Gas Barrier Performance of Hexagonal Boron Nitride Monolayers Grown on Copper Foils with Electrochemical Polishing
Abstract
1. Introduction
2. Methods
2.1. Electro-Chemical Polishing
2.2. Synthesis of h-BN Layers
2.3. Structure Characterizations
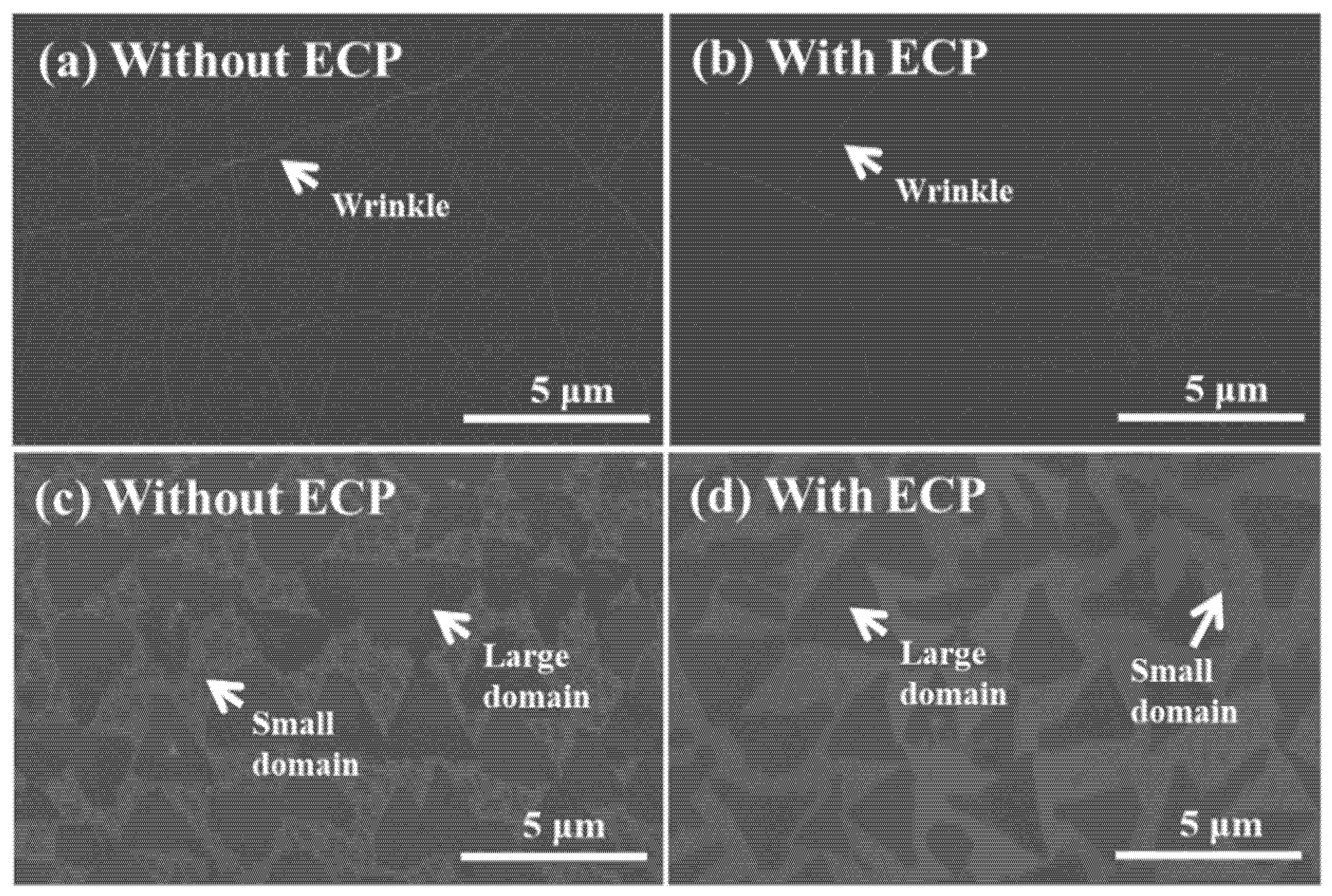
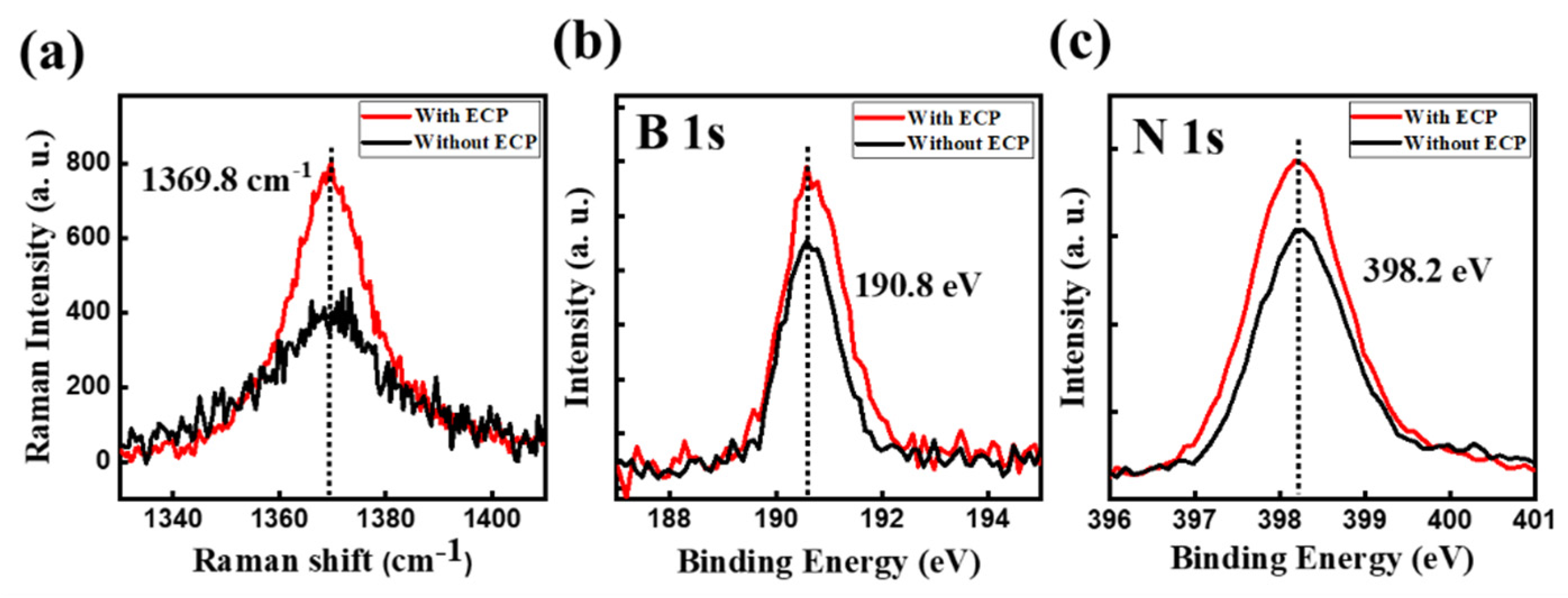
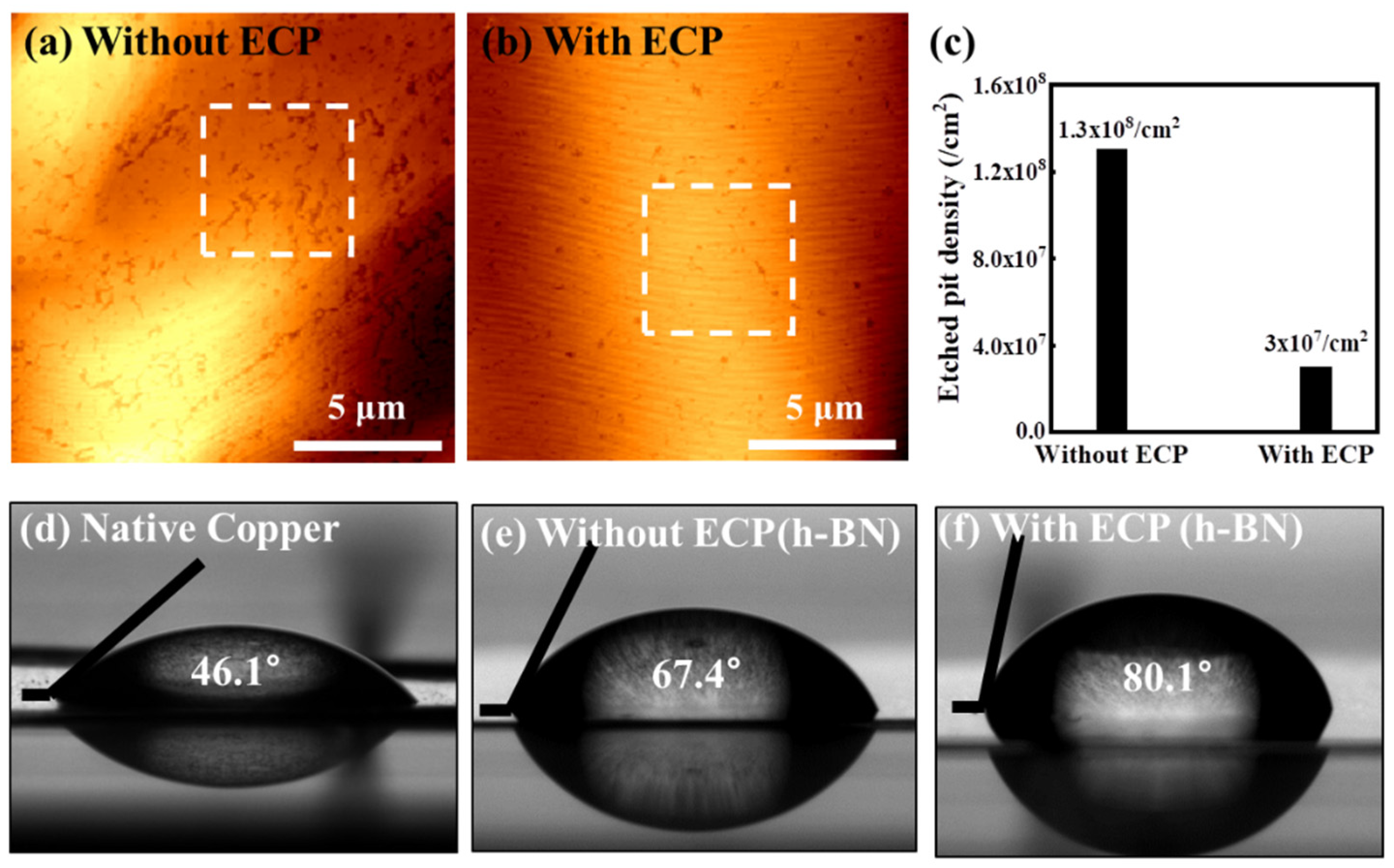
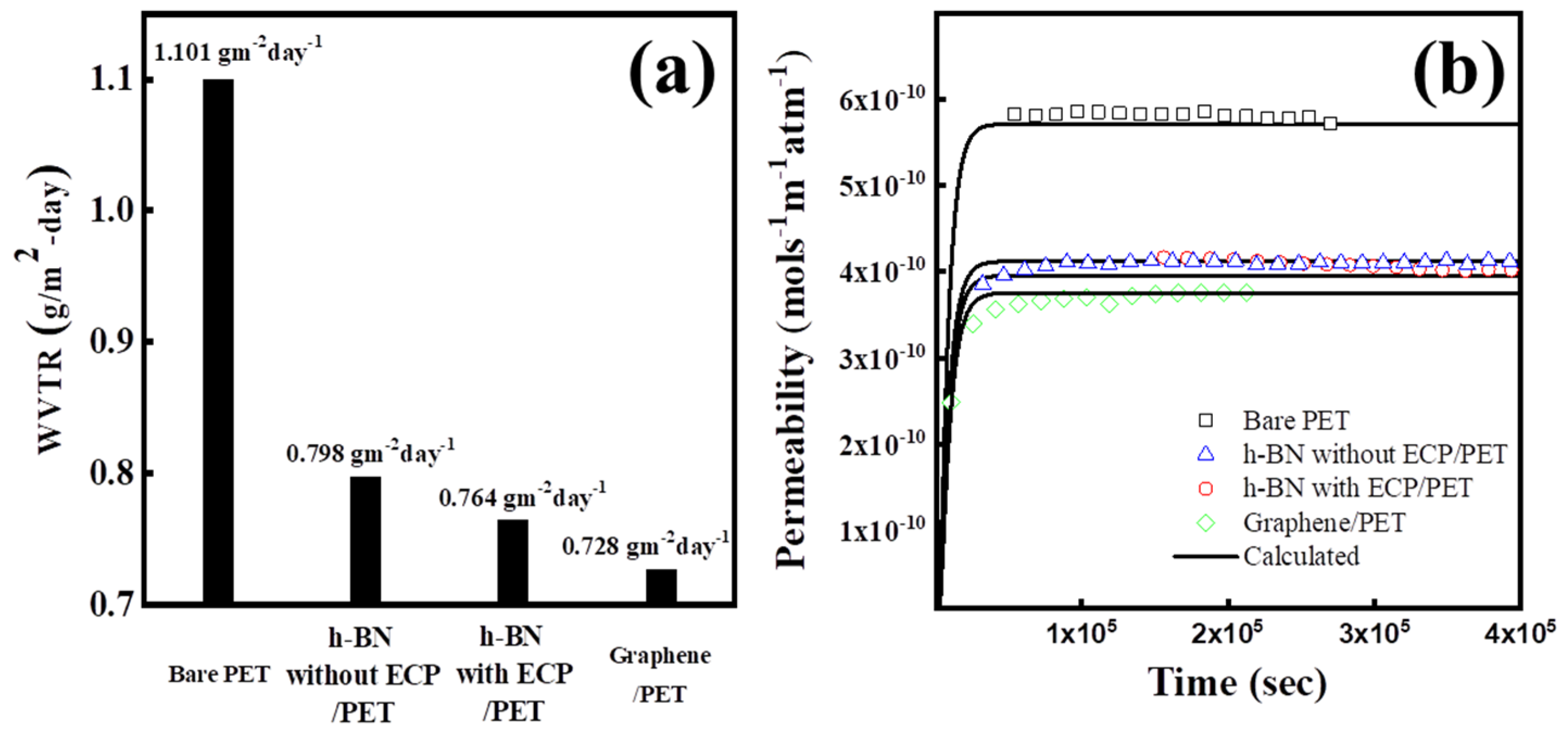
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zhou, D.; Huang, J.; Yu, J. High performance organic ultraviolet photodetector with efficient electroluminescence realized by a thermally activated delayed fluorescence emitter. Appl. Phys. Lett. 2015, 107, 043303. [Google Scholar] [CrossRef]
- Pradana, A.; Gerken, M. Photonic crystal slabs in flexible organic light-emitting diodes. Photon. Res. 2015, 3, 32–37. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.-Q.; Chen, Z.; Tao, Y.; Liu, Y.-F. Recent progress on thin-film encapsulation technologies for organic electronic devices. Opt. Commun. 2016, 362, 43–49. [Google Scholar] [CrossRef]
- Xiao, W.; Hui, D.Y.; Zheng, C.; Yu, D.; Qiang, Y.Y.; Ping, C.; Xiang, C.L.; Yi, Z. A flexible transparent gas barrier film employing the method of mixing ALD/MLD-grown Al2O3 and alucone layers. Nanoscale Res. Lett. 2015, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Rieth, L.; Williams, L.; Negi, S.; Bhandari, R.; Caldwell, R.; Sharma, R.; Tathireddy, P.; Solzbacher, F. Long-term relia-bility of Al2O3 and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J. Neural Eng. 2014, 11, 26016. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Hibino, H.; Ago, H. Grain Boundaries and Gas Barrier Property of Graphene Revealed by Dark-Field Optical Mi-croscopy. J. Phys. Chem. C 2018, 122, 902–910. [Google Scholar] [CrossRef]
- Seo, T.H.; Lee, S.; Cho, H.; Chandramohan, S.; Suh, E.-K.; Lee, H.S.; Bae, S.K.; Kim, S.M.; Park, M.; Lee, J.K.; et al. Tailored CVD graphene coating as a transparent and flexible gas barrier. Sci. Rep. 2016, 6, 24143. [Google Scholar] [CrossRef]
- Cui, C.; Lim, A.T.O.; Huang, J. A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 2017, 12, 834–835. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a Long-Term Metal Oxidation Barrier: Worse Than Nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef]
- Tanjil, R.-E.; Jeong, Y.; Yin, Z.; Panaccione, W.; Wang, M.C. Ångstrom-Scale, Atomically Thin 2D Materials for Corrosion Mitigation and Passivation. Coatings 2019, 9, 133. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Tong, X.; Wang, Z. Graphene-based optical modulators. Nanoscale Res. Lett. 2015, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Laturia, A.; Van De Put, M.L.; Vandenberghe, W.G. Dielectric properties of hexagonal boron nitride and transition metal dichalcogenides: From monolayer to bulk. 2D Mater. Appl. 2018, 2, 6. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Sun, J.; Lu, C.; Song, Y.; Ji, Q.; Song, X.; Li, Q.; Zhang, Y.; Zhang, L.; Kong, J.; Liu, Z. Recent progress in the tailored growth of two-dimensional hexagonal boron nitride via chemical vapour deposition. Chem. Soc. Rev. 2018, 47, 4242–4257. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, M.S.H.; Sun, C.; O’Hern, S.C.; Au, H.; Hadjiconstantinou, N.G.; Karnik, R. Implications of Permeation through Intrinsic Defects in Graphene on the Design of Defect-Tolerant Membranes for Gas Separation. ACS Nano 2014, 8, 841–849. [Google Scholar] [CrossRef]
- Giesbers, A.; Bouten, P.; Cillessen, J.; Van Der Tempel, L.; Klootwijk, J.; Pesquera, A.; Centeno, A.; Zurutuza, A.; Balkenende, A. Defects, a challenge for graphene in flexible electronics. Solid State Commun. 2016, 229, 49–52. [Google Scholar] [CrossRef]
- Lu, G.; Wu, T.; Yuan, Q.; Wang, H.; Wang, H.; Ding, F.; Xie, X.; Jiang, M. Synthesis of large single-crystal hexagonal boron nitride grains on Cu–Ni alloy. Nat. Commun. 2015, 6, 6160. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Park, S.; Won, D.I.; Kang, S.O.; Pyo, S.S.; Kim, D.I.; Kim, S.M.; Kim, H.C.; Kim, M.J. Growth kinetics of white gra-phene (h-BN) on a planarised Ni foil surface. Sci. Rep. 2015, 5, 11985. [Google Scholar] [CrossRef]
- Tay, R.Y.; Griep, M.H.; Mallick, G.; Tsang, S.H.; Singh, R.S.; Tumlin, T.; Teo, E.H.T.; Karna, S.P. Growth of large single-crystalline two-dimensional boron nitride hexagons on electropolished copper. Nano Lett. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Braeuninger-Weimer, P.; Brennan, B.; Pollard, A.J.; Hofmann, S. Understanding and Controlling Cu-Catalyzed Graphene Nucleation: The Role of Impurities, Roughness, and Oxygen Scavenging. Chem. Mater. 2016, 28, 8905–8915. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Güneş, F.; Bae, J.J.; Kim, E.S.; Chae, S.J.; Shin, H.-J.; Choi, J.-Y.; Pribat, D.; Lee, Y.H. Influence of Copper Morphology in Forming Nucleation Seeds for Graphene Growth. Nano Lett. 2011, 11, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Griep, M.H.; Sandoz-Rosado, E.; Tumlin, T.M.; Wetzel, E. Enhanced Graphene Mechanical Properties through Ultrasmooth Copper Growth Substrates. Nano Lett. 2016, 16, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Li, W.; Yu, W.; Liu, X. A Review on Lattice Defects in Graphene: Types, Generation, Effects and Regulation. Micromachines 2017, 8, 163. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Hu, H.; Cao, C.; Xu, Y.-J. Anisotropic vacancy-defect-induced fracture strength loss of graphene. RSC Adv. 2015, 5, 13623–13627. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.; Guo, Y.; Yuan, J.; Si, Y.; Zhang, S.; Chen, H. Strength and failure behavior of a graphene sheet containing bi-grain-boundaries. RSC Adv. 2014, 4, 54677–54683. [Google Scholar] [CrossRef]
- Stehle, Y.; Meyer, I.H.M.; Unocic, R.R.; Kidder, M.; Polizos, G.; Datskos, P.G.; Jackson, R.; Smirnov, S.N.; Vlassiouk, I.V. Synthesis of Hexagonal Boron Nitride Monolayer: Control of Nucleation and Crystal Morphology. Chem. Mater. 2015, 27, 8041–8047. [Google Scholar] [CrossRef]
- Mahvash, F.; Eissa, S.; Bordjiba, T.; Tavares, A.C.; Szkopek, T.; Siaj, M. Corrosion resistance of monolayer hexagonal boron nitride on copper. Sci. Rep. 2017, 7, 42139. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, H.; Okello, O.F.N.; Xiao, S.; Moon, S.; Kim, D.Y.; Kim, G.Y.; Lo, J.I.; Peng, Y.C.; Cheng, B.M.; et al. Improvements in structural and optical properties of wafer-scale hexagonal boron nitride film by post-growth annealing. Sci. Rep. 2019, 9, 10590. [Google Scholar] [CrossRef]
- Haider, A.; Ozgit-Akgun, C.; Goldenberg, E.; Okyay, A.K.; Biyikli, N. Low-Temperature Deposition of Hexagonal Boron Nitride via Sequential Injection of Triethylboron and N2/H2 Plasma. J. Am. Ceram. Soc. 2014, 97, 4052–4059. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar] [CrossRef]
- Belyaeva, L.A.; van Deursen, P.M.G.; Barbetsea, K.I.; Schneider, G.F. Hydrophilicity of Graphene in Water through Trans-parency to Polar and Dispersive Interactions. Adv. Mater. 2018, 30, 1703274. [Google Scholar] [CrossRef]
- Prydatko, A.V.; Belyaeva, L.A.; Jiang, L.; Lima, L.M.C.; Schneider, G.F. Contact angle measurement of free-standing square-millimeter single-layer graphene. Nat. Commun. 2018, 9, 4185. [Google Scholar] [CrossRef] [PubMed]
- Parobek, D.; Liu, H. Wettability of graphene. 2D Mater. 2015, 2. [Google Scholar] [CrossRef]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.-W.; Venkatesan, T. Surface energy and wettability of van der Waals structures. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, S.H.; Yun, S.J.; Kim, Y.I.; Boandoh, S.; Park, J.-H.; Shin, B.G.; Ko, H.; Lee, S.H.; Lee, Y.H.; et al. Wafer-scale single-crystal hexagonal boron nitride film via self-collimated grain formation. Science 2018, 362, 817–821. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, J.K.; Lee, H.S. Transparent and high gas barrier films based on poly(vinyl alcohol)/graphene oxide composites. Thin Solid Films 2011, 519, 7766–7771. [Google Scholar] [CrossRef]
| Bare PET | h-BN Without ECP/PET | h-BN with ECP/PET | Graphene/PET | |
|---|---|---|---|---|
| Permeability [×10−14 mol s−1 m−1 atm−1] | 7.43 | 5.36 | 5.14 | 4.88 |
| Diffusivity [×10−14 m2 s−1] | 0.30 | 0.28 | 0.28 | 0.27 |
| Solubility [mol m−3 atm−1] | 24.8 | 18.5 | 18.2 | 18.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.H.; Choi, G.B.; Kim, E.M.; Lee, J.; Lee, J.; Moon, H.G.; Kim, M.J.; Kim, Y.A.; Seo, T.H. Gas Barrier Performance of Hexagonal Boron Nitride Monolayers Grown on Copper Foils with Electrochemical Polishing. Appl. Sci. 2021, 11, 4599. https://doi.org/10.3390/app11104599
Lee CH, Choi GB, Kim EM, Lee J, Lee J, Moon HG, Kim MJ, Kim YA, Seo TH. Gas Barrier Performance of Hexagonal Boron Nitride Monolayers Grown on Copper Foils with Electrochemical Polishing. Applied Sciences. 2021; 11(10):4599. https://doi.org/10.3390/app11104599
Chicago/Turabian StyleLee, Chil Hyoung, Go Bong Choi, Eun Mi Kim, Jongho Lee, Jaegeun Lee, Hi Gyu Moon, Myung Jong Kim, Yoong Ahm Kim, and Tae Hoon Seo. 2021. "Gas Barrier Performance of Hexagonal Boron Nitride Monolayers Grown on Copper Foils with Electrochemical Polishing" Applied Sciences 11, no. 10: 4599. https://doi.org/10.3390/app11104599
APA StyleLee, C. H., Choi, G. B., Kim, E. M., Lee, J., Lee, J., Moon, H. G., Kim, M. J., Kim, Y. A., & Seo, T. H. (2021). Gas Barrier Performance of Hexagonal Boron Nitride Monolayers Grown on Copper Foils with Electrochemical Polishing. Applied Sciences, 11(10), 4599. https://doi.org/10.3390/app11104599








