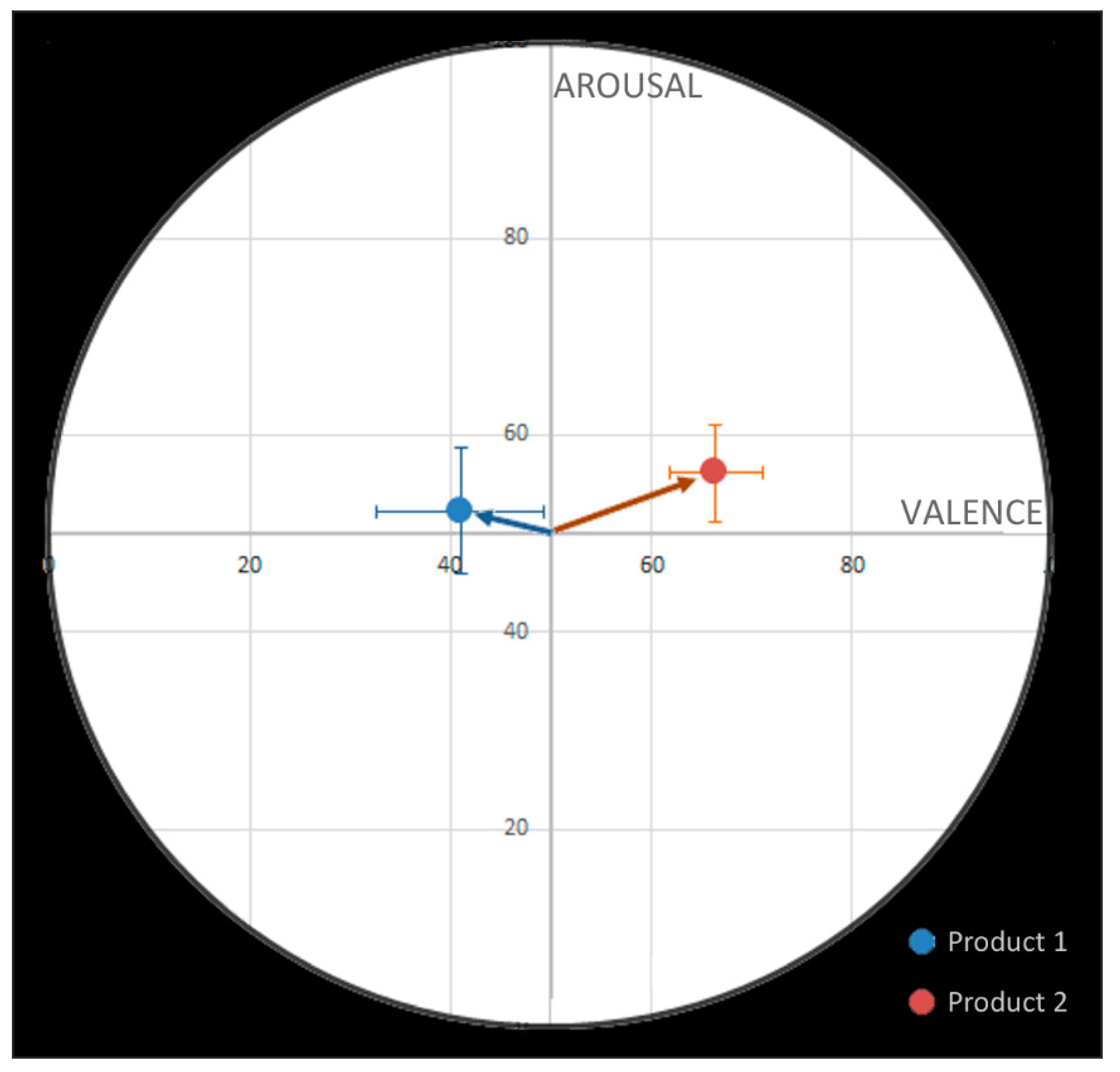
We analyzed the neural responses related to valence and arousal during the application of each product. During the application of product 1, the emotions detected had a rather negative valence, whereas, during the application of product 2, the emotions detected had a rather positive valence (
Figure 3).
A difference in valence was found when comparing the 2 creams (F(1,9) = 6.28;
p = 0.02). The measurement of brain activity during the application of product 2 had a significantly more positive valence than for product 1. To explore more precisely what type of emotion was generated by the application of the products, the emotions were divided into four parts: high positive (e.g., joy, happiness), low positive (e.g., calm, relaxation), low negative (e.g., sadness), and high negative (e.g., stress, anger). The percentages of emotions detected in each of these parts are shown in
Table 2.
A difference in particle movement towards positive emotions at high arousal was observed when comparing the two products (F(1,9) = 4.80; p = 0.04). Trends were observed for high negative emotions and low negative emotions, with product 1 causing more of these categories than product 2.
As a control, the order in which the creams were applied was analyzed to see if it might influence the emotions felt by the participants, i.e., whether participants consistently preferred the cream they applied first or second. There was no effect on the order of application on emotion detection and particle displacement (p > 0.1 for all comparisons).
3.3. Real-Time Representation of the Emotions
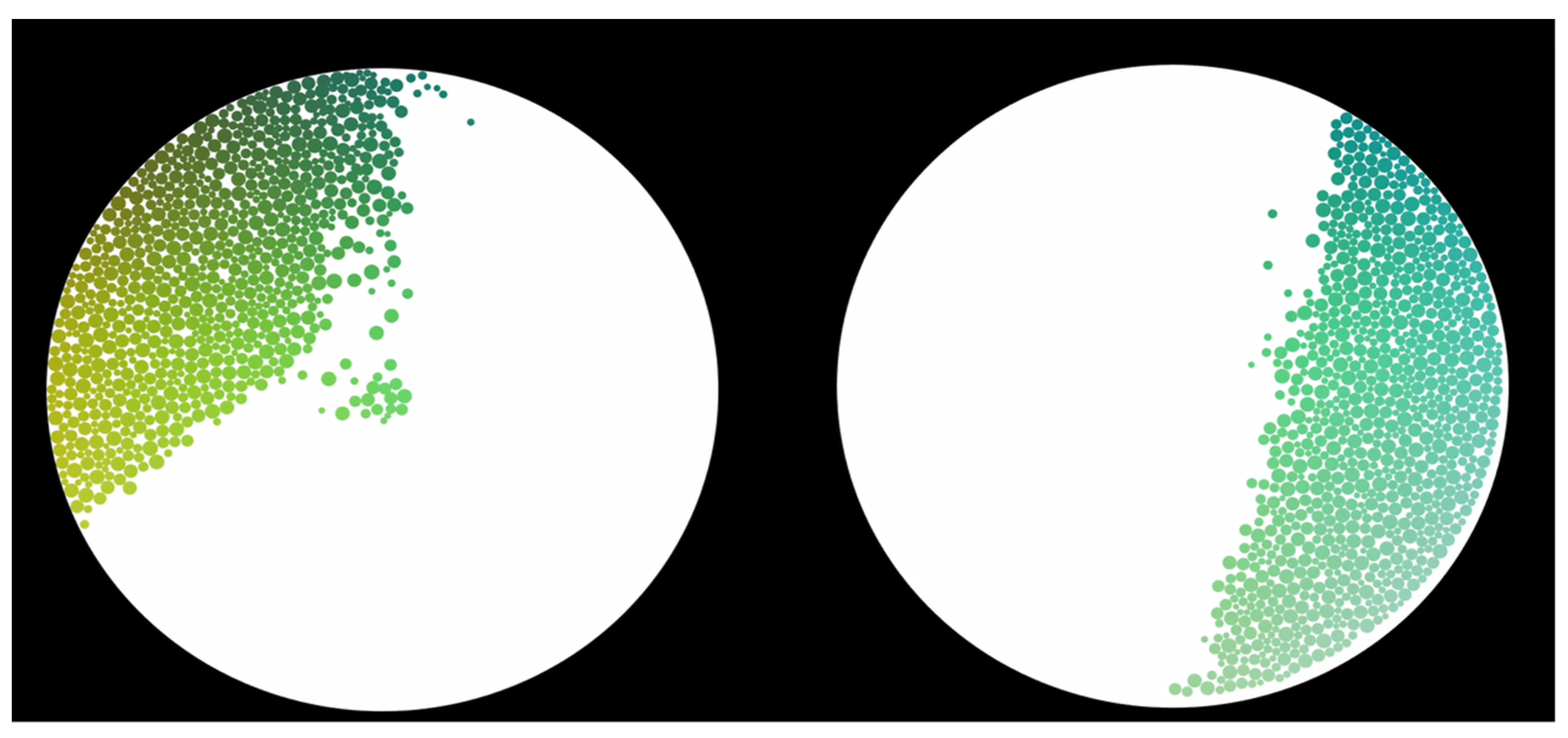
Finally, we analyzed at the individual level whether the emotions generated by the application of the cosmetic products were reflected by particles moving in specific parts of our artistic representation (
Figure 4). We observed that the measurement of brain activity during the application of CP2 caused a shift of the particles towards more positive and stronger emotions than for CP1. See
https://youtu.be/R11_LvKBovQ (accessed on 20 May 2021) for an example video of positive emotions felt during the application of CP2 and
https://youtu.be/0rj9HsNeECs (accessed on 20 May 2021) for an example video of negative emotions after the application of CP1 in one participant. In this subject, we can observe that the application of CP1 rapidly induced negative and positive emotions, but after a few seconds the particles remained in the left part of the screen. The application of CP2 induced the same first emotions, but then remained on the right part of the screen.
4. Discussion
This exploratory study aimed to assess whether EEG could be a useful tool to evaluate consumers’ emotions during the real-time topical application of a cosmetic product on the skin. Such an objective measure of emotions would be of major importance to the industry because it is well known that the emotional component of such products has a significant impact on consumer satisfaction [
34]. We compared two cosmetics creams that have been used previously in hedonic studies. We noted differences of valence that were visualized in real-time in the form of moving particles. The cosmetic product 2 was the most appreciated by participants and was also the one with the most positive valence, as measured by EEG.
The capacity of EEG to detect differences of valence is new in the application of a cosmetic product, but not in certain other domains, such as viewing movies [
35] or listening to music [
36]. The detection of such emotions during the application was not unexpected because it has been suggested that different tactile textures evoke highly selective activity in the secondary somatosensory cortex [
37], which in turn is known to provide input to the insula [
38]. The insula is associated with basic emotions such as anger, fear, disgust, joy, or sadness. Efferents from the insula project onto other brain areas such as the anterior cingulate [
39] and prefrontal cortices [
40]. Activity in the right and left prefrontal areas can then be measured with EEG and analyzed to detect the corresponding emotion. The recorded alpha prefrontal asymmetry reflected functional differences between positive and negative emotions (see as reviews [
18,
41,
42,
43,
44]). Because alpha power is assumed to reflect a decrease in metabolic activity [
45,
46], reduced alpha activity at the right prefrontal electrodes is associated with negative emotions. Compared with valence, the EEG measures of arousal alone were not sufficient to discriminate between the two cosmetic products. However, we were able to detect that the two products differed in terms of positive high-arousal emotional responses, corresponding to emotions such as excitement, happiness, and delight, according to the circumplex model of emotions [
30]. Participants were more excited and happy during the application of CP2 than during the application of CP1.
The results we obtained using the dimensional valence-arousal model of emotions further validated the notion that the application of a cosmetic product has effects on the brain. Other researchers have investigated the neural consequences of the application of a cosmetic product, but on other aspects than emotions. One study used functional magnetic resonance imaging—a technique that produces images with high spatial resolution—to compare the response of sensory areas during a passive touch, either after application of a cosmetic product on the skin or when the skin alone was touched [
10]. The first procedure activated different sensory areas in the brain than the second, but the responses of emotional areas were not examined. One EEG study demonstrated differences in various mental states (i.e., engagement, excitement, frustration, and meditation) after the application of three lip balms with different emollients [
7].
Because emotions are very complex phenomena with multiple manifestations, measurements of brain responses (and also skin conductance and heart rate) can be a useful adjunct to other methods, e.g., surveys, focus groups. Other original methods that have been developed include the measurement of salivary oxytocin. Oxytocin is one of the hormones synthesized in the hypothalamus. It contributes to the formation of emotional attachment in relationships, reduces stress, and stabilizes emotions. It has been suggested that the oxytocin level within the body increases and skin texture improves as more pleasant feelings are created by tactile stimulation during skincare, e.g., when the face is covered with the palms (
https://www.kao.com/global/en/news/rd/2018/20181115-001/ (accessed on 1 May 2021)).
The second purpose of our study was to evaluate the possibility of visualizing participants’ emotions in real-time. The emotions felt during the application of a cosmetic product are not necessarily constant, so it would be useful for the experimenter to observe continuously what the consumer is feeling. Historically, the real-time representation of emotions in EEG has been displayed—notably in neurofeedback experiments—in the form of histograms, where the levels rise or fall in real-time. We believe that such a representation, although accurate, is difficult for the researcher to follow. Less abstract methods have the advantage of providing an immediate response to something as complex as the visual representation of an emotional state. In the present study, the movement of particles was dictated by the dynamism of emotions. This approach has been validated by audiences at various scientific and artistic events [
28].
As has been noted, the two products compared herein have been previously submitted to consumer panels in hedonic studies, but in different conditions. We used a standardized environment in a neutral room, which is necessary for a rigorous analysis but far from an individual bathroom or cocooning spa room. Our next objective is to set up the protocol in real-life mimicable conditions or in-use test conditions. Indeed, the congruence of different elements has been demonstrated to be a determining factor in the appreciation of consumer products [
47,
48]. However, before being adapted for real-life conditions, the present study will have to be replicated using a larger sample of participants and online artefact correction/rejection methods. In that respect, other materials are currently being tested with artifact correction algorithms. From our initial sample of 15 participants, the EEG data of three had to be removed because they were bothered by the odor of the products. It is well known that odor exposure can impact cognition both positively and negatively [
49]. Here, the EEG recordings suggested that odor had a stronger impact on emotions than touch. As a consequence, it would be necessary for future to inform prospective participants that the products have a pleasant odor. A larger group of participants would also help define which parameters are responsible for the differences in emotional responses, which was not the case with our post-experiment questionnaire. In future research, the recordings of emotions during the application of a cosmetic product could also be investigated by high-density EEG methods. First, because the use of classic Ag/AgCl electrodes, which are saturated with gel or saline electrolyte solution for better electrical conduction, would keep the best impedance through the experiment. Second, because it would combine the advantages of an EEG technique as an optimal temporal resolution with the spatial resolution of the neuroimaging, allowing to discover the succession of cerebral activations at the origin of the surface EEG response {31].