Abstract
The cleidoic eggs of oviparous reptiles are protected from the external environment by membranes and a parchment shell permeable to water and dissolved molecules. As a consequence, not only physical but also chemical insults can reach the developing embryos, interfering with gene expression. This review provides information on the impact of the exposure to cadmium contamination or thermal stress on gene expression during the development of Italian wall lizards of the genus Podarcis. The results obtained by transcriptomic analysis, although not exhaustive, allowed to identify some stress-reactive genes and, consequently, the molecular pathways in which these genes are involved. Cadmium-responsive genes encode proteins involved in cellular protection, metabolism and proliferation, membrane trafficking, protein interactions, neuronal transmission and plasticity, immune response, and transcription regulatory factors. Cold stress changes the expression of genes involved in transcriptional/translational regulation and chromatin remodeling and inhibits the transcription of a histone methyltransferase with the probable consequence of modifying the epigenetic control of DNA. These findings provide transcriptome-level evidence of how terrestrial vertebrate embryos cope with stress, giving a key to use in population survival and environmental change studies. A better understanding of the genes contributing to stress tolerance in vertebrates would facilitate methodologies and applications aimed at improving resistance to unfavourable environments.
1. Introduction
The mid-blastula transition is a crucial event in vertebrate development as it represents the moment in which the transcription of embryonic genes begins [1]. This event is finely regulated, and any disturbance of embryonic gene expression will cause malformations, arrested development or even the death of the embryo. In this scenario, few master genes control and direct the correct expression of a cascade of genes determining cell specialization in structure and functions [2].
The dysregulation of embryo gene expression can occur as a result of different insults, of a chemical and physical nature, of environmental or, most often, of anthropogenic origin. In placental mammals, these stress factors are able to modify embryonic gene expression changing the feto-maternal interface at the placental level [3]. Conversely, for a long time, the cleidoic eggs of reptiles were considered a closed system with respect to the environment, protected from toxic substances present in the soil where they are usually laid [4]. Nowadays, it is known that this is not completely true, since the shell and the amniotic membranes are permeable to water and any toxic substances dissolved in it can reach the embryonic cells [5,6,7]. It is also clear that substances entering the developing egg may strongly interfere with their correct development at a morphological and molecular level, not least influencing and altering embryonic gene expression [8,9,10].
Many studies have described reptilian developmental stages [11,12,13] and the morpho/functional effects of environmental disturbances such as humidity and temperature [14,15]. Not surprisingly, great attention has been dedicated to the mechanisms controlling sex determination [16,17] but other aspects of reptilian development have been completely neglected.
This is what happened to research on the effects of contaminants. Though the eggs have permeable membranes and absorb contaminated water, very few investigations have addressed the problem of how these contaminants impact on the developmental program. Studies usually report on contaminant accumulation by ovaries and eggs and on their effects on fecundity [18,19]. This lack of interest is surprising considering that reptiles are a very ancient taxa, the first to develop an amniotic egg and ancestors of birds and mammals. In addition, they play a relevant ecological role, being top predators controlling soil communities. Their long lifespan, entirely in contact with the soil, makes reptiles preferential victims of soil contamination.
An explanation for the disinterest may come from the fact that reptiles usually nest in soil shelters and therefore might have been considered sufficiently protected against pollution and changes in environmental conditions. However, it is clear that this is not the case. In recent years, in fact, we assisted to a rapid decline in the population trend due to habitat disturbances with a consequent detrimental effect on biodiversity.
Stress-linked gene expression changes could arise for many reasons and they could be reactive to alterations in cellular and organismal physiology. If these alterations are reproducible among different stress factors, it can be assumed that these genetic-responsive events form a stress-associated gene expression program, which could mitigate degenerative processes associated with environmental contamination and pollution. Furthermore, the alterations in gene expression observed in cells and tissues of embryos exposed to environmental stimuli could be an ancillary consequence, since many of these toxic insults lead primarily to translational or post-translational modifications and epigenetic dysregulations such as DNA and/or histone methylation [20,21]. It follows that past and ongoing studies on the modulation of stress-induced gene expression in lizard embryos may help to understand the cellular basis and biochemical mechanisms underlying the tissue alterations observed as a result of stress factors affecting the development of embryos considered sufficiently sheltered from environmental pollution and other harmful stimuli.
This article examines the impact of environmental stresses on gene expression during the development of oviparous Lacertidae belonging to the genus Podarcis. In particular, the transcriptomic responses are analysed following exposure to a chemical insult, represented by the heavy metal cadmium, and to physical stress, a sudden drop in the incubation temperature. Podarcis lizards are terrestrial oviparous species endemic of the Mediterranean regions; they reproduce in late spring/early summer, and the eggs, usually 7/8 per brood [22], are laid in holes dug in the ground or in walls, sheltered from excessive sun exposure to avoid the risk of dehydration [23]. These lizards are the only reptiles on which the transcriptomic effects of stressors during embryonic development have been investigated.
Cadmium-Responsive Genes in Podarcis Siculus Embryos
The incubation of fertilised eggs in cadmium-contaminated soil (50 mg/kg soil) is not lethal for embryos, which develop without appreciable delays; however, it causes the onset of morphological alterations that are hardly compatible with life in nature once hatched [24]. In these embryos, skeletal malformations such as the failure to close the cranial vault and palatal alterations are evident; vice versa, the limbs are perfectly formed. Changes are also evident in the organization of the brain vesicles, which show various types of malformations: open and/or dilated vesicles protruding outward or, conversely, ventricles invaded by collapsed encephalic walls and/or eyes with over-proliferating and folded retinal layers. The spinal cord retains its normal structure, as do organs such as the lung, liver and kidney, where haemorrhagic foci and, in some cases, cells with vacuolised cytoplasm and/or pyknotic nuclei are observed [7].
Malformations are correlated with changes in gene expression, as determined by using northern blot, mRNA differential display and reverse dot-blot techniques. Differential display (DD) is a PCR-based technique widely used to assess changes in gene modulation between samples at the level of mRNA [25,26]. Differentially expressed mRNAs are identified by 4% polyacrylamide gel electrophoresis followed by automated sequencing and, finally, results are validated by reverse dot blot analysis [8]. Comparison of transcripts present in control and Cd-incubated P. siculus embryos allows for the identification of some differentially Cd-modulated mRNAs (Figure 1).
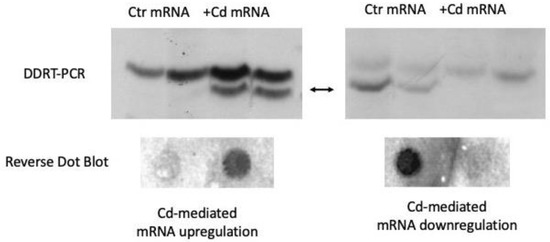
Figure 1.
Representative identification of cadmium-responsive genes by using the mRNA differential display reverse transcriptase-polymerase chain reaction analysis (DDRT-PCR) performed on total RNAs extracted from control- (Ctr) and Cd-treated (+Cd) embryos incubated for 20 days after laying in Cd-contaminated soil. The result of such analysis was validated by subsequent reverse dot blot hybridization [8].
Studies were performed on embryos incubated in Cd-contaminated soil and maintained under natural conditions of temperature and humidity, such as the control embryos incubated in uncontaminated soil. Twenty days after laying, embryos were collected, freed from the egg membranes and processed for gene expression analysis. This stage represents an intermediate stage of development, during which organogenesis has begun and embryonic genes are working to ensure correct differentiation.
The results obtained from these analyses demonstrated that Cd increases and decreases the expression of genes involved in development and transcription regulation, cell growth and communication, brain and eye development, metabolism and detoxification (Table 1).

Table 1.
Cadmium-responsive genes identified in 20-days-old P. siculus embryos.
2. Cd-Mediated Genes Downregulation
2.1. Transcription Regulation
Basic Transcription Factor 3
The basic transcription factor 3 (BTF3), also known as nascent polypeptide-associated complex β (NACβ), is involved in various biotic and abiotic stress processes, as well as different physiological and developmental mechanisms [27]. It promotes the initiation of transcription by RNA polymerase from proximal promoter elements such as the TATA box [28]. In addition to its function as a transcriptional regulator, BTF3 also aids the regulation of the cell cycle and apoptosis [29]. BTF3 loss-of-function mutation in mice leads to death in the early stages of development, indicating its pivotal function in development [30]. Decreased BTF3 expression is associated with a generalised inhibition of transcription and protein synthesis, together with increased apoptosis; on the contrary, the overexpression of BTF3, observed in several tumorigenic cells, prevents cell apoptosis [31].
2.2. Cellular Metabolism
Three-hydroxy-3-methylglutaryl coenzyme A reductase
The enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) is the rate-limiting enzyme in the cholesterol biosynthesis. In vertebrates, cholesterol is fundamental in both embryos and adults. Its main function is to maintain the integrity and fluidity of cell membranes and to serve as a precursor for the synthesis of steroid hormones, bile acids, and vitamin D [32]. During embryogenesis, cholesterol and its products also mediate cell–cell signalling via activation of nuclear hormone receptors and by controlling the diffusion or reception of members of the hedgehog growth factor family [33]. In mammalian embryos, mutations in genes encoding cholesterol biosynthetic enzymes are associated with patterning defects; the lack of HMGR results in early embryonic lethality [34].
2.3. Cell Cycle Regulation
Fizzy and Cell Division Cycle 20 Related 1
The folding of retinal layers and periventricular walls of encephalic vesicles in P. siculus embryos exposed to cadmium is ascribable to the increased proliferation rate in neuronal cells, thus demonstrating that Cd interferes with the regulation of the cell cycle by increasing mitosis [9]. The protein fizzy and cell division cycle 20 related 1 (Cdh1 o fizzy-related 1, fzr1) is an activator of the anaphase-promoting complex/cyclosome (APC/C), which plays a role in the mitotic cell cycle. Indeed, APC/C regulates proteolysis, a process that is essential for cell-cycle progression, signal transduction, and development [35]. Cdh1 is a conserved protein identified as a limiting, substrate-specific activator of APC-dependent proteolysis. Proteolysis mediated by the APC triggers chromosomal segregation and exit from mitosis. In the cell, a decrease in the level of Cdh1 protein causes APC/C inactivation leading, consequently, to cell cycle progression and mitosis. By using a P. siculus-specific Cdh1 cDNA fragment as a probe, Northern blot analysis demonstrates the downregulation of this gene in embryos incubated in Cd-treated soil [36], thus suggesting that cadmium alters the expression of at least one of the players regulating mitotic rate and cell proliferation.
3. Cd-Mediated Genes Upregulation
3.1. Transcription Regulation
3.1.1. RLF Zinc Finger Protein
The zinc finger RLF, known as Rearranged L-myc fusion, is a classical C2H2-type (Cys2Hys2) zinc finger (Znf). The C2H2 Znf is the most common DNA-binding motif found in eukaryotic transcription factors; it can also bind to RNAs and proteins [37]. Znf domains are relatively small protein motifs which contain multiple finger-like protrusions that make tandem contacts with their target molecule. Znf-containing proteins play a role in gene transcription, translation, mRNA trafficking, cytoskeleton organization, epithelial development, cell adhesion, protein folding, chromatin remodelling and zinc (or more in general metal) sensing [38,39].
3.1.2. Topoisomerase 1-Binding Protein with a BTB/POZ Domain
This partially uncharacterised protein at the C-terminus binds the Topoisomerase 1 (Top 1); it is assumed to regulate the expression levels and distribution of TOP 1, through an unknown mechanism [40]. It may play a role in mesenchymal differentiation promoting myogenic differentiation and suppressing adipogenesis. The N-terminus contains a proline-rich region and a BTB/POZ domain (broad-complex, Tramtrack and bric a brac/Pox virus and Zinc finger), both of which are typically involved in protein–protein interactions [41]. The BTB domain is found in proteins involved in a wide variety of regulatory events throughout development. Many BTB proteins are transcriptional regulators that mediate gene expression through the control of chromatin conformation [42]. The functions of several BTB genes have been shown to be necessary for normal eye development [43]; the tramtrack gene overexpression results in a transformation of photoreceptor neurons into non-neuronal cone cells [43].
3.2. Master Regulatory Genes in Eye Development
Otx 2 and Pax6
In vertebrates, eye development starts with the specification of retinal progenitor cells in the anterior neural plate by the overlapping expression of several transcription factors. Among them, the proper expression of the two homeobox genes Otx2 (ortho-denticle protein homolog 2) and Pax6 (Paired Box 6) is essential for normal eye development. Otx2 upregulation in mice induces ephitelial–mesenchimal transition and alteration of the lens cells [44]; in the same embryos, loss of Otx2 function leads to severely altered cranial phenotype [45], whereas heterozygous deletion leads to variable phenotypes [46]. Additionally, Pax6 overexpression results in optic disc malformation, progressive retinal dysplasia, and microphthalmia [47]. In Pax6-deficient mice and in heterozygous mutants, eyes develop with different severe defects [48,49,50].
Incubation in Cd-contaminated soil has been observed to be highly toxic to the retina of Podarcis embryos, causing significant developmental anomalies; in particular, a marked retinal folding is observed [9]. To evaluate the possible involvement of a dysregulation of Pax6 and Otx2, the levels of gene expression and localization of Pax6 and Otx2 transcripts in the developing eyes of control and Cd-treated embryos were investigated. Northern blot analysis shows an increased expression of both Pax6 and Otx2 genes in the cranial region of embryos incubated in Cd-contaminated soil. In parallel, immunocytochemical analysis shows that, in embryos exposed to cadmium, the distribution of messengers does not undergo any significant difference compared to controls, both in the intact or folded retina [9].
3.3. Membrane Trafficking
Development and Differentiation-Enhancing Factor 1
The Development and Differentiation-Enhancing Factor 1 (DDEF1) gene encodes an ADP-ribosylation factor GTPase-Activating Protein (ArfGAP) protein, also known as centaurin, and two ankyrin repeats. ArfGAP1 is an enzyme that promotes hydrolysis of GTP bound to ADP-ribosylation factor 1 and is required for the dissociation of coat proteins from Golgi-derived vesicles, a critical step in vesicle formation and intracellular vesicle trafficking [51]. Centaurins are also known to activate phosphoinositide 3-kinase, a key regulator of cell proliferation and motility in addition to vesicular trafficking [52]. It has been demonstrated that Arfs are susceptible to the presence of cell stressors, including cadmium [53,54] contributing to Cd resistance, probably by maintaining membrane integrity and by modulating membrane trafficking. The ankyrin repeat is a 33 aminoacidic residues motif that mediates the protein–protein interaction in eukaryotes. The repeat has been found in transcriptional initiators, cell-cycle regulators, ion transporters, signal transducers and cytoskeletal proteins [55]. Ankyrin overexpression in plants is associated with resistance to stressors, such as salt tolerance [56], while in mammals it is associated with increased apoptosis that promotes p53 activation and tumour cell proliferation [57].
3.4. Sensory Transduction and Synaptic Plasticity
3.4.1. Voltage-Dependent Sodium Channel
The voltage-gated sodium channels are membrane proteins typical of excitable cells such as neurons and myocytes. They are involved in controlling nerve transmission, sensory transduction, muscle contraction and synaptic plasticity, and, during embryogenesis, play a pivotal role in axon elongation and neuronal circuit formation [58]. Anomalies in voltage-dependent sodium channels are always associated with neurological disorders [59]. The upregulation of the gene encoding the voltage-dependent sodium channel may be a consequence of the alteration in calcium metabolism induced by cadmium [60], as occurs following exposure to valproic acid, an antiepileptic that causes, in mammalian embryos, defects in the closure of the neural tube [61]. Interestingly, similar damage is observed in P. siculus embryos exposed to cadmium, suggesting a shared mechanism between cadmium and valproic acid, which may indeed involve a dysregulation of voltage gated sodium channels.
3.4.2. γ-Aminobutyric Acid Type B Receptor
Metabotropic γ-aminobutyric acid type B receptors (GABABR) are G protein-coupled inhibitory receptors widely distributed in the central and peripheral nervous system [62]. Their activation causes an inhibition of adenylate cyclase and calcium channels activities and the opening of potassium channels in neuronal membranes. As with voltage-gated sodium channels, GABA-B receptor signalling during mammalian embryogenesis is not only crucial for modulation of nascent and mature synapses but is also involved in developmental processes such as neuronal migration and axon growth [63]. As observed for the modulation of the voltage-gated sodium channel gene expression, exposure of mammalian embryos to Cd ions and valproic acid shares the same final effects on the nervous system [64], i.e., an increase in the activity on GABA-B receptors.
3.5. Immune Response
Lymphocyte Function Associated Antigen 3
The lymphocyte function-associated antigen 3 (LFA-3, also known as CD58) is a cell adhesion molecule expressed, in human and other mammals, on the surface of hemopoietic cells, dendritic cells, macrophages, endothelial cells, and erythrocytes in a transmembrane and glycosylated form [65]. CD58 is a ligand for the CD2 glycoprotein, present on membranes of T-cells and natural killer (NK) cells. It has been demonstrated that the CD58/CD2 interaction is important to activate the NK cell lytic activity and inflammatory cytokine production [66]. Blockade of CD58 by anti-CD58 antibodies reduces inflammatory responses and diminishes the recognition and cytolysis of target cells by cytotoxic T cells and NK cells [67].
3.6. Cellular Detoxification
3.6.1. Metallothionein
Metallothionein (MT) is a low-molecular weight, cysteine-rich, metal-binding protein that regulates metal homeostasis and detoxification by binding essential and non-essential metal ions [68,69]; it is also involved in the immune response and in free radical scavenging [70,71,72]. MT gene expression is readily upregulated by exposure to heavy metals [73,74,75,76], providing a mechanism by which the metal can be sequestered in a relatively inert, non-toxic state [77].
MT is expressed during the embryonic development of many vertebrates and performs its detoxifying function by protecting embryos from toxic metals such as cadmium [7,78,79,80].
Under normal conditions, in P. siculus MT transcripts accumulate during oogenesis [81,82]; in embryos, in situ hybridizations elucidated the precise tissue distribution of MT transcripts during the development [82], but failed to discriminate between maternal and embryonic transcripts, making it difficult to determine when expression of the embryonic MT gene begins. However, the same analyses show that at 40 days from oviposition the embryonic gene is active in the brain and lungs; before hatching, at about 60 days of development, the gene is also expressed in hepatic monocytes and in Kupffer cells [82].
Northern blot analysis demonstrates an appreciable increase in MT transcripts in P. siculus embryos developed for 20 days in Cd-contaminated soil when compared with control embryos, thus demonstrating the induction of embryonic MT gene expression by heavy metals. In situ hybridization shows markedly increased localization of MT mRNA in liver sinusoids and in the intestine [7]. However, this de novo expression of the MT gene is not able to completely protect the embryo from damage induced by Cd; hence, as described above, lizard embryos incubated in Cd contaminated soil show serious morphological alterations [7].
3.6.2. Cold Stress-Responsive Genes in Podarcis Embryos
Temperature plays a pivotal role in the reproduction of heterothermic vertebrates [83]. It is responsible for the beginning of the breeding season by promoting the maturation of oocytes, spermatogenesis and the predisposition of the animals to mating; in these animals, the temperature also controls the correct progression of the differentiation processes underlying embryonic development. In heterotherms, a slight decrease in the incubation temperature is quite tolerated and generally leads to a slowdown in development [84]. The increase in incubation temperature has more serious effects, leading in some cases to embryonic lethality [85,86]. In reptiles, the incubation temperature of the egg can affect the sex of the embryo [17].
Recently, it has been demonstrated that embryos of the lizard Podarcis muralis developing at a stressfully low (15 °C) incubation temperature expressed on average 20% less total RNA than those incubated at the optimal temperature (24 °C), because of the lower rates of transcription at cool temperature; approximately 50% of all transcripts showed different rates of expression between the two incubation temperatures [87]. Changes in expression profiles were found in genes involved in transcriptional and translational regulation and chromatin remodelling, suggesting possible epigenetic mechanisms underlying the acclimatization of developing embryos to a cool temperature.
In the lizard P. siculus even a slight increase in temperature (3 to 4 °C) leads to the death of the embryo, regardless of the incubation period during which the heat shock occurs [88]. P. siculus development is affected differently by cold shock. If the drop in incubation temperature occurs at least 15 days after laying, the embryos are viable and in the corresponding stage of development, but they show varying degrees of morphological alterations; if the cold shock occurs in the early stages of development, from day 0 to 15 days after laying, development stops and the animal dies, as observed after the heat shock [88]. It is noteworthy that the morphological changes observed following cold stress (in particular, a five-day incubation at a temperature of 10 °C) are very similar to those observed in embryos incubated in cadmium-contaminated soil. In fact, even in this case, morphological alterations in the brain and eye are evident (breakage of the vesicular walls, folding of the walls of the encephalic ventricles, hyperproliferation of the retinal layers); the organs of the trunk region show oedema and haemorrhagic sites, while maintaining normal morphology of the tissues [88].
A preliminary differential display analysis highlighted few changes in gene expression between control and cold-shock embryos. Interestingly, the expression and localization of the heat-shock protein HSP70, considered the primary member of the multigenic family of proteins involved in protecting the cell from thermal shock (both heat and cold), are the same in P. siculus embryos developed under the natural thermal regime or subjected to a cold shock [88]. However, in non-mammalian vertebrates, another specific member of the HSP family called HSP30 is induced in response to thermal shock, both during embryogenesis and in adult cells [89,90]. The HSP70 in Podarcis embryos could represent a constitutive molecular chaperone, while HSP30 could be a stress-inducible cytoprotective protein. Unfortunately, the gene expression analyses carried out so far have not allowed us to identify the HSP30 of P. siculus, so it is not known whether the expression of this gene can change following a thermal shock during P. siculus development.
Conversely, in lizard embryos incubated for five days at a temperature of 10 °C, the gene encoding the Suppressor of Variegation 4-20 Homolog 1 (SUV4-20H1, also known as KMT5B) undergoes a marked downregulation of expression, which cannot be explained by the general slowing of transcription that is observed in heterothermic animals exposed to a cold environment. The SUV4-20H1 gene encodes a methyltransferase methylating the lysine in position 20 of the histone H4 [91]. It is well-established that DNA methylation modulates the expression of genes associated with embryonic development and aging [92,93]; alterations in DNA methylation occur frequently in many types of cancer [94]. Histone modifications are powerful regulators of cell cycle progression, DNA replication and DNA damage repair, chromosomal stability, and cellular lineage specification [95]. Recently, it has been demonstrated that the histone H4 lysine 20 methylation plays a crucial role in brain development [96]. In particular, the authors demonstrated that, during human development, KMT5B expression is positively correlated with neurogenesis, with the highest levels up to the first 20 weeks, steadily declining until birth and beyond. Studies on mice suggest a role for this gene in the maintenance of stem cell pools; it has been hypothesised that KMT5B is involved in neurogenesis through maintenance of the neural stem cell [96].
The evident morphological malformations observed in the brain of P. siculus embryos incubated in a cold environment may be due to alterations in neurogenesis; in this context, it is conceivable to assume that these alterations are partly a consequence of the inhibition of the transcription of SUV4-20H1, leading to a lack of its regulatory effect on brain development. This finding agrees with the DNA hypomethylation detected in the brains of P. muralis lizards developed in a cold environment, i.e., 20 °C versus 24 °C, considered the optimal incubation temperature for this species [97].
4. Conclusions
The stress-linked changes in gene expression observed in Podarcis lizards offer transcriptome-level evidence of how terrestrial vertebrate embryos cope with stress, providing a key to use in population survival and environmental change studies. Indeed, a better understanding of the genes/proteins that contribute to stress tolerance in different types of organisms could facilitate methodologies and applications aimed at improving organismal tolerance to unfavourable environments.
From this point of view, it is unfortunate that these studies in reptiles are very few, as evidenced by the paucity of available literature. It would be interesting to prove that the responses are reproducible among different taxa and different stress factors, i.e., that they represent a shared program resulting from a long adaptation to environmental changes and are now also engaged in counteracting the anthropic interferences.
In conclusion, the results collected here, which are far from exhaustive, allow us to identify some stress-reactive genes and, consequently, the molecular pathways in which these genes are involved. Cd-responsive genes encode proteins involved in cellular protection, metabolism and proliferation, membrane trafficking, protein interactions, neuronal transmission and plasticity, immune response and transcription regulatory factors. The latter in particular can generate a cascade response, which, in turn, is able to modify the transcriptional or post-translational activity of many genes and proteins, respectively. A similar effect appears to have changes in gene expression profiles induced by thermal stress. Differences were found in genes involved in transcriptional and translational regulation and chromatin remodelling. The inhibition of SUV4-20H1 methyltransferase expression observed in P. siculus embryos falls within this context. The drop in histone methylation could modify the epigenetic control of DNA, with consequent effects on the transcription of other messengers, with a cascade mechanism, as demonstrated by the increasing number of diseases caused by the dysregulation of post-translational modifications of histone proteins.
Author Contributions
Conceptualization, C.M.M., P.S., R.S.; data curation, R.S.; writing—original draft, R.S.; writing—review and editing, C.M.M., P.S., R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weigel, D.; Izaurralde, E. A tiny helper lightens the maternal load. Cell 2006, 124, 1117–1118. [Google Scholar] [CrossRef]
- Davis, T.L.; Rebay, I. Master regulators in development: Views from the Drosophila retinal determination and mammalian pluripotency gene networks. Dev. Biol. 2017, 421, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic Programming and Fetal Metabolic Programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Packard, G.C.; Packard, M.J. Evolution of the Cleidoic Egg Among Reptilian Antecedents of Birds. Am. Zool. 1980, 20, 351–362. [Google Scholar] [CrossRef]
- Marco, A.; López-Vicente, M.; Pérez-Mellado, V. Arsenic uptake by reptile flexible-shelled eggs from contaminated nest substrates and toxic effect on embryos. Bull. Environ. Contam. Toxicol. 2004, 72, 983–990. [Google Scholar] [CrossRef]
- Gómara, B.; Gómez, G.; Diaz-Paniagua, C.; Marco, A.; Gonzalez, M.J. PCB, DDT, arsenic, and heavy metal (Cd, Cu, Pb, and Zn) concentrations in chameleon (Chamaeleo chamaeleon) eggs from Southwest Spain. Chemosphere 2007, 68, 25–31. [Google Scholar] [CrossRef]
- Simoniello, P.; Motta, C.M.; Scudiero, R.; Trinchella, F.; Filosa, S. Cadmium-induced teratogenicity in lizard embryos: Correlation with metallothionein gene expression. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 119–127. [Google Scholar] [CrossRef]
- Trinchella, F.; Cannetiello, M.; Simoniello, P.; Filosa, S.; Scudiero, R. Differential gene expression profiles in embryos of the lizard Podarcis sicula under in ovo exposure to cadmium. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Simoniello, P.; Trinchella, F.; Filosa, S.; Scudiero, R.; Magnani, D.; Theil, T.; Motta, C.M. Cadmium contaminated soil affects retinogenesis in lizard embryos. J. Exp. Zool. 2014, 321A, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, R.; Verderame, M.; Motta, C.M.; Simoniello, P. Unravelling the role of metallothionein on development, reproduction and detoxification in the wall lizard Podarcis sicula. Int. J. Mol. Sci. 2017, 18, 1569. [Google Scholar] [CrossRef]
- Dufaure, J.P.; Hubert, J. Table de developpement du lezard vivipare: Lacerta (Zootoca) vivipara Jacquin. Arch. Anat. Microscop. Morphol. Exp. 1961, 50, 309–328. [Google Scholar]
- Sanger, T.J.; Losos, J.B.; Gibson-Brown, J.J. A developmental staging series for the lizard genus Anolis: A new system for the integration of evolution, development, and ecology. J. Morphol. 2008, 269, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Boback, S.M.; Dichter, E.K.; Mistry, H.L. A developmental staging series for the African house snake, Boaedon (Lamprophis) fuliginosus. Zoology 2012, 115, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Lazić, M.M.; Carretero, M.A.; Crnobrnja-Isailović, J.; Kaliontzopoulou, A. Effects of environmental disturbance on phenotypic variation: An integrated assessment of canalization, developmental stability, modularity, and allometry in lizard head shape. Am. Nat. 2015, 185, 44–58. [Google Scholar] [CrossRef]
- Singh, S.K.; Das, D.; Rhen, T. Embryonic Temperature Programs Phenotype in Reptiles. Front. Physiol. 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.E.; Wilson, H.R.; McPherson, B.N.; Mather, F.B.; Wilcox, C.J. Low temperature effects on embryonic development and hatch time. Poult. Sci. 1996, 75, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Schroeder, A. Molecular mechanisms of sex determination in reptiles. Sex Dev. 2010, 4, 16–28. [Google Scholar] [CrossRef]
- Simoniello, P.; Filosa, S.; Scudiero, R.; Trinchella, F.; Motta, C.M. Cadmium impairment of reproduction in the female wall lizard Podarcis sicula. Environ. Toxicol. 2013, 28, 553–562. [Google Scholar] [CrossRef]
- Yang, L.; Shen, Q.; Zeng, T.; Li, J.; Li, W.; Wang, Y. Enrichment of imidacloprid and its metabolites in lizards and its toxic effects on gonads. Environ. Pollut. 2020, 258, 113748. [Google Scholar] [CrossRef]
- Elhamamsy, A.R. DNA methylation dynamics in plants and mammals: Overview of regulation and dysregulation. Cell Biochem. Funct. 2016, 34, 289–298. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Murphy, A.J.; Costa, M.; Zhao, X.; Sun, H. Downregulation of hedgehog-interacting protein (HHIP) contributes to hexavalent chromium-induced malignant transformation of human bronchial epithelial cells. Carcinogenesis 2021, 42, 136–147. [Google Scholar] [CrossRef]
- Motta, C.M.; Simoniello, P.; Filosa, S. Control of oocyte recruitment and selection in Podarcis sicula, the Italian wall lizard. In Advances in Medicine and Biology; Nova Science Publishers: Happauge, NY, USA, 2011; Volume 24, pp. 247–263. [Google Scholar]
- Verderame, M.; Scudiero, R. Health status of the lizard Podarcis siculus (Rafinesque-Schmaltz, 1810) subject to different anthropogenic pressures. CR Biol. 2019, 342, 81–89. [Google Scholar] [CrossRef]
- Scudiero, R.; Filosa, S.; Motta, C.M.; Simoniello, P.; Trinchella, F. Cadmium in the wall lizard Podarcis Sicula: Morphological and molecular effects on embryonic and adult tissues. In Reptiles: Biology, Behavior and Conservation; Baker, K.J., Ed.; Nova Science Publishers: Happauge, NY, USA, 2011; pp. 147–162. [Google Scholar]
- Liang, P.; Bauer, D.; Averboukh, L.; Warthoe, P.; Rohrwild, M.; Muller, H.; Strauss, M.; Pardee, A.B. Analysis of altered gene expression by differential display. Methods Enzymol. 1995, 254, 304–321. [Google Scholar]
- Carginale, V.; Capasso, C.; Scudiero, R.; Parisi, E. Identification of cadmium-sensitive genes in the Antarctic fish Chionodraco hamatus by mRNA differential display. Gene 2002, 299, 117–124. [Google Scholar] [CrossRef]
- Kirstein-Miles, J.; Scior, A.; Deuerling, E.; Morimoto, R.I. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J. 2013, 32, 1451–1468. [Google Scholar] [CrossRef]
- Zheng, X.M.; Moncollin, V.; Egly, J.M.; Chambon, P. A general transcription factor forms a stable complex with RNA polymerase B (II). Cell 1987, 50, 361–368. [Google Scholar] [CrossRef]
- Thiede, B.; Dimmler, C.; Siejak, F.; Rudel, T. Predominant identification of RNA-binding proteins in Fas-induced apoptosis by proteome analysis. J. Biol. Chem. 2001, 276, 26044–26050. [Google Scholar] [CrossRef]
- Deng, J.M.; Behringer, R.R. An insertional mutation in the BTF3 transcription factor gene leads to an early postimplantation lethality in mice. Transgenic Res. 1995, 4, 264–269. [Google Scholar] [CrossRef]
- Li, X.; Sui, J.; Xing, J.; Cao, F.; Wang, H.; Fu, C.; Wang, H. Basic transcription factor 3 expression silencing attenuates colon cancer cell proliferation and migration in vitro. Oncol. Lett. 2019, 17, 113–118. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Cooper, M.K.; Wassif, C.A.; Krakowiak, P.A.; Taipale, J.; Gong, R.; Kelley, R.I.; Porter, F.D.; Beachy, P.A. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 2003, 33, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Osuga, J.; Tozawa, R.; Kitamine, T.; Yagyu, H.; Sekiya, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; Yahagi, N.; et al. Early embryonic lethality caused by targeted disruption of the 3- hydroxy-3-methylglutaryl-CoA reductase gene. J. Biol. Chem. 2003, 278, 42936–42941. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, L.; Gamper, A.M.; Fujita, T.; Wan, Y. APC/C-Cdh1. From cell cycle to cellular differentiation and genomic integrity. Cell Cycle 2010, 9, 3904–3912. [Google Scholar] [CrossRef] [PubMed]
- Simoniello, P.; Trinchella, F.; Borrelli, L.; De Stasio, R.; Motta, C.M.; Filosa, S.; Scudiero, R. Effects of cadmium on retinal development in lizard embryo: A molecular and morphological study. Comp. Biochem. Physiol. 2009, 154A, S21–S22. [Google Scholar] [CrossRef]
- .Razin, S.V.; Borunova, V.V.; Maksimenko, O.G.; Kantidze, O.L. Cys2His2 zinc finger protein family: Classification, functions, and major members. Biochemistry 2012, 77, 217–226. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Hashimoto, K.; Anderson, M.; Kohlhagen, G.; Pommier, Y.; D’Arpa, P. Characterization of BTBD1 and BTBD2, two similar BTB-domain-containing Kelch-like proteins that interact with Topoisomerase I. BMC Genom. 2002, 3, 1. [Google Scholar] [CrossRef]
- Bardwell, V.J.; Treisman, R. The POZ domain: A conserved protein–protein interaction motif. Genes Dev. 1994, 8, 1664–1677. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, X.; Zhang, H.K.; Yang, H.M.; Zhou, Y.B.; Han, Z.G. ZBTB34, a novel human BTB/POZ zinc finger protein, is a potential transcriptional repressor. Mol. Cell. Biochem. 2006, 290, 159–167. [Google Scholar] [CrossRef]
- Wen, Y.; Nguyen, D.; Li, Y.; Lai, Z.C. The N-terminal BTB/POZ domain and C- terminal sequences are essential for Tramtrack69 to specify cell fate in the developing Drosophila eye. Genetics 2000, 156, 195–203. [Google Scholar] [CrossRef]
- Yoshitomi, Y.; Osada, H.; Satake, H.; Kojima, M.; Saito-Takatsuji, H.; Ikeda, T.; Yoshitake, Y.; Ishigaki, Y.; Kubo, E.; Sasaki, H.; et al. Ultraviolet B-induced Otx2 expression in lens epithelial cells promotes epithelial-mesenchymal transition. Biol. Open 2019, 8, bio035691. [Google Scholar] [CrossRef]
- Acampora, D.; Mazan, S.; Lallemand, Y.; Avantaggiato, V.; Maury, M.; Simeone, A.; Brûlet, P. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 1995, 121, 3279–3290. [Google Scholar] [CrossRef]
- Wyatt, A.; Bakrania, P.; Bunyan, D.J.; Osborne, R.J.; Crolla, J.A.; Salt, A.; Ayuso, C.; Newbury-Ecob, R.; Abou-Rayyah, Y.; Collin, J.R.O.; et al. Novel heterozygous Otx2 mutations and whole gene deletions in anophthalmia, microphthalmia and coloboma. Hum. Mutat. 2008, 29, E278–E283. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M.; Pratt, T.; Liu, M.; Jeffery, G.; Price, D.J. Overexpression of Pax6 results in microphthalmia, retinal dysplasia and defective retinal ganglion cell axon guidance. BMC Dev. Biol. 2008, 8, 59. [Google Scholar] [CrossRef]
- Grindley, J.C.; Hargett, L.K.; Hill, R.E.; Ross, A.; Hogan, B.L. Disruption of PAX6 function in mice homozygous for the Pax6Sey-1Neu mutation produces abnormalities in the early development and regionalization of the diencephalon. Mech. Dev. 1997, 64, 111–126. [Google Scholar] [CrossRef]
- Collinson, J.M.; Quinn, J.C.; Buchanan, M.A.; Kaufman, M.H.; Wedden, S.E.; West, J.D.; Hill, R.E. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc. Natl. Acad. Sci. USA 2001, 98, 9688–9693. [Google Scholar] [CrossRef]
- Mort, R.L.; Bentley, A.J.; Martin, F.L.; Collinson, J.M.; Douvaras, P.; Hill, R.E.; Morley, S.D.; Fullwood, N.J.; West, J.D. Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis. PLoS ONE 2011, 6, e28895. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, S.Y.; Gao, M.; Bourgoin, S.; Randazzo, P.A.; Premont, R.T.; Hsu, V.W. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell Biol. 2002, 159, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, M.; Yang, X.; Elkins, J.M.; Sobott, F.; Doyle, D.A. The centaurin gamma-1 GTPase-like domain functions as an NTPase. Biochem. J. 2007, 401, 679–688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zuk, M.; Prescha, A.; Kepczynski, J.; Szopa, J. ADP ribosylation factor regulates metabolism and antioxidant capacity of transgenic potato tubers. J. Agric. Food Chem. 2003, 51, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Minglin, L.; Yuxiu, Z.; Tuanyao, C. Identification of genes up-regulated in response to Cd exposure in Brassica juncea L. Gene 2005, 363, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Voronin, D.A.; Kiseleva, E.V. Functional role of proteins containing ankyrin repeats. Tsitologiia 2007, 49, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Lu, Z.W.; Sun, Y.; Fang, Z.W.; Chen, J.; Zhou, Y.B.; Chen, M.; Ma, Y.Z.; Xu, Z.S.; Min, D.H. The Ankyrin-Repeat Gene GmANK114 Confers Drought and Salt Tolerance in Arabidopsis and Soybean. Front. Plant Sci. 2020, 11, 584167. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, C.; Wei, X.; Li, X.; Luo, G.; Zhang, J.; Bin, J.; Huang, X.; Cao, S.; Li, G.; et al. Overexpression of ankyrin repeat domain 1 enhances cardiomyocyte apoptosis by promoting p53 activation and mitochondrial dysfunction in rodents. Clin. Sci. 2015, 128, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure–function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Wada, A. Roles of voltage-dependent sodium channels in neuronal development, pain, and neurodegeneration. J. Pharmacol. Sci. 2006, 102, 253–268. [Google Scholar] [CrossRef]
- Lévesque, M.; Martineau, C.; Jumarie, C.; Moreau, R. Characterization of cadmium uptake and cytotoxicity in human osteoblast-like MG-63 cells. Toxicol. Appl. Pharmacol. 2008, 15, 308–317. [Google Scholar] [CrossRef]
- Ogawa, T.; Kuwagata, M.; Hori, Y.; Shioda, S. Valproate-induced developmental neurotoxicity is affected by maternal conditions including shipping stress and environmental change during early pregnancy. Toxicol. Lett. 2007, 174, 18–24. [Google Scholar] [CrossRef]
- Evenseth, L.; Gabrielsen, M.; Sylte, I. The GABABReceptor—Structure, Ligand Binding and Drug Development. Molecules 2020, 25, 3093. [Google Scholar] [CrossRef]
- Kornau, H.C. GABA(B) receptors and synaptic modulation. Cell Tissue Res. 2006, 326, 517–533. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Nadi, N.S. Mechanism of action of antiepileptic and antimyoclonic drugs. Adv. Neurol. 1995, 67, 329–360. [Google Scholar]
- Dengler, T.J.; Hoffmann, J.C.; Knolle, P.; Albert-Wolf, M.; Roux, M.; Wallich, R.; Meuer, S.C. Structural and functional epitopes of the human adhesion receptor CD58 (LFA-3). Eur. J. Immunol. 1992, 22, 2809–2817. [Google Scholar] [CrossRef]
- Binder, C.; Cvetkovski, F.; Sellberg, F.; Berg, S.; Paternina Visbal, H.; Sachs, D.H.; Berglund, E.; Berglund, D. CD2 Immunobiology. Front. Immunol. 2020, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Shi, W.; Zheng, J.Y.; Xu, X.X.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Costimulatory Function of Cd58/Cd2 Interaction in Adaptive Humoral Immunity in a Zebrafish Model. Front. Immunol. 2018, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.; Stillman, M.J. The “magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. [Google Scholar] [CrossRef]
- Waeytens, A.; De Vos, M.; Laukens, D. Evidence for a potential role of metallothioneins in inflammatory bowel diseases. Mediat. Inflamm. 2009, 2009, 729172. [Google Scholar] [CrossRef]
- Kumari, M.V.; Hiramatsu, M.; Ebadi, M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic Res. 1998, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.; Kaur, M.; Ansari, R.A.; Raisuddin, S. Channa punctata brain metallothionein is a potent scavenger of superoxide radicals and prevents hydroxyl radical-induced in vitro DNA damage. J. Biochem. Mol. Toxicol. 2008, 22, 202–208. [Google Scholar] [CrossRef]
- Carginale, V.; Scudiero, R.; Capasso, C.; Capasso, A.; Kille, P.; di Prisco, G.; Parisi, E. Cadmium-induced differential accumulation of metallothionein isoforms in the Antarctic icefish which exhibits no basal protein but high endogenous mRNA levels. Biochem. J. 1998, 332, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Gedamu, L. Molecular analyses of metallothionein gene regulation. Prog. Nucleic Acid Res. Mol. Biol. 1998, 59, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Trinchella, F.; Riggio, M.; Filosa, S.; Volpe, M.G.; Parisi, E.; Scudiero, R. Cadmium distribution and metallothionein expression in lizard tissues following acute and chronic cadmium intoxication. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Positive and negative regulators of the metallothionein gene (review). Mol. Med. Rep. 2015, 12, 795–799. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Günes, C.; Heuchel, R.; Georgiev, O.; Müller, K.H.; Lichtlen, P.; Blüthmann, H.; Marino, S.; Aguzzi, A.; Schaffner, W. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 1998, 17, 2846–2854. [Google Scholar] [CrossRef]
- Durliat, M.; Muller, J.P.; André, M.; Wegnez, M. Expression of the Xenopus laevis metallothionein gene during ontogeny. Int. J. Dev. Biol. 1999, 43, 575–578. [Google Scholar]
- Romero, M.B.; Polizzi, P.; Chiodi, L.; Das, K.; Gerpe, M. The role of metallothioneins, selenium and transfer to offspring in mercury detoxification in Franciscana dolphins (Pontoporia blainvillei). Mar. Pollut. Bull. 2016, 109, 650–654. [Google Scholar] [CrossRef]
- Riggio, M.; Trinchella, F.; Parisi, E.; Filosa, S.; Scudiero, R. Accumulation of zinc, copper and metallothionein mRNA in lizard ovary proceeds without a concomitant increase in metallothionein content. Mol. Repr. Dev. 2003, 66, 347–382. [Google Scholar] [CrossRef]
- Simoniello, P.; Motta, C.M.; Scudiero, R.; Trinchella, F.; Filosa, S. Spatiotemporal changes in metallothionein gene expression during embryogenesis in the wall lizard Podarcis sicula. J. Exp. Zool. 2010, 313A, 410–420. [Google Scholar] [CrossRef]
- Borrelli, L.; De Stasio, R.; Motta, C.M.; Parisi, E.; Filosa, S. Seasonal-dependent effect of temperature on the response of adenylate cyclase to FSH stimulation in the oviparous lizard, Podarcis sicula. J. Endocrinol. 2000, 167, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, R.; Esposito, M.G.; Simoniello, P. Tolerance to Thermal Stress in Lizard Embryos. In Lizards: Thermal Ecology, Genetic Diversity and Functional Role in Ecosystems; Kiernan, M.P., Ed.; Nova Science Publishers: Happauge, NY, USA, 2014; pp. 29–44. [Google Scholar]
- Simoniello, P.; Esposito, M.G.; Trinchella, F.; Motta, C.M.; Scudiero, R. Alterations in brain morphology and HSP70 expression in lizard embryos exposed to thermal stress. CR Biol. 2016, 339, 380–390. [Google Scholar] [CrossRef]
- Dayananda, B.; Webb, J.K. Incubation under climate warming affects learning ability and survival in hatchling lizards. Biol. Lett. 2017, 13, 20170002. [Google Scholar] [CrossRef]
- Feiner, N.; Rago, A.; While, G.M.; Uller, T. Developmental plasticity in reptiles: Insights from temperature-dependent gene expression in wall lizard embryos. J. Exp. Zool. A Ecol. Integr. Physiol. 2018, 329, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, R.; Verderame, M.; Motta, C.M.; Migliaccio, V.; Simoniello, P. HSP70 localization in Podarcis siculus embryos under natural thermal regime and following a non-lethal cold shock. CR Biol. 2019, 342, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.; Heikkila, J.J. Identification of members of the hsp30 small heat shock protein family and characterization of their developmental regulation in heat-shocked Xenopus laevis embryos. Dev. Genet. 1995, 17, 331–339. [Google Scholar] [CrossRef]
- Heikkila, J.J. The expression and function of hsp30-like small heat shock protein genes in amphibians, birds, fish, and reptiles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 203, 179–192. [Google Scholar] [CrossRef]
- Yang, H.; Pesavento, J.J.; Starnes, T.W.; Cryderman, D.E.; Wallrath, L.L.; Kelleher, N.L.; Mizzen, C.A. Preferential dimethylation of histone H4 lysine 20 by Suv4-20. J. Biol. Chem. 2008, 283, 12085–12092. [Google Scholar] [CrossRef]
- Yizhar-Barnea, O.; Valensisi, C.; Jayavelu, N.D.; Kishore, K.; Andrus, C.; Koffler-Brill, T.; Ushakov, K.; Perl, K.; Noy, Y.; Bhonker, Y.; et al. DNA methylation dynamics during embryonic development and postnatal maturation of the mouse auditory sensory epithelium. Sci. Rep. 2018, 8, 17348. [Google Scholar] [CrossRef]
- Fan, L.H.; Wang, Z.B.; Li, Q.N.; Meng, T.G.; Dong, M.Z.; Hou, Y.; Ouyang, Y.C.; Schatten, H.; Sun, Q.Y. Absence of mitochondrial DNA methylation in mouse oocyte maturation, aging and early embryo development. Biochem. Biophys. Res. Commun. 2019, 513, 912–918. [Google Scholar] [CrossRef]
- Klutstein, M.; Nejman, D.; Greenfield, R.; Cedar, H. DNA Methylation in Cancer and Aging. Cancer Res. 2016, 76, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.; Schotta, G.; Sørensen, C.S. Histone H4 lysine 20 methylation: Key player in epigenetic regulation of genomic integrity. Nucleic Acids Res. 2013, 41, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Wickramasekara, R.N.; Stessman, H.A.F. Histone 4 Lysine 20 Methylation: A Case for Neurodevelopmental Disease. Biology 2019, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Paredes, U.; Radersma, R.; Cannell, N.; While, G.M.; Uller, T. Low Incubation Temperature Induces DNA Hypomethylation in Lizard Brains. J. Exp. Zool. A Ecol. Genet. Physiol. 2016, 325, 390–395. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).