Abstract
Pectinolytic enzymes are an important tool for sustainable food production, with a wide range of applications in food processing technologies as well as the extraction of bioactive compounds from pectin-rich raw materials. In the present study, we immobilized commercial pectinase preparation onto pellet and thread shaped nylon 6/6 carriers and assessed its stability and reusability. Five commercial pectinase preparations were tested for different pectin de-polymerizing activities (pectinase, polygalacturonase, and pectin lyase activities). Thereafter, Pectinex® Ultra Tropical preparation, exhibiting the highest catalytic activities among the studied preparations (p < 0.0001), was immobilized on nylon 6/6 using dimethyl sulfate and glutaraldehyde. The immobilization yield was in accordance with the carrier surface area available for enzyme attachment, and it was 1.25 ± 0.10 U/g on threads, which was over 40 times higher than that on pellets. However, the inactivation of immobilized enzymes was not dependent on the shape of the carrier, indicating that the attachment of the enzymes on the surface of nylon 6/6 carriers was similar. The half-life of enzyme inactivation fast phase at 4 °C was 12.8 days. After 5 weeks, the unused threads retained 63% of their initial activity. Reusability study showed that after 20 successive cycles the remaining activity of the immobilized pectinase was 22%, indicating the good prospects of reusability of the immobilized enzyme preparations for industrial application.
1. Introduction
Pectinolytic enzymes, also known as pectinases, are a heterogeneous group of enzymes catalyzing different steps of the hydrolysis of pectin, a major component of plant cell walls [1]. Based on their mode of action, pectinases are classified as (i) de-esterifying enzymes (pectin esterase), and (ii) de-polymerizing enzymes (hydrolases, lyases). De-polymerizing enzymes are further classified based on their specific action site to endo- and exo-polygalacturonases and rhamnogalacturonases [2], or separated as per the nature of action mechanism, primary substrate, and products [3].
Pectinases have a wide range of applications in different industries including food, textile, and paper industries. Microbial pectinases are one of the most commercially produced enzymes, with 25% share in the global food and beverage enzyme market [4]. Pectinases are considered as a sustainable and environmentally-friendly industrial catalyst as they are produced by fungi (Aspergillus, Penicillium, Trichoderma, Rhizomucor, etc.) and bacterial (Bacillus, Streptomyces, Erwina) fermentation of different by-products like wheat bran, citrus peels, and sugar beet pulp [2,5]. Commercial pectinase preparations are widely utilized in the extraction and clarification of fruits and vegetables juices [6]. They are also used for the extraction of vegetable oil [7] and the fermentation of coffee beans and tea leaves [2]. Moreover, along with cellulases, pectinases are an important tool for ‘green’ enzyme-assisted extraction of bioactive compounds from different plant matrices including citrus peel, carrot pomace, tomato, etc. [8,9].
Several studies have highlighted the attractiveness of pectinases’ immobilization allowing multiple reuses and increasing the efficiency of production processes, easy separation of the enzyme from the final product [10], but also the development of continuous automated processes particularly relevant in juice production and winemaking [11]. Various techniques have been used for the immobilization of different pectinase preparations including adsorption [12,13,14], entrapment [15,16,17], covalent binding to insoluble carriers [18,19,20,21], as well as to magnetic nanoparticles [22,23,24,25]. Despite the variety of immobilization methods used, the most stable enzyme coupling is achieved using covalent attachment. Commonly, enzymes are coupled via their amino groups, but other functional groups like carboxylic, thiol, sulfhydryl, and other groups can be also used.
Carriers for the immobilization of pectinase and other enzymes which are used in food industry must be non-toxic, biocompatible, and insoluble under reaction conditions, and thermally stable [26]. Functional groups for protein binding onto an insoluble carrier are usually generated through chemical activation of the support surface. In addition, linker molecules (e.g., glutaraldehyde, carbodiimide, etc.) are often used to enhance enzyme flexibility and activity [27]. For the technological production of immobilized enzymes, important criteria are the durability of the carrier, but also its availability and low cost [26]. Nylon 6/6 is an interesting, inexpensive, and convenient carrier for enzyme immobilization. It is inert, non-toxic, mechanically strong, and readily available in different forms (pellets, discs, threads, fabric, etc.) [28,29]. The inertness of nylon 6/6 makes it unable to bind proteins as it is, so it should be activated by partial acid hydrolysis or O-alkylation with dimethyl sulfate (DMS) [30]. Nylon 6/6 activation generates free amino (-NH2) and carboxyl (-COOH) groups on its surface, allowing the covalent binding of enzymes using glutaraldehyde as a crosslinking agent [31].
In the present study, we used nylon 6/6 pellets and threads as carriers for the immobilization of multi-enzyme pectinase commercial preparation used for degrading fibrous plant material and providing higher juice yields and clarity. We optimized the protocols for the attachment of enzyme onto nylon 6/6 and assessed the stability of immobilized pectinase in the course of its storage and during application for multiple successive cycles.
2. Materials and Methods
2.1. Materials
Pectinase preparations Pectinex® Ultra SPL (≥3800 U/mL, Sigma-Aldrich, Steinheim, Germany), Pectinex® BE XXL (13,600 PECTU/g), Pectinex® Ultra Mash (9500 PECTU/g) and Pectinex® Ultra Tropical (5000 PECTU/g) from Novozymes (Bagsvaerd, Denmark), and Panzym® Univers (11,000 PECTU/mL) from Begerow (Langenlonsheim, Germany) were used in this work. Apple pectin, polygalacturonic acid, and galacturonic acid were all purchased from Sigma-Aldrich (Steinheim, Germany). Nylon 6/6 pellets were from Sigma-Aldrich and threads consisting of 60 filaments with a diameter of 25 µm twisted together from Oja, Turkey. All chemical reagents and solvents used were of analytical grade.
2.2. Determination of Pectinolytic Activity
Pectinase activity was measured using apple pectin and polygalacturonase activity was measured using polygalacturonic acid as substrates. Aqueous substrate solution (0.5%, w/v) was prepared by adding purified water to polygalacturonic acid or apple pectin, heating the solution under continuous stirring until complete dissolution of compounds, and then cooling the solution to 25 °C. The pH value of the substrate solution was adjusted to 4.8 by adding NaOH solution (1N). Enzyme preparation 40-fold diluted (0.1 mL) or a definite amount of immobilized enzyme was added to 5 mL apple pectin or polygalacturonic acid solution (0.5%, w/v, pH 4.8) and incubated 5–30 min in a water bath at 40 °C. All activity measurements were carried out in triplicates.
2.2.1. Galacturonic acid Assay
The amount of galacturonic acid released after the enzymatic reaction was quantified spectrophotometrically using the copper reduction procedure reported by Rajdeo et al. [14]. Briefly, 0.3 mL of 10 mM copper sulfate solution (in 0.2 M CH3COONa buffer containing 2 M NaCl, pH 4.8) was mixed with 0.3 mL of sample in a capped 15-mL tube and incubated at 80 °C for 30 min. After incubation, 2.4 mL of diluted Folin-Ciocalteu reagent (40-fold, final concentration 0.05 N) was added. A blue-colored product immediately formed and the color intensity was determined by measuring the absorbance at 750 nm with a spectrophotometer (UV−1800, Shimadzu, Kyoto, Japan). Galacturonic acid content in the sample was calculated based on galacturonic acid standard curve in concentration range from 30 to 300 nmol.
2.2.2. Iodine Titration Method
The other method used for the measurement of galacturonic acid produced was the standard process involving galacturonic acid oxidation in the presence of I2 and titration of excess I2 with thiosulfate [32]. The enzyme reaction was stopped by adding 0.1 M I2 solution (5 mL) and 1 M Na2CO3 solution (1 mL) and the mixture was incubated for 20 min in the dark at room temperature. After adding 2 N sulphuric acid (2 mL), the excess I2 was titrated with 0.1 M Na2S2O3 solution until the solution became colorless. The following equation was used for the calculation of enzyme activity:
where:
- 1 = µmole galacturonic acid is oxidized by 1 microequivalent of I2
- 100 = microequivalents of S2O3 per mL of titrant
- df = dilution factor
- 2 = microequivalents of S2O3 oxidized per microequivalent of I2 reduced
- Venzyme = volume (in mL) of enzume used
- treaction = time of incubation (in minutes) for the enzyme reaction
2.2.3. Pectin lyase Activity
Pectin lyase activity was measured using apple pectin as substrate. The activity was determined spectrophotometrically according to the method described by Dal Magro et al. [33] by monitoring the increase in the absorbance at 235 nm due to the formation of unsaturated uronide. An enzyme preparation 40-fold diluted (0.05 mL) was mixed with 1.95 mL pectin solution (0.5%, w/v, pH4.8) and incubated for 1 min at 40 °C. The reaction was stopped by adding 3 mL of 0.5 M HCl and the absorbance at 235 nm was measured using spectrophotometer (UV-1800, Shimadzu, Japan). Unsaturated uronide concentration was calculated according to the molar attenuation coefficient ε = 5500 M−1.cm−1 and one unit of pectin lyase activity is defined as one nmol of unsaturated uronide released per minute per mL enzyme preparation.
2.3. Enzyme Immobilization onto Nylon 6/6
Pectinase preparation Pectinex Ultra Tropical was immobilized on different nylon 6/6 carriers (pellets and thread). Nylon thread was cut into 2 m length fragments and coiled around Teflon rings (4 cm outer diameter, 3.5 cm inner diameter).
First, nylon carriers were activated with dimethyl sulfate (DMS) at 60 °C for 1 to 7.5 min, washed two times with ice-cold methanol and several times with ice-cold 0.1 M phosphate buffer (pH 7.0). Then, the carriers were treated for 60 min with glutaraldehyde solution (2.5% or 12.5% in 0.1 M phosphate buffer, pH 7) at room temperature. After washing several times with phosphate buffer, the carriers were incubated with diluted pectinase preparation (20-fold in 0.2 M sodium acetate buffer pH 4.8) for 24 h at 4 °C. After 24 h, nylon carriers were removed from the enzyme solution, washed thoroughly 6 times with ~30 mL 0.2 M sodium acetate buffer (pH 4.8), and stored in the same buffer at 4 °C. For easier handling of threads during the immobilization process and assessment of the activity of immobilized pectinases on threads, we used threads coiled around Teflon rings (Ø 4 cm) (Figure 1).
Figure 1.
Nylon threads coiled around Teflon rings.
2.4. Statistical Analysis
The statistical analysis was conducted using Prism version 5 (GraphPad Software, San Diego, CA, USA). Data were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s test. Differences at p < 0.05 were considered to be significant.
3. Results and Discussion
3.1. Comparison of Different Methods for the Assessment of Pectinase Activity
There are several methods for the determination of pectinase hydrolytic activity. In many studies, it has been assessed by measuring the amount of reducing sugars released during the reaction by the 3,5-dinitrosalicyclic acid (DNS) method [15,33,34]. However, this method is not specific to galacturonic acid as it is sensitive to other reducing sugars like glucose and galactose as well. Other methods for the determination of the produced galacturonic acid are iodometric titration method [32] and spectrophotometric method based on copper-reducing reaction [35]. We evaluated the latter two methods for the determination of pectinase and polygalacturonase activities expressed in international units U (µmol galacturonic acid released per one minute). The results for Pectinex Ultra SPL preparation as an example are shown in Table 1.

Table 1.
Pectinase and polygalacturonase activities of Pectinex Ultra SPL preparation determined using titration and spectrophotometric methods.
Compared to pectinase activity declared by the producer (≥3800 U/mL), the activity determined was considerably lower (≥3 times) with both methods used. Compared to the titration assay, the spectrophotometric method indicated approximately 20% lower estimations of both pectinase and polygalacturonase activity. As the relative activities are similar, we selected the spectrophotometric method for the determination of the activity of immobilized enzymes in the current study because the assessment procedure was less time-consuming and the experimental errors were smaller. In addition, this method is selective for uronic acids over neutral sugars like glucose, galactose, and rhamnose [35].
3.2. Characterization of Different Commercial Pectinase Preparations
Five commercial preparations of pectinolytic enzymes: Pectinex Ultra SPL (from Sigma-Aldrich), Pectinex BE XXL, Ultra Mash, Ultra Tropical (from Novozymes), and Panzym-Univers (from Begerow) were evaluated regarding their specific pectin depolymerizing activity (Figure 2).
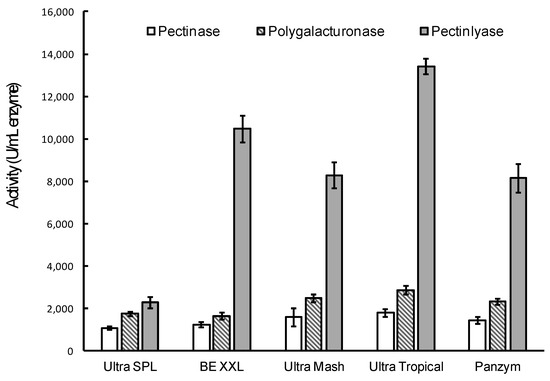
Figure 2.
Pectinase, polygalacturonase, and pectin lyase activities of commercial pectinolytic enzyme preparations. All values are expressed as means ± standard deviation (n = 3).
All five commercial pectinase preparations used are recommended for use in fruit mash for increasing juice yield as well as for juice clarification and improved color stability. Using the above-described spectrophotometric method, pectinase activity of the analyzed preparations was found to be between 1065 and 1784 U/mL enzyme (Figure 2, white bars). The determined pectinase activity was considerably lower (3–10 times lower than the activity declared by the producer (see Section 2.1), which ranged between 3800 and 13,600 PECTU/mL. This can be related to the difference in the method and conditions used for activity measurement. For instance, Biz et al. [34] demonstrated that depending on the particular combination of incubation time, pectin concentration, and reaction temperature, the same extract could be reported to have activities that differ by an order of magnitude. All preparations exhibited a polygalacturonase activity higher than their pectinase activities. The detected polygalacturonase activities measured towards polygalacturonic acid ranged from 1633 to 2861 U/mL enzyme (Figure 2, dashed bars). The difference between the two activities can be explained by the nature of the substrate, as pectin has a more complex structure than polygalacturonic acid, which includes rhamnogalacturonan regions with side chains containing rhamnose, galactose, arabinose, and xylose [37]. In addition, polygalacturonic acid is characterized by low content of methyl esters which facilitates its hydrolysis by polygalacturonases.
The analyzed commercial preparations also exhibited pectin lyase activity ranging between 2283 and 13,417 U/mL enzyme (Figure 2, grey bars). Pectin lyases act on pectin with high degree of methylation without the need of pectin de-esterifying enzymes [33]. Pectin lyases catalyze the cleavage of α (1–4) glycosidic bonds by trans-elimination, resulting in a double bond at the non-reducing end of the galacturonic acid.
Statistical analysis showed that pectinase activities of the analyzed preparations were not significantly different (p = 0.1750), whereas, considering polygalacturonase and pectin lyase activities determined, Pectinex® Ultra Tropical preparation exhibited significantly higher enzymatic activities than the remaining four preparations (p < 0.0001). Thus, we selected this preparation for further immobilization experiments.
3.3. Optimization of Immobilization Process
Nylon 6/6 is a polyamide of which secondary amide groups can be converted into secondary imidate groups by O-alkylation with DMS. This process introduces a reactive functional group into nylon without any accompanying polymerization, and the formed functional groups react directly with amino groups of enzymes to produce a stable amidine link [38]. However, additional use of glutaraldehyde as a linker results in more stable immobilization of enzymes [28].
Nylon threads were first treated with DMS at 60 °C, which has been previously reported as the optimum temperature for nylon activation [30]. We examined the effect of DMS treatment duration (1–7.5 min) on pectinase immobilization, as in addition to generating active groups, the mechanical strength of nylon 6/6 can decrease after a longer treatment over 5 min. Assuming that the thread with immobilized enzyme is homogenous, the activities of immobilized enzymes can be expressed in meters, which is extremely convenient for technological applications [39]. The effect of the length of DMS treatment on the activity of immobilized on nylon 6/6 threads pectinase is presented in Table 2.

Table 2.
The effect of incubation time in DMS on the activity of immobilized pectinase.
Increasing incubation time in DMS resulted in a decreased immobilized pectinase activity. In fact, over O-alkylation of nylon carrier can result in intra-cross-linkage of the amino groups with glutaraldehyde hindering the attachment of the enzyme [30]. For further enzyme immobilization procedure, we incubated nylon carriers in DMS for 2 min at 60 °C, as this treatment time resulted also in more stable immobilized enzymes (as measured after five working cycles). Two different concentrations of glutaraldehyde, 2.5% [40] and 12.5% [28], were used for enzyme immobilization. (Table 3). We did not use higher glutaraldehyde concentrations, as, in high rate immobilization processes, glutaraldehyde promotes crosslinking between enzyme molecules [41].

Table 3.
The effect of glutaraldehyde concentration on pectinase immobilization.
Treatment with 12.5% glutaraldehyde solution resulted in higher immobilized pectinase activity and also residual pectinase activity after five cycles was 2.5 times higher compared to the threads treated with 2.5% glutaraldehyde solution. So, for further immobilization experiments, nylon carriers were treated with 12.5% glutaraldehyde solution for 1 h at room temperature.
After the immobilization process, the carriers should be washed carefully for several times with buffer using an orbital shaker (150 rpm) for 5 to 15 min each time to remove all non-covalently attached enzymes.
3.4. Nylon 6/6 Carriers
Nylon 6/6 is one of the most common polymers for textile and plastic industries and it is available in different forms including pellets, discs, threads, nets, fabric, etc. that have been previously used also for enzyme immobilization [28,29,30,40]. In the current work, we compared the immobilization of pectinase enzyme preparation onto two different nylon 6/6 carriers—threads and pellets (Figure 3). The surface area per 1 g thread- and pellet-shaped carrier was calculated considering the micrometer scale carrier dimensions, and was found to be 0.5760 m2/g and 0.00702 m2/g, respectively. This difference for ~80 times indicated that the higher enzyme loads per amount of carrier can be expected using nylon 6/6 threads.
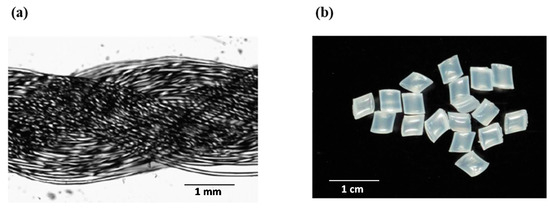
Figure 3.
Nylon 6/6 threads ((a), shot with Olympus CX31) and pellets ((b), shot with UV transilluminator, Cleaver Scientific Ltd., Warwickshire, UK).
3.5. Pectinase Immobilization Yield
Pectinex Ultra Tropical preparation was immobilized onto nylon 6/6 threads and pellets using the selected protocol. Carriers were first soaked into DMS at 60 °C for 2 min, after that, washed with ice-cold methanol and buffer, and kept in 12.5% glutaraldehyde solution 1 h at room temperature. Finally, activated nylon carriers were incubated in 20-fold diluted enzyme solution (89 U/mL) for 24 h at 4 °C. After careful washing to remove unbound enzyme, we measured the pectinase activity of immobilized enzymes and the immobilization yield was expressed as pectinase activity per g of carrier. Results are presented in Table 4.

Table 4.
Pectinase immobilization yield on different nylon 6/6 carriers.
As expected from the comparison of the surface area of thread and pellets, the immobilization yield of pectinase per 1 g carrier was over 40 times higher for threads than pellets, although the pectinase activity per 1 mm2 surface area was ~2 times higher for pellets (262 and 140 µU/mm2 for pellets and threads, respectively).
Up to now, few studies have investigated the immobilization of pectinases on nylon carriers. Shukla et al. [40] immobilized purified polygalacturonase from Aspergillus niger (MTCC 3323) on nylon-6 beads and reported an immobilization yield of 17.54 U/g. This immobilization yield is higher than the measured yield obtained in the present study. This apparent lower yield can be related to the use of the commercial enzyme preparation, which contains different enzymes that are potentially immobilized onto the carrier, although only pectinase activity was assessed.
3.6. Stability and Reusability of the Immobilized Pectinase
Stability and reusability are some of the major features of immobilized enzymes to characterize their industrial applicability. We studied the inactivation of the immobilized enzymes during storage and throughout the active use of both the threads and pellets (Figure 4).
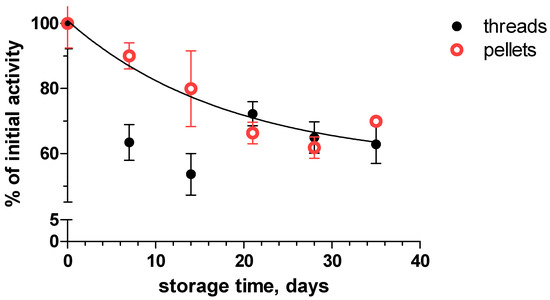
Figure 4.
The storage stability of immobilized pectinase in 0.2 M sodium acetate buffer (pH 4.8) at 4 °C.
The activity decreased in an exponential mode and the relative inactivation was not dependent on the shape of the carrier, indicating that the attachment of the enzymes on the surface of nylon 6/6 carriers was similar. The inactivation process was characterized with a 2-exponential decay model, as there is a stabilization phase after the first quick decay of the activity. The F test revealed that there was no statistically relevant difference in the rate of inactivation process of immobilized pectinase (p = 0.9791) on nylon 6/6 threads or pellets. The half-life of the enzyme fast phase inactivation in 0.2 M sodium acetate buffer (pH 4.8) at 4 °C was 12.8 days and after 5 weeks, the remaining pectinase activity of the unused threads was 63% of the initial activity. Pectinase immobilized on nylon 6/6 threads and pellets exhibits storage stability comparable to other covalent immobilization carriers like sodium alginate/graphene oxide beads via amide bonds, which retained 52% of initial pectinase activity after 30 days in 0.25 M citrate buffer (pH 4.0) at 4 °C [20]. Meanwhile, pectinase immobilized onto chitosan-grafted magnetic nanoparticles has exhibited a better storage stability, retaining 74% of initial activity after 75 days in 5 mM sodium acetate buffer (pH 4.5) at 4 °C [42].
To determine the reusability of immobilized pectinase onto nylon 6/6 threads and pellets, enzyme activity was measured at 40 °C for 30 min for 20 successive cycles (Figure 5). After each cycle, substrate solution was removed and nylon 6/6 carriers were washed with 0.2 M sodium acetate buffer (pH 4.8) for 10 min before adding new substrate solution. Additionally, in this case, the enzyme inactivation obeyed exponential decay and there was no statistically significant difference in inactivation of immobilized pectinase onto nylon 6/6 threads or pellets, but the activity dropped to 40% after five cycles. After 20 successive cycles, the remaining activity of the immobilized pectinase was 22.0 ± 1.6%, indicating the good reusability of the immobilized enzyme. An earlier study by Shukla et al. [40] reported that polygalacturonase immobilized onto nylon-6 particles retained 50% of its initial activity for three cycles and only 17% after seven cycles. Recent studies on covalent immobilization of pectinase onto magnetic nanoparticle carriers reported better reusability potential where immobilized enzymes retained 50 to 60% of initial pectinase activity after 15 cycles [25,42].
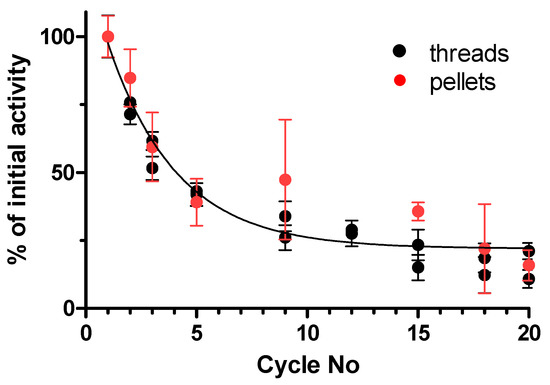
Figure 5.
The reusability of immobilized pectinase onto nylon 6/6 threads and beads during 20 successive cycles.
4. Conclusions
In the present work, commercial pectinase preparation was immobilized onto DMS/glutaraldehyde-activated nylon 6/6 carriers. The obtained results showed that pectinase immobilization yield per g of carrier was more than 40 times higher in nylon 6/6 thread than pellets in accordance with a larger surface area per gram of thread compared to pellets. However, the stability and reusability studies showed that the immobilized enzyme inactivation was independent from the shape of the nylon carrier, indicating similar attachment of the enzyme to the carriers. Immobilized pectinase was relatively stable during storage, retaining more than 60% of its initial activity after 35 days at 4 °C. In addition, the immobilized pectinase exhibited good reusability, retaining 40% of its initial activity after five successive cycles and more than 20% after twenty successive cycles. The obtained results indicate the high potential of immobilized pectinase on nylon 6/6 pectinase preparation for industrial application, as it retains sufficient activity after numerous working cycles and the nylon 6/6 carrier is extremely suitable for operating in food production processes due to its inertness, sturdiness, and availability. However, further studies of practical utilization are required to assess the effect of potentially available enzyme inhibitors present in plant material.
Author Contributions
Conceptualization, T.R. and S.B.-O.; methodology, S.B.-O. and T.R.; investigation, S.B.-O. and T.R.; writing—original draft preparation, S.B.-O. and T.R.; writing—review and editing, S.B.-O. and T.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by VALORTECH project, which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 810630. Funding received from Mobilitas Pluss ERA-Chair support (Grant no. MOBEC006 ERA Chair for Food (By-) Products Valorisation Technologies of the Estonian University of Life Sciences) is also acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A review. Process Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Garg, G.; Singh, A.; Kaur, A.; Singh, R.; Kaur, J.; Mahajan, R. Microbial pectinases: An ecofriendly tool of nature for industries. 3 Biotech 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Rout, J.R.; Kerry, R.G.; Thatoi, H.; Sahoo, S.L. Biochemical Prospects of Various Microbial Pectinase and Pectin: An Approachable Concept in Pharmaceutical Bioprocessing. Front. Nutr. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M. Recent advances in the production strategies of microbial pectinases—A review. Int. J. Biol. Macromol. 2019, 122, 1017–1026. [Google Scholar] [CrossRef]
- John, J.; Kaimal, K.K.S.; Smith, M.L.; Rahman, P.K.S.M.; Chellam, P.V. Advances in upstream and downstream strategies of pectinase bioprocessing: A review. Int. J. Biol. Macromol. 2020, 162, 1086–1099. [Google Scholar] [CrossRef]
- Sharma, H.P.; Patel, H. Sugandha Enzymatic added extraction and clarification of fruit juices—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.E.; Ponce-Mora, M.C.; Noseda, D.G.; Cazabat, G.; Saravalli, C.; López, M.C.; Gil, G.P.; Blasco, M.; Albertó, E.O. Pectinase production by Aspergillus giganteus in solid-state fermentation: Optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J. Ind. Microbiol. Biotechnol. 2017, 44, 197–211. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Kumari Dubey, K.; Singhal, R.S. Improvements in the extraction of bioactive compounds by enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and characterization of a Combi-CLEAs from pectinases and cellulases: A potential biocatalyst for grape juice clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Ottone, C.; Romero, O.; Aburto, C.; Illanes, A.; Wilson, L. Biocatalysis in the winemaking industry: Challenges and opportunities for immobilized enzymes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 595–621. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, H.L.; Briones, A.I.; Úbeda, J.; Arevalo, M. Immobilization of pectinase by adsorption on an alginate-coated chitin support. Biotecnol. Apl. 2013, 30, 101–104. [Google Scholar]
- Chauhan, S.; Vohra, A.; Lakhanpal, A.; Gupta, R. Immobilization of Commercial Pectinase (Polygalacturonase) on Celite and Its Application in Juice Clarification. J. Food Process. Preserv. 2015, 39, 2135–2141. [Google Scholar] [CrossRef]
- Rajdeo, K.; Harini, T.; Lavanya, K.; Fadnavis, N.W. Immobilization of pectinase on reusable polymer support for clarification of apple juice. Food Bioprod. Process. 2016, 99, 12–19. [Google Scholar] [CrossRef]
- Cerreti, M.; Markošová, K.; Esti, M.; Rosenberg, M.; Rebroš, M. Immobilisation of pectinases into PVA gel for fruit juice application. Int. J. Food Sci. Technol. 2017, 52, 531–539. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; Dias, J.L.; da Silva, O.S.; Porto, T.S. Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod. Process. 2018, 109, 9–18. [Google Scholar] [CrossRef]
- Martín, M.C.; López, O.V.; Ciolino, A.E.; Morata, V.I.; Villar, M.A.; Ninago, M.D. Immobilization of enological pectinase in calcium alginate hydrogels: A potential biocatalyst for winemaking. Biocatal. Agric. Biotechnol. 2019, 18, 101091. [Google Scholar] [CrossRef]
- Mohammadi, M.; Khakbaz Heshmati, M.; Sarabandi, K.; Fathi, M.; Lim, L.T.; Hamishehkar, H. Activated alginate-montmorillonite beads as an efficient carrier for pectinase immobilization. Int. J. Biol. Macromol. 2019, 137, 253–260. [Google Scholar] [CrossRef]
- Abdel Wahab, W.A.; Karam, E.A.; Hassan, M.E.; Kansoh, A.L.; Esawya, M.A.; Awad, G.E.A. Optimization of pectinase immobilization on grafted alginate-agar gel beads by 24 full factorial CCD and thermodynamic profiling for evaluating of operational covalent immobilization. Int. J. Biol. Macromol. 2018, 113, 159–170. [Google Scholar] [CrossRef]
- Dai, X.Y.; Kong, L.M.; Wang, X.L.; Zhu, Q.; Chen, K.; Zhou, T. Preparation, characterization and catalytic behavior of pectinase covalently immobilized onto sodium alginate/graphene oxide composite beads. Food Chem. 2018, 253, 185–193. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Dai, X.-Y.; Wei, X.-Y.; Zhu, Q.; Zhou, T. Co-immobilization of pectinase and glucoamylase onto sodium aliginate/graphene oxide composite beads and its application in the preparation of pumpkin–hawthorn juice. J. Food Biochem. 2019, 43, e122741. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016, 213, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Ladole, M.R.; Nair, R.R.; Bhutada, Y.D.; Amritkar, V.D.; Pandit, A.B. Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason. Sonochem. 2018, 48, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. A co-immobilization of pectinase and cellulase onto magnetic nanoparticles for antioxidant extraction from waste fruit peels. Biocatal. Agric. Biotechnol. 2019, 17, 470–479. [Google Scholar] [CrossRef]
- Dal Magro, L.; de Moura, K.S.; Backes, B.E.; de Menezes, E.W.; Benvenutti, E.V.; Nicolodi, S.; Klein, M.P.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization of pectinase on chitosan-magnetic particles: Influence of particle preparation protocol on enzyme properties for fruit juice clarification. Biotechnol. Rep. 2019, 24, e00373. [Google Scholar] [CrossRef]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of Immobilized Enzymes in Food Industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef]
- Wu, X.; Fraser, K.; Zha, J.; Dordick, J.S. Flexible Peptide Linkers Enhance the Antimicrobial Activity of Surface-Immobilized Bacteriolytic Enzymes. ACS Appl. Mater. Interfaces 2018, 10, 36746–36756. [Google Scholar] [CrossRef]
- Kivirand, K.; Rinken, T. Preparation and characterization of cadaverine sensitive nylon threads. Sens. Lett. 2009, 7, 580–585. [Google Scholar] [CrossRef]
- Damle, M.; Badhe, P.; Mahajan, G.; RV, A. Immobilisation of marine pectinase on nylon 6,6. J. Text. Eng. Fash. Technol. 2018, 4, 181–187. [Google Scholar] [CrossRef]
- Nan, C.; Zhang, Y.; Zhang, G.; Dong, C.; Shuang, S.; Choi, M.M.F. Activation of nylon net and its application to a biosensor for determination of glucose in human serum. Enzym. Microb. Technol. 2009, 44, 249–253. [Google Scholar] [CrossRef]
- Pahujani, S.; Kanwar, S.S.; Chauhan, G.; Gupta, R. Glutaraldehyde activation of polymer Nylon-6 for lipase immobilization: Enzyme characteristics and stability. Bioresour. Technol. 2008, 99, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Enzymatic Assay of Pectinase|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/technical-documents/protocols/biology/enzymatic-assay-of-pectinase.html (accessed on 25 March 2021).
- Dal Magro, L.; Goetze, D.; Ribeiro, C.T.; Paludo, N.; Rodrigues, E.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Identification of Bioactive Compounds From Vitis labrusca L. Variety Concord Grape Juice Treated With Commercial Enzymes: Improved Yield and Quality Parameters. Food Bioprocess Technol. 2016, 9, 365–377. [Google Scholar] [CrossRef]
- Biz, A.; Farias, F.C.; Motter, F.A.; De Paula, D.H.; Richard, P.; Krieger, N.; Mitchell, D.A. Pectinase activity determination: An early deceleration in the release of reducing sugars throws a spanner in the works! PLoS ONE 2014, 9, e109529. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; The “Gold Book”; Blackwell Science: Oxford, UK, 1997; Volume 1. [Google Scholar]
- Tapre, A.R.; Jain, R.K. Pectinases: Enzymes for fruit processing industry. Int. Food Res. J. 2014, 21, 447–453. [Google Scholar]
- Morris, D.L.; Campbell, J.; Hornby, W.E. A chemistry for the immobilization of enzymes on nylon. The preparation of nylon tube supported hexokinase and glucose 6 phosphate dehydrogenase and the use of the co immobilized enzymes in the automated determination of glucose. Biochem. J. 1975, 147, 593–603. [Google Scholar] [CrossRef]
- Rinken, T.; Järv, J.; Rinken, A. Production of biosensors with exchangeable enzyme-containing threads. Anal. Chem. 2007, 79, 6042–6044. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, S.; Thakur, J.; Gupta, R. Immobilization of polygalacturonase from Aspergilus niger onto glutaraldehyde activated Nylon-6 and its application in apple juice clarification. Acta Aliment. 2010, 39, 277–292. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Alonso-Morales, N.; Dellamora, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Glutaraldehyde in Protein Immobilization. In Immobilization of Enzymes and Cells; Guisan, J.M., Ed.; Humana Press, 2006; pp. 57–64. Available online: https://link.springer.com/protocol/10.1007/978-1-59745-053-9_5#citeas (accessed on 25 March 2021). [CrossRef]
- Soozanipour, A.; Taheri-Kafrani, A.; Barkhori, M.; Nasrollahzadeh, M. Preparation of a stable and robust nanobiocatalyst by efficiently immobilizing of pectinase onto cyanuric chloride-functionalized chitosan grafted magnetic nanoparticles. J. Colloid Interface Sci. 2019, 536, 261–270. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).