Assessment of Imaging Protocol and Patients Radiation Exposure in Computed Tomography Colonography
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. CT Equipment
2.3. CTC Protocol
2.4. Radiation Dosimetry
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saudi Cancer Registry, Cancer Incidence Report Saudi Arabia. Available online: https://nhic.gov.sa/eServices/Documents/ESCRfinal6NOV.pdf (accessed on 2 January 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Graff, R.E.; Möller, S.; Passarelli, M.N.; Witte, J.S.; Skytthe, A.; Christensen, K.; Tan, Q.; Adami, H.-O.; Czene, K.; Harris, J.R.; et al. Familial Risk and Heritability of Colorectal Cancer in the Nordic Twin Study of Cancer. Clin. Gastroenterol. Hepatol. 2017, 15, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updat. Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Murphy, C.C.; Harlan, L.C.; Lund, J.L.; Lynch, C.F.; Geiger, A.M. Patterns of colorectal cancer care in the United States: 1990–2010. J. Natl. Cancer Inst. 2015, 107, djv198. [Google Scholar] [CrossRef]
- Alsanea, N.; Abduljabbar, A.S.; Alhomoud, S.; Ashari, L.H.; Hibbert, D.; Bazarbashi, S. Colorectal cancer in Saudi Arabia: Incidence, survival, demographics and implications for national policies. Ann. Saudi Med. 2015, 35, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Chen, J.; Lv, X.; Liu, S.; Chen, R.; Zhang, Z. Computed tomography colonography versus colonoscopy for detection of col-orectal cancer: A diagnostic performance study. BMC Med. Imaging 2020, 20. [Google Scholar] [CrossRef]
- A Issa, I.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- de Haan, M.C.; van Gelder, R.E.; Graser, A.; Bipat, S.; Stoker, J. Diagnostic value of CT-colonography as compared to colon-oscopy in an asymptomatic screening population: A meta-analysis. Eur. Radiol. 2011, 21, 1747–1763. [Google Scholar] [CrossRef]
- Moreno, C.; Kim, D.H.; Bartel, T.B.; Cash, B.D.; Chang, K.J.; Feig, B.W.; Fowler, K.J.; Garcia, E.M.; Kambadakone, A.R.; Lambert, D.L.; et al. ACR Appropriateness Criteria® Colorectal Cancer Screening. J. Am. Coll. Radiol. 2018, 15, S56–S68. [Google Scholar] [CrossRef]
- Yee, J.; McFarland, E. Extracolonic findings and radiation at CT colonography: What the referring provider needs to know. Abdom. Radiol. 2018, 43, 554–565. [Google Scholar] [CrossRef]
- Flicek, K.T.; Hara, A.K.; Silva, A.C.; Wu, Q.; Peter, M.B.; Johnson, C.D. Reducing the Radiation Dose for CT Colonography Using Adaptive Statistical Iterative Reconstruction: A Pilot Study. Am. J. Roentgenol. 2010, 195, 126–131. [Google Scholar] [CrossRef]
- Sulieman, A.; Mahmoud, M.; Serhan, O.; Alonazi, B.; Alkhorayef, M.; Alzimami, K.; Bradley, D. CT examination effective doses in Saudi Arabia. Appl. Radiat. Isot. 2018, 141, 261–265. [Google Scholar] [CrossRef] [PubMed]
- AAPM. Adult CT Colonography Protocols Version 10. 2017, pp. 1–16. Available online: https://www.aapm.org/pubs/CTProtocols/documents/AdultCTColonography.pdf (accessed on 14 January 2021).
- Lieberman, D. Colorectal Cancer Screening: Practice Guidelines. Dig. Dis. 2012, 30, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Pizzi, A.D.; Esposito, G.; Timpani, M.; Tavoletta, A.; Pulsone, P.; Basilico, R.; Cotroneo, A.R.; Filippone, A. Ultra-low dose CT colonography with automatic tube current modulation and sinogram-affirmed iterative reconstruction: Effects on radiation exposure and image quality. J. Appl. Clin. Med Phys. 2018, 20, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Fujiwara, M.; Kanazawa, H.; Mogi, T.; Iida, N.; Mitsushima, T.; Lefor, A.; Sugimoto, H. Evaluation of dose reduc-tion and image quality in CT colonography: Comparison of low-dose CT with iterative reconstruction and routine-dose CT with filtered back projection. Eur. Radiol. 2015, 25, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Liedenbaum, M.H.; Venema, H.W.; Stoker, J. Radiation dose in CT colonography—Trends in time and differences be-tween daily practice and screening protocols. Eur. Radiol. 2008, 18, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.; Ourednicek, P.; Briza, J.; Giepmans, W.; Jahoda, J.; Hruska, L.; Danes, J. Sub-milliSievert ultralow-dose CT colonography with iterative model reconstruction technique. PeerJ 2016, 4, e1883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, K.J.; Yee, J. Dose reduction methods for CT colonography. Abdom. Imaging 2012, 38, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Millerd, P.J.; Paden, R.G.; Lund, J.T.; Hara, A.K.; Stiles, W.L.; He, M.; Wu, Q.; Johnson, C.D. Reducing the radiation dose for computed tomography colonography using mod-el-based iterative reconstruction. Abdom. Imaging 2015, 40, 1183–1189. [Google Scholar] [CrossRef]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection; ICRP Publication Annals; ICRP: Ottawa, ON, Canada, 2007; Volume 37, pp. 1–399. [Google Scholar]
- Söderberg, M.; Gunnarsson, M. Automatic exposure control in computed tomography-an evaluation of systems from different manufacturers. Acta Radiol. 2010, 51, 625–634. [Google Scholar] [CrossRef]
- Martin, C.J.; Sookpeng, S. Setting up computed tomography automatic tube current modulation systems. J. Radiol. Prot. 2016, 36, R74–R95. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, A.; Adam, H.; Elnour, A.; Tamam, N.; Alhaili, A.; Alkhorayef, M.; Alghamdi, S.; Khandaker, M.U.; Bradley, D.A. Patient radiation dose reduction using a commercial iterative reconstruction technique package. Radiat. Phys. Chem. 2021, 178, 108996. [Google Scholar] [CrossRef]
- Moro, L.; Panizza, D.; D’Ambrosio, D.; Carne, I. Considerations on an automated computed tomography tube current modulation system. Radiat. Prot. Dosim. 2013, 156, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Sookpeng, S.; Martin, C.J.; Gentle, D.J.; Lopez-Gonzalez, M.R. Relationships between patient size, dose and image noise under automatic tube current modulation systems. J. Radiol. Prot. 2014, 34, 103–123. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Miglioretti, D.L. CTDIvol, DLP, and Effective Dose Are Excellent Measures for Use in CT Quality Improvement. Radiology 2011, 261, 999. [Google Scholar] [CrossRef] [PubMed]
- AAPM, American Association of Physicists in Medicine. Size-Specific Dose Estimates (SSDE) in Pediatric and Adult Body CT Examinations (Task Group 204); American Association of Physicists in Medicine: College Park, MD, USA, 2011. [Google Scholar]
- Fujii, K.; McMillan, K.; Bostani, M.; Cagnon, C.; McNitt-Gray, M. Patient Size–Specific Analysis of Dose Indexes from CT Lung Cancer Screening. Am. J. Roentgenol. 2017, 208, 144–149. [Google Scholar] [CrossRef]
- Lubner, M.G.; Pooler, B.D.; Kitchin, D.R.; Tang, J.; Li, K.; Kim, D.H.; Del Rio, A.M.; Chen, G.-H.; Pickhardt, P.J. Sub-milliSievert (sub-mSv) CT colonography: A prospective comparison of image quality and polyp conspicuity at reduced-dose versus standard-dose imaging. Eur. Radiol. 2015, 25, 2089–2102. [Google Scholar] [CrossRef]
- Lambert, L.; Danes, J.; Jahoda, J.; Masek, M.; Lisy, J.; Ourednicek, P. Submilisievert ultralow-dose CT colonography using iterative reconstruction technique: A feasibility study. Acta Radiol. 2015, 56, 517–525. [Google Scholar] [CrossRef]
- Chang, K.; Caovan, D.B.; Grand, D.J.; Huda, W.; Mayo-Smith, W.W. Reducing radiation dose at CT colonography: Decreas-ing tube voltage to 100 kVp. Radiology 2013, 266, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, J.; Shi, J.; Wu, Z.; Wang, Q.; Tang, Z.; He, K.; Shi, Y.; Shen, D. Review of Artificial Intelligence Techniques in Imaging Data Acquisition, Segmentation, and Diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2021, 14, 4–15. [Google Scholar] [CrossRef]
- Ahmad, O.F.; Soares, A.S.; Mazomenos, E.; Brandao, P.; Vega, R.; Seward, E.; Stoyanov, D.; Chand, M.; Lovat, L.B. Artificial intelligence and computer-aided diagnosis in colonoscopy: Current evidence and future directions. Lancet Gastroenterol. Hepatol. 2019, 4, 71–80. [Google Scholar] [CrossRef]

| Parameters | Philips | GE |
|---|---|---|
| Scan type | Helical | Helical |
| kVp * | 120 | 120 |
| mA * | 100 | 120 |
| Collimation (mm) | 40 (64 * 0.625) | 40 (64 * 0.625) |
| Rotation time (s) | 0.5 | 0.5 |
| Pitch | 1.375 | 1.375 |
| SFOV | Large | Large |
| Coverage | Diaphragm to ischium | Diaphragm to ischium |
| IRT | 40% ASIR | 50% ASIR |
| Protocol | Tube Current (mA) | CTDIVOL (mGy) | Effective Dose (MSV) | ||
|---|---|---|---|---|---|
| Supine | Prone/Decubitus | Supine | Prone/Decubitus | ||
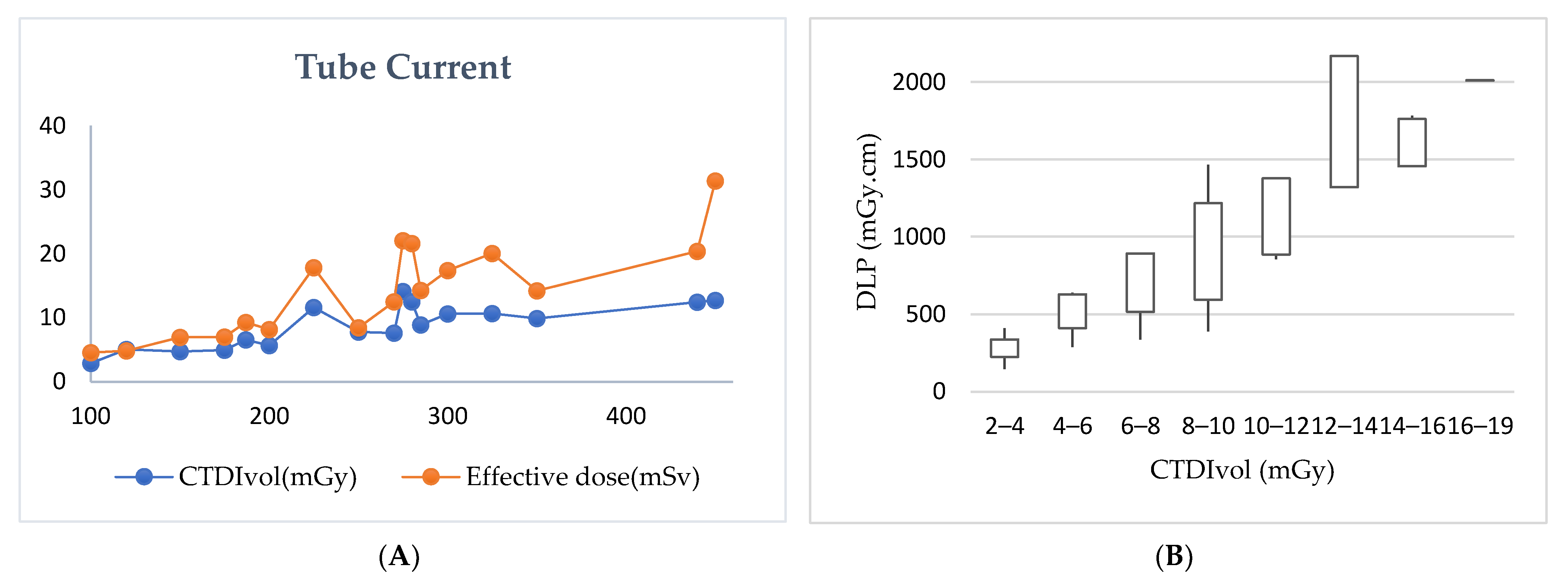
| Supine and one decubitus | 266.6 ± 144 (100–350) | 266.6 ± 144 (100–350) | 7.5 ± 4 (2.8–9.8) | 7.5 ± 4 (2.8–9.8) | 13 ± 5.9 (6.1–16.8) |
| Supine and two decubitus | 375 ± 106 (300–450) | 375 ± 106 (300–450) | 13.4 ± 1 (12.6–14.2) | 12.2 ± 0.57 (11.8–12.6) | 31.6 ± 0.38 (31.3–31.9) |
| Auto tube current (mA) | 253.1 ± 63 (150–325) | 256.2 ± 59 (150–325) | 11.2 ± 5.2 (2.2–18.3) | 10.5 ± 4.7 (2.1–16.5) | 18.7 ± 9.9 (3.06–31.9) |
| Routine (manual) | 203 ± 96.3 (100–440) | 202.2 ± 96 (100–440) | 6.4 ± 3.3 (2.7–15.5) | 6.51 ± 3.9 (2.8–19.7) | 9.6 ± 6 (2.1–26.7) |
| (A) | ||||||
| Mode | Tube Voltage (kVp) | Tube current (mA) | Rotation Time (s) | Pitch | Slice Thickness (mm) | |
| Supine | Prone | |||||
| Auto mA (Philips) | 120 | 287.5 ± 17 (275–300) | 287.5 ± 17 (275–300) | 0.85 ± 0.02 (0.84–0.87) | 0.9 * | 0.67 * |
| Auto mA (GE) | 116.6 ± 8.1 (100–120) | 241.6 ± 70 (150–325) | 245.8 ± 65.9 (150–325) | 0.5 ± 0.1 (0.4–0.7) | 1.1 ± 0.2 (0.98–1.37) | 0.62 * |
| Manual mA (GE) | 120 | 212.3 ± 104 (100–450) | 213.2 ± 105.5 (100–450) | 0.52 ± 0.06 (0.5–0.7) | 1.32 ± 0.1 (0.98–1.37) | 0.62 * |
| (B) | ||||||
| Mode | CTDIvol (mGy) | DLP (mGy.cm) | Effective Dose (mSv) | |||
| Supine | Prone | |||||
| Auto mA (Philips) | 16.2 ± 2.9 (14.2–18.3) | 14.6 ± 2.6 (12.7–16.5) | 1702.8 ± 436.7 (1394–2011.6) | 25.5 ± 6.5 (20.9–30.1) | ||
| Auto mA (GE) | 9.6 ± 4.8 (2.2–14.5) | 9.1 ± 4.6(2.2–15) | 1098.1 ± 686.5 (204–2128.8) | 16.4 ± 10.2 (3.06–31.9) | ||
| Manual mA (GE) | 6.6 ± 3.4 (2.7–15.5) | 6.7 ± 3.9 (2.8–19.7) | 690.1 ± 447.3 (145.2–2092.9) | 10.3 ± 6.7 (2.1–31.3) | ||
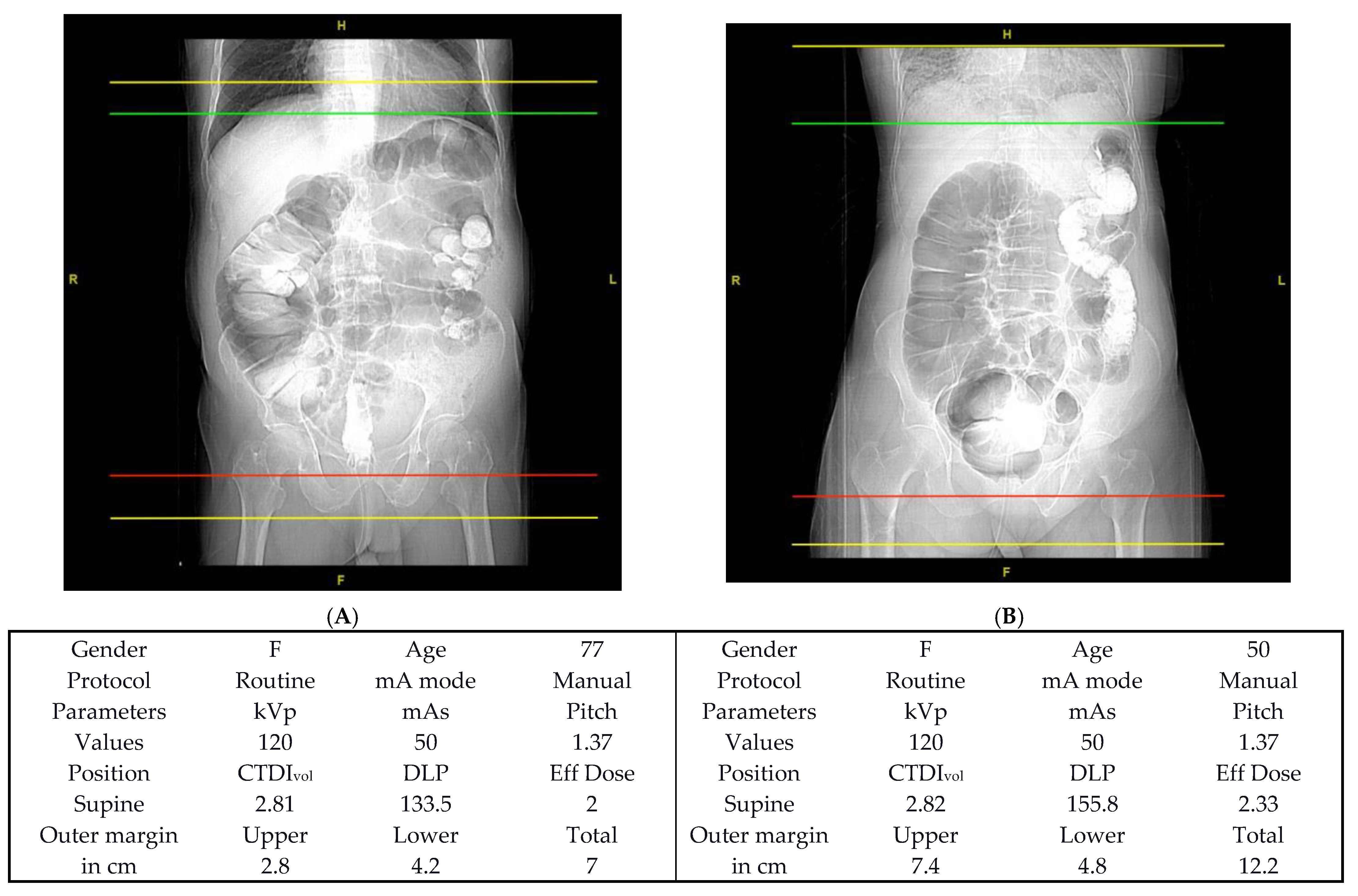
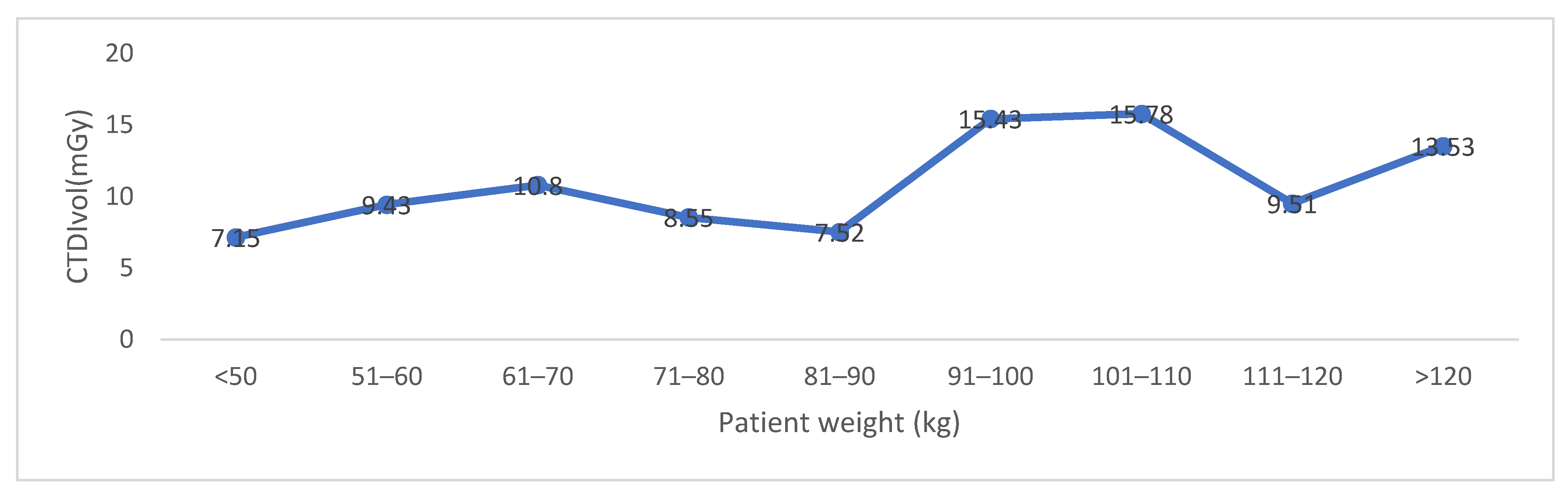
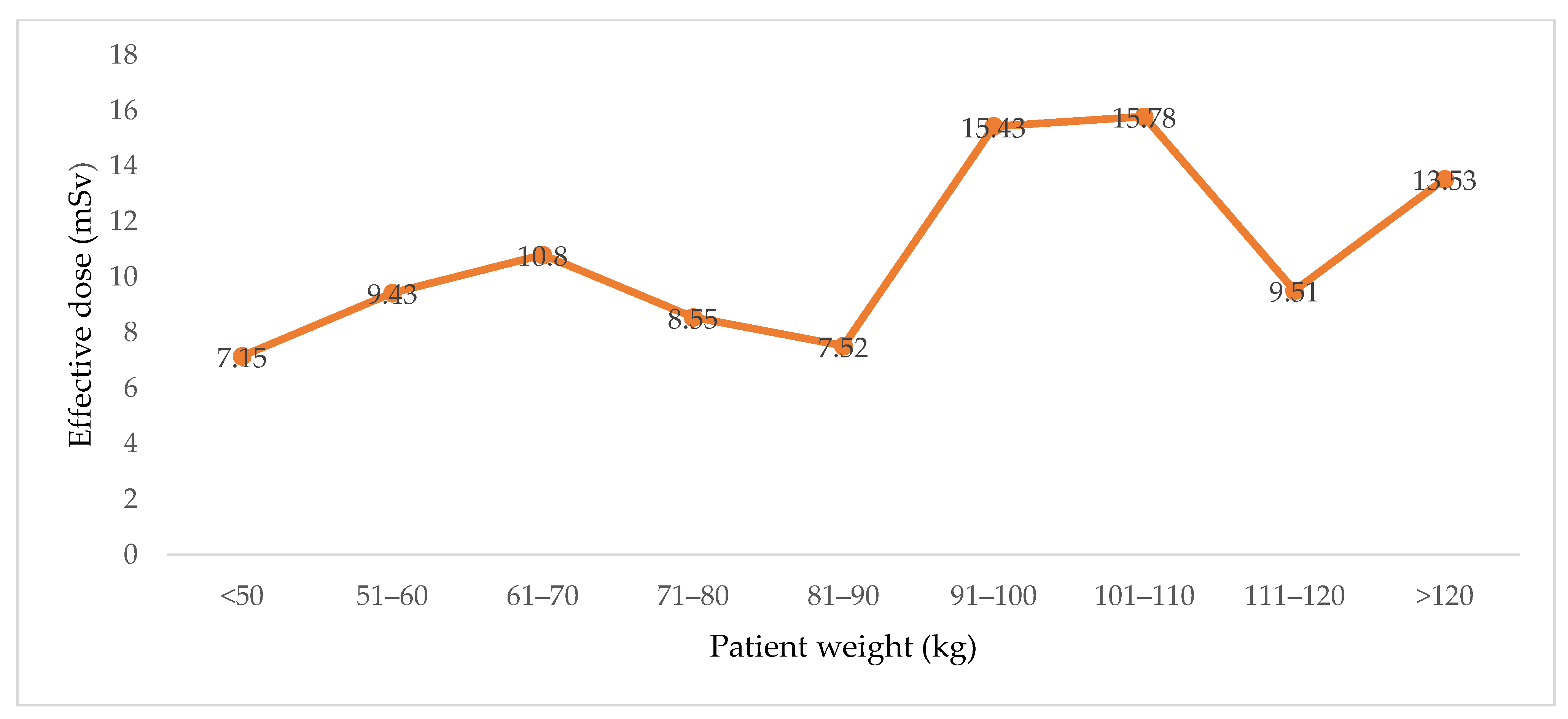
| Gender | Number | Age (Y) | CTDIVOL (mGy) Supine | CTDIVOL (mGy) Prone | Effective Dose (mSv) |
|---|---|---|---|---|---|
| M | 25 | 58.64 ± 15.2, 58 (29–83) | 6.7 ± 4.42, 5.63 (2.27–15.56) | 6.5 ± 4.1, 5.6 (2.1–15.58) | 10.3 ± 7.95, 6.92 (2.17–31.9) |
| F | 30 | 57.4 ± 12.3, 55 (24–82) | 7.84 ± 3.64, 8.02 (2.79–18.3) | 8.06 ± 4.3, 7.61 (2.8–19.76) | 12.6 ± 7.55, 10.99 (3.88–31.39) |
| Total | 55 | 57.96 ± 13.6, 56 (24–83) | 7.33 ± 4, 7.02 (2.2–18.3) | 7.37 ± 4.3, 7.11 (2.2–19.7) | 11.57 ± 7.75, 9.25 (2.17–31.93) |
| Standard Imaging Protocol | Modified * | ||||||
|---|---|---|---|---|---|---|---|
| Authors | Country | CTDIvol (mGy) | DLP (mGy.cm) | Effective Dose (mSv) | CTDIvol (mGy) | DLP (mGy.cm) | Effective Dose (mSv) |
| Chang et al. [22] | USA | 5.3 | 239 | 3.6 | 4.1 | 197 | 3.0 |
| Nagata et al. [19] | Japan | 2.37 | 118.8 | 1.78 | 1.22 | 47.4 | 0.9 |
| Nagata et al. [19] | Japan | 2.53 | 128.2 | 1.92 | 0.92 | 42.2 | 0.7 |
| Nagata et al. [19] | Japan | 2.63 | 130.4 | 1.96 | 0.77 | 36.4 | 0.6 |
| Nagata et al. [19] | Japan | 2.51 | 123.9 | 1.86 | 0.62 | 29 | 0.5 |
| Cianci et al. [18] | Italy | 3.87 | 179.3 | 2.69 | 1.32 | 65.3 | 1.0 |
| Liedenbaum et al. [20] | The Netherlands | N/A | 303.3 | 4.55 | N/A | 193.3 | 3.0 |
| Millerd et al. [23] | USA | 6.7 | 327.8 | 4.91 | 2.7 | 129.1 | 1.9 |
| Current study | Saudi Arabia | 7.3 | 385.5 | 5.7 | 7.3 | 385.5 | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsufayan, M.; Sulieman, A.; Moslem, R.; Asiri, A.; Alomary, A.; Alanazi, B.M.; Aldossari, H.; Alonazi, B.; Bradley, D.A. Assessment of Imaging Protocol and Patients Radiation Exposure in Computed Tomography Colonography. Appl. Sci. 2021, 11, 4761. https://doi.org/10.3390/app11114761
Alsufayan M, Sulieman A, Moslem R, Asiri A, Alomary A, Alanazi BM, Aldossari H, Alonazi B, Bradley DA. Assessment of Imaging Protocol and Patients Radiation Exposure in Computed Tomography Colonography. Applied Sciences. 2021; 11(11):4761. https://doi.org/10.3390/app11114761
Chicago/Turabian StyleAlsufayan, Mohammed, Abdelmoneim Sulieman, Rayan Moslem, Abdullah Asiri, Abdullah Alomary, Bandar M. Alanazi, Hassan Aldossari, Batil Alonazi, and David A. Bradley. 2021. "Assessment of Imaging Protocol and Patients Radiation Exposure in Computed Tomography Colonography" Applied Sciences 11, no. 11: 4761. https://doi.org/10.3390/app11114761
APA StyleAlsufayan, M., Sulieman, A., Moslem, R., Asiri, A., Alomary, A., Alanazi, B. M., Aldossari, H., Alonazi, B., & Bradley, D. A. (2021). Assessment of Imaging Protocol and Patients Radiation Exposure in Computed Tomography Colonography. Applied Sciences, 11(11), 4761. https://doi.org/10.3390/app11114761






